Figure 1.
Location of Coulet des Roches. Map modified from Geoatlas.
Figure 1.
Location of Coulet des Roches. Map modified from Geoatlas.
Figure 2.
Coulet des Roches. Cross section. Topographical survey: Gandelet S. and Bérard Ch.
Figure 2.
Coulet des Roches. Cross section. Topographical survey: Gandelet S. and Bérard Ch.
Figure 3.
Scheme of measurements of mustelid calvarium: 1, total length (prosthion–acrocranion); 2, condylobasal length (prosthion–occipital condyles); 3, basal length (prosthion–basion); 4, viscerocranium length (prosthion–point F); 5, neurocranium length (point F—acrocranion); 6, facial length (prosthion–point F); 7, rostrum length (prosthion–infraorbital); 8, rostrum length (anterior margin of I1 to posterior margin of M1); 9, palatal length (prosthion–staphylion); 10, C1-M1 length on alveoli (anterior margin of C1 alveolus to the posterior margin of M1 alveolus); 11, upper premolar row length on alveoli (anterior margin of P2 alveolus to the posterior margin of P4 alveolus); 12, breadth at zygomatic arches (zygion–zygion); 13, incisor row breadth (I3-I3 breadth); 14, maximal breadth at the canine alveoli; 15, maximal breadth at the P4 alveoli; 16, maximal breadth at the M1 alveoli; 17, lowest palatal breadth; 18, lowest breadth between infraorbital foramina; 19, lowest breadth between orbits (entorbital–entorbital); 20, frontal breadth (ectorbital–ectorbital); 21, postorbital lowest breadth (postorbital bar); 22, maximal neurocranium breadth (euryon–euryon); 23, mastoid breadth (otion–otion); 24, maximal breadth of occipital condyles; 25, nasal aperture height; 26, nasal aperture breadth; 27, height of foramen magnum (basionopisthion); 28, breadth of foramen magnum; 29, bullae ossae length; 30, bullae ossae breadth; 31, cranial height (acrocranion–basion); 32, maximal cranial height (staphylion-frontal).
Figure 3.
Scheme of measurements of mustelid calvarium: 1, total length (prosthion–acrocranion); 2, condylobasal length (prosthion–occipital condyles); 3, basal length (prosthion–basion); 4, viscerocranium length (prosthion–point F); 5, neurocranium length (point F—acrocranion); 6, facial length (prosthion–point F); 7, rostrum length (prosthion–infraorbital); 8, rostrum length (anterior margin of I1 to posterior margin of M1); 9, palatal length (prosthion–staphylion); 10, C1-M1 length on alveoli (anterior margin of C1 alveolus to the posterior margin of M1 alveolus); 11, upper premolar row length on alveoli (anterior margin of P2 alveolus to the posterior margin of P4 alveolus); 12, breadth at zygomatic arches (zygion–zygion); 13, incisor row breadth (I3-I3 breadth); 14, maximal breadth at the canine alveoli; 15, maximal breadth at the P4 alveoli; 16, maximal breadth at the M1 alveoli; 17, lowest palatal breadth; 18, lowest breadth between infraorbital foramina; 19, lowest breadth between orbits (entorbital–entorbital); 20, frontal breadth (ectorbital–ectorbital); 21, postorbital lowest breadth (postorbital bar); 22, maximal neurocranium breadth (euryon–euryon); 23, mastoid breadth (otion–otion); 24, maximal breadth of occipital condyles; 25, nasal aperture height; 26, nasal aperture breadth; 27, height of foramen magnum (basionopisthion); 28, breadth of foramen magnum; 29, bullae ossae length; 30, bullae ossae breadth; 31, cranial height (acrocranion–basion); 32, maximal cranial height (staphylion-frontal).
![Quaternary 01 00019 g003]()
Figure 4.
Scheme of measurements of mustelid mandible: 1, total length (condyle to infradentale); 2, distance of angular process to infradentale; 3, distance of infradentale to anterior margin of masseter fossa; 4, distance of anterior margin of c1 to posterior margin of m2; 5, cheek teeth row length (anterior margin of p1 to posterior margin of m2); 6, premolar row length (anterior margin of p1 to posterior margin of p4); 7, molar row length (anterior margin of m1 to posterior margin of m2); 8, distance between mental foramina; 9, distance of posterior margin of m2 to condyle; 10, distance of angular process to coronoid process; 11, mandible maximum height; 12, mandible body height between p3 and p4; 13, mandible body thickness between p3 and p4; 14, mandible body height between m1 and m2; 15, mandible body thickness between m1 and m2; 16, condyle height; 17, condyle breadth; 18, symphysis maximum diameter; 19, symphysis minimum diameter.
