Quercetin Suppresses CYR61-Mediated Multidrug Resistance in Human Gastric Adenocarcinoma AGS Cells
Abstract
:1. Introduction
2. Results
2.1. Multidrug Resistance in AGS-cyr61 Cells
2.2. Quercetin Downregulates Drug Resistance-Related Proteins in AGS-cyr61 Cells
2.3. Quercetin Induces Apoptosis and Inhibits Colony Formation in AGS-cyr61 Cells
2.4. Quercetin Inhibits Cell Migration and Downregulates the Epithelial–Mesenchymal Transition (EMT) in AGS-cyr61 Cells
2.5. Quercetin Synergizes the Actions of the Standard Chemotherapeutic Agents 5-FU and ADR
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Gene Transfection
4.4. Measurement of Cell Viability
4.5. Western Blotting
4.6. Microscopic Observation of Nuclear Morphology
4.7. Wound-Healing Assays
4.8. Clonogenic Assay
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.R.; Chang, C.C.; Chen, L.R.; Wu, M.H.; Wang, M.Y.; Kuo, I.H.; Chu, C.Y.; Chang, K.J.; Lee, P.H.; Chen, W.J.; et al. Cysteine-rich 61 (CCN1) enhances chemotactic migration, transendothelial cell migration, and intravasation by concomitantly up-regulating chemokine receptor 1 and 2. Mol. Cancer Res. 2007, 5, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Chang, C.C.; Lin, B.R.; Yang, H.Y.; Chu, C.Y.; Wu, M.H.; Kuo, M.L. Elevated expression of Cyr61 enhances peritoneal dissemination of gastric cancer cells through integrin alpha2beta1. J. Biol. Chem. 2007, 282, 34594–34604. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Zuon, C.Y.; Chang, C.C.; Chen, S.T.; Chen, C.P.; Lin, B.R.; Wang, M.Y.; Jeng, Y.M.; Chang, K.J.; Lee, P.H.; et al. Cyr61 induces gastric cancer cell motility/invasion via activation of the integrin/nuclear factor-kappaB/cyclooxygenase-2 signaling pathway. Clin. Cancer Res. 2005, 11, 5809–5820. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vellon, L.; Mehmi, I.; Teng, P.K.; Griggs, D.W.; Lupu, R. A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene 2005, 24, 761–779. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Ho, K.C.; Hao, Y.; Yang, X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011, 71, 2728–2738. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.H.; Noh, S.H.; Shin, D.W.; Choi, S.H.; Min, J.S. Recurrence following curative resection for gastric carcinoma. Br. J. Surg. 2000, 87, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Wu, M.S.; Hua, K.T.; Kuo, M.L.; Lin, M.T. Cyr61/CTGF/Nov family proteins in gastric carcinogenesis. World J. Gastroenterol. 2014, 20, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Gallus, J.; Juvale, K.; Wiese, M. Characterization of 3-methoxy flavones for their interaction with ABCG2 as suggested by ATPase activity. Biochim. Biophys. Acta 2014, 1838, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.; Ahn, K.S.; Song, Y.W.; Moon, J.Y.; Cho, M.; Lim, Y.; Cho, S.K. Mechanism of 2′,3′-dimethoxyflavanone-induced apoptosis in breast cancer stem cells: Role of ubiquitination of caspase-8 and LC3. Arch. Biochem. Biophys. 2014, 562, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, R.C.; Kamachi, H.; Kikumori, M.; Tokuda, H.; Suzuki, N.; Suenaga, K.; Nagai, H.; Irie, K. Effects of the methoxy group in the side chain of debromoaplysiatoxin on its tumor-promoting and anti-proliferative activities. Bioorg. Med. Chem. Lett. 2013, 23, 4319–4323. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Anticancer properties of the flavone wogonin. Toxicology 2013, 314, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jo, B.W.; Kim, Y.C. Enhanced paclitaxel bioavailability after oral administration of paclitaxel or prodrug to rats pretreated with quercetin. Eur. J. Pharm. Biopharm. 2004, 57, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Paduch, R.; Piersiak, T.; Głowniak, K.; Gawron, A.; Kandefer-Szerszen, M. The effect of quercetin on pro-apoptotic activity of cisplatin in HeLa cells. Biochem. Pharmacol. 2004, 69, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Cho, M.; Ahn, K.S.; Cho, S.K. Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutr. Cancer 2013, 65, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Huang, Y.; Sadee, W. Growth factor signaling and resistance to cancer chemotherapy. Curr. Top. Med. Chem. 2004, 4, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, N.; Nishimoto, A.; Hosoyama, T.; Ueno, K.; Enoki, T.; Li, T.S.; Hamano, K. The c-MYC-ABCB5 axis plays a pivotal role in 5-fluorouracil resistance in human colon cancer cells. J. Cell. Mol. Med. 2015, 19, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Gouazé, V.; Yu, J.Y.; Bleicher, R.J.; Han, T.Y.; Liu, Y.Y.; Wang, H.; Gottesman, M.M.; Bitterman, A.; Giuliano, A.E.; Cabot, M.C. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol. Cancer Ther. 2004, 5, 633–638. [Google Scholar]
- Song, M.; Zang, W.; Zhang, B.; Cao, J.; Yang, G. GCS overexpression is associated with multidrug resistance of human HCT-8 colon cancer cells. J. Exp. Clin. Cancer Res. 2012, 31, 23. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Chang, C.C.; Chen, S.T.; Chang, H.L.; Su, J.L.; Chau, Y.P.; Kuo, M.L. Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-kappaB-dependent XIAP up-regulation. J. Biol. Chem. 2004, 279, 24015–24023. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, Y.; Yang, J.; Gong, L.; Zhao, P.; Zhang, Y.; Su, H. The up-regulation of cysteine-rich protein 61 induced by transforming growth factor beta enhances osteosarcoma cell migration. Mol. Cell. Biochem. 2013, 384, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, B.P. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin. J. Cancer 2001, 30, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.L.; Heckford, S.E.; Skerker, J.M.; Worland, P.; Torri, J.A.; Thompson, E.W.; Byers, S.W.; Gelmann, E.P. Loss of epithelial markers and acquisition of vimentin expression in adriamycin-resistant and vinblastine-resistant human breast-cancer cell-lines. Cancer Res. 1992, 52, 5190–5197. [Google Scholar] [PubMed]