Figure 4.
Scheme of measurements of mustelid mandible: 1, total length (condyle to infradentale); 2, distance of angular process to infradentale; 3, distance of infradentale to anterior margin of masseter fossa; 4, distance of anterior margin of c1 to posterior margin of m2; 5, cheek teeth row length (anterior margin of p1 to posterior margin of m2); 6, premolar row length (anterior margin of p1 to posterior margin of p4); 7, molar row length (anterior margin of m1 to posterior margin of m2); 8, distance between mental foramina; 9, distance of posterior margin of m2 to condyle; 10, distance of angular process to coronoid process; 11, mandible maximum height; 12, mandible body height between p3 and p4; 13, mandible body thickness between p3 and p4; 14, mandible body height between m1 and m2; 15, mandible body thickness between m1 and m2; 16, condyle height; 17, condyle breadth; 18, symphysis maximum diameter; 19, symphysis minimum diameter.
Figure 5.
Lower carnassial (m1) measurements (left) and cusp terminology (right). L, total length; L tri, trigonid length; L tal, talonid length; B tri, trigonid breadth; B tal, talonid breadth; tri, trigonid; tal, talonid; par, paraconid; pro, protoconid; met, metaconid; hyp, hypoconid.
Figure 5.
Lower carnassial (m1) measurements (left) and cusp terminology (right). L, total length; L tri, trigonid length; L tal, talonid length; B tri, trigonid breadth; B tal, talonid breadth; tri, trigonid; tal, talonid; par, paraconid; pro, protoconid; met, metaconid; hyp, hypoconid.
Figure 6.
Schematic presentation of cranial features distinguishing Mustela eversmanii (1) from Mustela putorius (2). The features are indicated by red arrows, the numbers next to them correspond to the number of characteristics described in the text.
Figure 6.
Schematic presentation of cranial features distinguishing Mustela eversmanii (1) from Mustela putorius (2). The features are indicated by red arrows, the numbers next to them correspond to the number of characteristics described in the text.
Figure 7.
Schematic presentation of dental features distinguishing Mustela putorius (1) from Mustela eversmanii (2). The features are indicated by red arrows, the numbers next to them correspond to the numbers of the characteristics described in the text.
Figure 7.
Schematic presentation of dental features distinguishing Mustela putorius (1) from Mustela eversmanii (2). The features are indicated by red arrows, the numbers next to them correspond to the numbers of the characteristics described in the text.
Figure 8.
Cranial material of polecats. Mustela putorius: ♂ skull from Coulet des Roches FdN R 53 (1a–c), ♀ skull from Aven des Planes (2a,b), right (4) and left mandible (5) of ♀ from Aven des Planes, right ♂ mandible from Coulet des Roches Fond nord (FdN R 53) (6), right R 7940 (7) and left ♂ mandible R 3994 (8) from Coulet des Roches heap. Mustela eversmanii: ♂ skull from Aven de la Terrasse (3a,b). Skulls showed in dorsal (a), ventral (b) and lateral (c) views, mandibles in buccal view. Scale bar 20 mm. Photos: C. Triat.
Figure 8.
Cranial material of polecats. Mustela putorius: ♂ skull from Coulet des Roches FdN R 53 (1a–c), ♀ skull from Aven des Planes (2a,b), right (4) and left mandible (5) of ♀ from Aven des Planes, right ♂ mandible from Coulet des Roches Fond nord (FdN R 53) (6), right R 7940 (7) and left ♂ mandible R 3994 (8) from Coulet des Roches heap. Mustela eversmanii: ♂ skull from Aven de la Terrasse (3a,b). Skulls showed in dorsal (a), ventral (b) and lateral (c) views, mandibles in buccal view. Scale bar 20 mm. Photos: C. Triat.
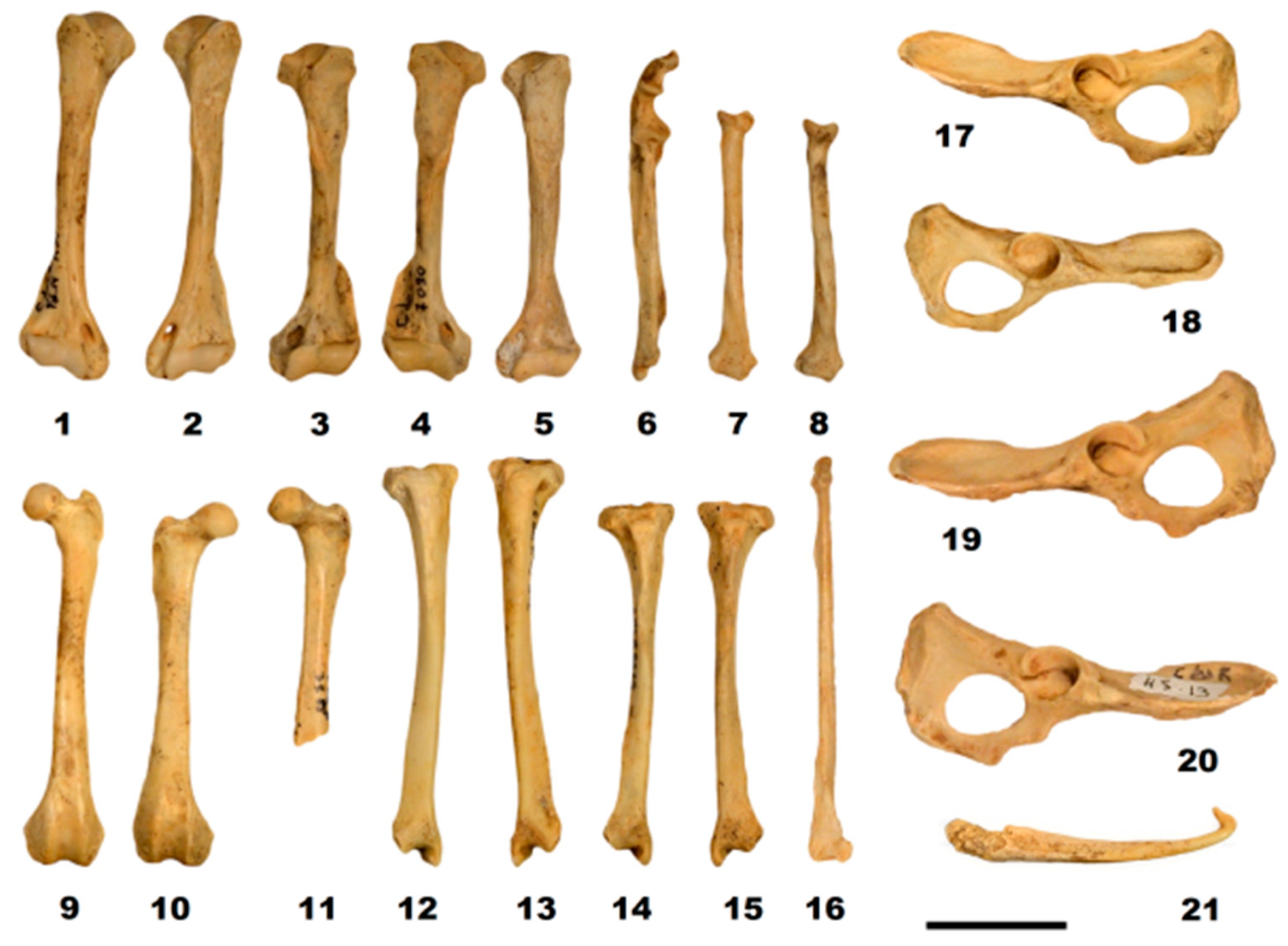
Figure 9.
Postcranial material of Mustela putorius: right humerus (1); left humerus FdN R 35 (2); left humerus FdN R 36 (3); right humerus R 7096 (4); left humerus R 7090 (5); right ulna R 9326 (6); right radius G5 16-4 (7); right radius R 9325 (8); left femur G5 16-1 (9); right femur R 3288 (10); left femur R 9328 (11); right tibia G5 28-1 (12); left tibia G5 16-2 (13); right tibia R 8874 (14); left tibia R 8875 (15); left fibula M5 432-1 (16); left pelvis G5 18-1 (17); right pelvis R 8876 (18); left pelvis G5 18-1 (19); right pelvis H5 13 (20); and baculum (21). Scale bar 20 mm. Photos: C. Triat.
Figure 9.