- Xavier, C.P.; Lima, C.F.; Rohde, M.; Pereira-Wilson, C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011, 68, 1449–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, T.; Fadlalla, K.; Mosley, L.; Katkoori, V.; Turner, T.; Manne, U. Dual-mode interaction between quercetin and DNA-damaging drugs in cancer cells. Anticancer Res. 2012, 32, 61–71. [Google Scholar] [PubMed]
- Sun, Z.J.; Wang, Y.; Cai, Z.; Chen, P.P.; Tong, X.J.; Xie, D. Involvement of Cyr61 in growth, migration, and metastasis of prostate cancer cells. Br. J. Cancer 2008, 99, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Radeff-Huang, J.; Matteo, R.; Hsiao, A.; Subramaniam, S.; Stupack, D.; Brown, J.H. Thrombin receptor and RhoA mediate cell proliferation through integrins and cysteine-rich protein 61. FASEB J. 2008, 22, 4011–4021. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Yu, Y.; Perlaky, L.; Man, T.K.; Redell, M.S. Stromal CYR61 Confers Resistance to Mitoxantrone via Spleen Tyrosine Kinase Activation in Human Acute Myeloid Leukaemia. Br. J. Haematol. 2015, 170, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R., Jr.; Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updates 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Eustermann, S.; Wu, W.F.; Langelier, M.F.; Yang, J.C.; Easton, L.E.; Riccio, A.A.; Pascal, J.M.; Neuhaus, D. Structural Basis of Detection and Signaling of DNA Single-Strand Breaks by Human PARP-1. Mol. Cell 2015, 60, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Curtin, N.J. The role of PARP in DNA repair and its therapeutic exploitation. Br. J. Cancer 2011, 105, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Soengas, M.S.; Lowe, S.W. Apoptosis and melanoma chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.N.; Bhoumik, A.; Ronai, Z. Death receptors and melanoma resistance to apoptosis. Oncogene 2003, 22, 3152–3161. [Google Scholar] [CrossRef] [PubMed]
- Higashitsuji, H.; Higashitsuji, H.; Nagao, T.; Nonoguchi, K.; Fujii, S.; Itoh, K.; Fujita, J. A novel protein overexpressed in hepatoma accelerates export of NF-kappa B from the nucleus and inhibits p53-dependent apoptosis. Cancer Cell 2002, 2, 335–346. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed]