Postcranial material of Mustela putorius: right humerus (1); left humerus FdN R 35 (2); left humerus FdN R 36 (3); right humerus R 7096 (4); left humerus R 7090 (5); right ulna R 9326 (6); right radius G5 16-4 (7); right radius R 9325 (8); left femur G5 16-1 (9); right femur R 3288 (10); left femur R 9328 (11); right tibia G5 28-1 (12); left tibia G5 16-2 (13); right tibia R 8874 (14); left tibia R 8875 (15); left fibula M5 432-1 (16); left pelvis G5 18-1 (17); right pelvis R 8876 (18); left pelvis G5 18-1 (19); right pelvis H5 13 (20); and baculum (21). Scale bar 20 mm. Photos: C. Triat.
Figure 10.
Postorbital skull breadth plotted against condylobasal length in
Mustela eversmanii and
Mustela putorius. Data from Marciszak [
21], Koby [
27], Zapfe [
31], Sickenberg [
33], Hugueney [
35], and Crégut-Bonnoure and Guérin [
38].
Figure 10.
Postorbital skull breadth plotted against condylobasal length in
Mustela eversmanii and
Mustela putorius. Data from Marciszak [
21], Koby [
27], Zapfe [
31], Sickenberg [
33], Hugueney [
35], and Crégut-Bonnoure and Guérin [
38].
Figure 11.
Length of m1 (L m1) plotted against length of m2 (L m2) in
Mustela eversmanii and
Mustela putorius. Data from Marciszak [
21], Koby [
27], Zapfe [
31], Sickenberg [
33], Hugueney [
35], and Crégut-Bonnoure and Guérin [
38].
Figure 11.
Length of m1 (L m1) plotted against length of m2 (L m2) in
Mustela eversmanii and
Mustela putorius. Data from Marciszak [
21], Koby [
27], Zapfe [
31], Sickenberg [
33], Hugueney [
35], and Crégut-Bonnoure and Guérin [
38].
Figure 12.
Schematic presentation of the cranial and dental features distinguishing Mustela erminea (1) from Mustela nivalis (2). The features are indicated by red arrows, the numbers next to them correspond to the number of characteristics described in the text.
Figure 12.
Schematic presentation of the cranial and dental features distinguishing Mustela erminea (1) from Mustela nivalis (2). The features are indicated by red arrows, the numbers next to them correspond to the number of characteristics described in the text.
Figure 13.
Skulls of Mustela nivalis from Coulet des Roches: M4 217 (1a,b); F5 R 1-1 (2a,b); J5 7 (3); G5 10-1 (4a,b); N3-N4 2 (5a,b); N4 35 (6a,b); M4 548 (7a,b); M3 188 (8a,b); R 8855 (9a,b); R 8856 (10a,b); R 8857 (11a,b); R 7057 (12a,b); and R 2878 (13a,b). Skulls are shown in dorsal (a) and ventral (b) views. Scale bar 20 mm. Photos: C. Triat.
Figure 13.
Skulls of Mustela nivalis from Coulet des Roches: M4 217 (1a,b); F5 R 1-1 (2a,b); J5 7 (3); G5 10-1 (4a,b); N3-N4 2 (5a,b); N4 35 (6a,b); M4 548 (7a,b); M3 188 (8a,b); R 8855 (9a,b); R 8856 (10a,b); R 8857 (11a,b); R 7057 (12a,b); and R 2878 (13a,b). Skulls are shown in dorsal (a) and ventral (b) views. Scale bar 20 mm. Photos: C. Triat.
Figure 14.
Skulls of Mustela erminea from Coulet des Roches: F5 5 (1a,b); J3 23 (2a,b); N4 164 (3a,b); R 7056 (4a,b); M5 512 (5a,b); and M6 400-4 (6a,b). Skulls are shown in dorsal (a) and ventral (b) view. Scale bar 20 mm. Photos: C. Triat.
Figure 14.
Skulls of Mustela erminea from Coulet des Roches: F5 5 (1a,b); J3 23 (2a,b); N4 164 (3a,b); R 7056 (4a,b); M5 512 (5a,b); and M6 400-4 (6a,b). Skulls are shown in dorsal (a) and ventral (b) view. Scale bar 20 mm. Photos: C. Triat.
Figure 15.
Postorbital skull breadth plotted against condylobasal length in Mustela erminea and Mustela nivalis.
Figure 15.
Postorbital skull breadth plotted against condylobasal length in Mustela erminea and Mustela nivalis.
Figure 16.
Length of m1 (L m1) plotted against length of m2 (L m2) in Mustela erminea and Mustela nivalis.