- Ryo, A.; Suizu, F.; Yoshida, Y.; Perrem, K.; Liou, Y.C.; Wulf, G.; Rottapel, R.; Yamaoka, S.; Lu, K.P. Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 2003, 12, 1413–1426. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Montagut, C.; Tusquets, I.; Ferrer, B.; Corominas, J.M.; Bellosillo, B.; Campas, C.; Suarez, M.; Fabregat, X.; Campo, E.; Gascon, P.; et al. Activation of nuclear factor-kappa B is linked to resistance to neoadjuvant chemotherapy in breast cancer patients. Endocr.-Relat. Cancer 2006, 13, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Bu, C.; Hu, W.; Zhang, H.; Liu, M.; Zhai, G. Preparation and in vitro and in vivo evaluation of quercetin-loaded mixed micelles for oral delivery. Biosci. Biotechnol. Biochem. 2018, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, Y.; Gao, C.; Li, Y.; Chen, S.; Xiong, T.; Li, J.; Du, M.; Gong, Z.; Chen, H.; et al. Characterization and biodistribution in vivo of quercetin-loaded cationic nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2014, 1, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Maity, S.; Mandal, S.; Chakraborti, A.S.; Prajapati, A.K.; Kundu, P.P. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym. 2018, 15, 42–51. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |






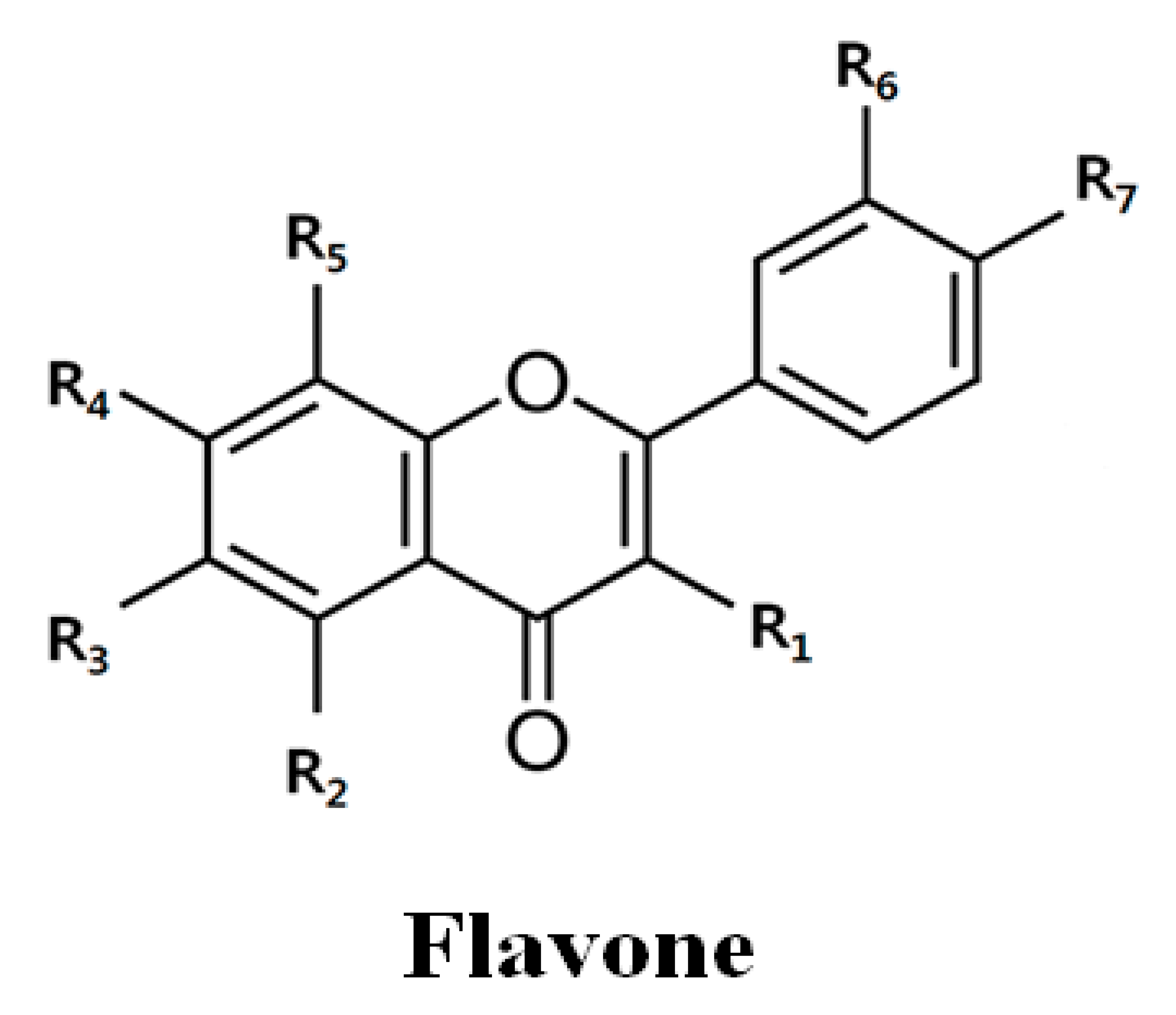
| Flavones | R1 | R2 | R3 | R4 | R5 | R6 | R7 | IC50 * | |
|---|---|---|---|---|---|---|---|---|---|
| AGS | AGS-cyr61 | ||||||||
| Quercetin | OH | OH | H | OH | H | OH | OH | 73.1 ± 9.1 | 46.5 ± 15.1 |
| Tangeretin | H | OCH3 | OCH3 | OCH3 | OCH3 | H | OCH3 | >100 | >100 |
| PMF | OCH3 | OCH3 | H | OCH3 | H | OCH3 | OCH3 | 49.8 ± 6.4 | >200 |
| Nobiletin | H | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | >100 | >100 |
| Quercetin | 25 μM | 50 µM | ||||
|---|---|---|---|---|---|---|
| Drugs |  |  | ||||
| 1 * | 2 | 3 | 1 | 2 | 3 | |
| 5-FU | 0.33 | 0.26 | 0.21 | 0.54 | 0.38 | 0.25 |
| ADR | 0.18 | 0.20 | 0.20 | 0.34 | 0.32 | 0.25 |
| TAM | 0.89 | 1.04 | 0.81 | 0.78 | 0.83 | 0.83 |
| PAC | 0.36 | 1.86 | 6.87 | 0.73 | 0.51 | 0.48 |
| DOC | 0.25 | 1.20 | 2.96 | 0.81 | 1.20 | 0.59 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, H.B.; Moon, J.Y.; Cho, S.K. Quercetin Suppresses CYR61-Mediated Multidrug Resistance in Human Gastric Adenocarcinoma AGS Cells. Molecules 2018, 23, 209. https://doi.org/10.3390/molecules23020209
Hyun HB, Moon JY, Cho SK. Quercetin Suppresses CYR61-Mediated Multidrug Resistance in Human Gastric Adenocarcinoma AGS Cells. Molecules. 2018; 23(2):209. https://doi.org/10.3390/molecules23020209
Chicago/Turabian StyleHyun, Ho Bong, Jeong Yong Moon, and Somi Kim Cho. 2018. "Quercetin Suppresses CYR61-Mediated Multidrug Resistance in Human Gastric Adenocarcinoma AGS Cells" Molecules 23, no. 2: 209. https://doi.org/10.3390/molecules23020209
APA StyleHyun, H. B., Moon, J. Y., & Cho, S. K. (2018). Quercetin Suppresses CYR61-Mediated Multidrug Resistance in Human Gastric Adenocarcinoma AGS Cells. Molecules, 23(2), 209. https://doi.org/10.3390/molecules23020209