Figure 16.
Length of m1 (L m1) plotted against length of m2 (L m2) in Mustela erminea and Mustela nivalis.
Figure 17.
Three basic types of
Mustela nivalis weasel, examined as “eco-types”: (
left) dwarf, short-tailed pygmy (least) weasel (eg., M4 217) forming the
pygmaea-
rixosa group, characteristic of northern, cold climates; (
middle) average, short-tailed common weasel (eg., R 8857, R 7057) from the
vulgaris-
nivalis group, typical of temperate climates; and (
right) large, long-tailed Transcaucasian weasel from the
boccamela-
numidica group, characteristic of warm climates. Note size differences. After King and Powell [
62] and Abramov and Baryshnikov [
67], modified.
Figure 17.
Three basic types of
Mustela nivalis weasel, examined as “eco-types”: (
left) dwarf, short-tailed pygmy (least) weasel (eg., M4 217) forming the
pygmaea-
rixosa group, characteristic of northern, cold climates; (
middle) average, short-tailed common weasel (eg., R 8857, R 7057) from the
vulgaris-
nivalis group, typical of temperate climates; and (
right) large, long-tailed Transcaucasian weasel from the
boccamela-
numidica group, characteristic of warm climates. Note size differences. After King and Powell [
62] and Abramov and Baryshnikov [
67], modified.
Figure 18.
Mustela erminea and
Mustela nivalis long bone size comparison (larger,
Mustela erminea; smaller,
Mustela nivalis; red, males; and black, females). Black circles indicate individuals from Coulet des Roches. Dimensions of comparative recent material according to Rechstein [
55,
56] and measurements according to A. Marciszak.
Figure 18.
Mustela erminea and
Mustela nivalis long bone size comparison (larger,
Mustela erminea; smaller,
Mustela nivalis; red, males; and black, females). Black circles indicate individuals from Coulet des Roches. Dimensions of comparative recent material according to Rechstein [
55,
56] and measurements according to A. Marciszak.
Figure 19.
Equus ferus gallicus, Stallion L6 41. Radiocarbon date: 22,190 ± 90 cal BP (GI 3). Photo: E. Crégut-Bonnoure.
Figure 19.
Equus ferus gallicus, Stallion L6 41. Radiocarbon date: 22,190 ± 90 cal BP (GI 3). Photo: E. Crégut-Bonnoure.
Figure 20.
Equus ferus gallicus. Stallion skull L6 41. Radiocarbon date: 22,190 ± 90 cal BP (GI 3). Photos: C. Triat.
Figure 20.
Equus ferus gallicus. Stallion skull L6 41. Radiocarbon date: 22,190 ± 90 cal BP (GI 3). Photos: C. Triat.
Figure 21.
Muzzle length in relation to maximal muzzle breadth. Coulet measurements in
Table 13. Comparative data: Przewalski’s horse, Siréjol, Jaurens, and Mezin [
79]; Fontaihnas [
80]; Mounoï [
81]; Quéroy I [
82].
Figure 21.
Muzzle length in relation to maximal muzzle breadth. Coulet measurements in
Table 13. Comparative data: Przewalski’s horse, Siréjol, Jaurens, and Mezin [
79]; Fontaihnas [
80]; Mounoï [
81]; Quéroy I [
82].
Figure 22.
Ratio diagram of bone length and third phalanx breadth of Pleistocene horses. Coulet des Roches measurements in
Table 13. Reference: Przewalski’s horse [
79]. Comparative data: Jaurens and Mezin [
79]; Portugal [
80]; Tournal B-D [
81]; Gönnersdorf [
86]; Pair-non-Pair, Saint-Germain-la-Rivière [
88]; Solutré [
89]; Quéroy I [
82]. Abbreviations: H, Humerus; F, Femur; R, Radius; T, Tibia; MC, Metacarpal; MT, Metatarsal; PH1A, First Anterior Phalanx; PH1P, First Posterior Phalanx; PH3A, Third Anterior Phalanx.
Figure 22.
Ratio diagram of bone length and third phalanx breadth of Pleistocene horses. Coulet des Roches measurements in
Table 13. Reference: Przewalski’s horse [
79]. Comparative data: Jaurens and Mezin [
79]; Portugal [
80]; Tournal B-D [
81]; Gönnersdorf [
86]; Pair-non-Pair, Saint-Germain-la-Rivière [
88]; Solutré [
89]; Quéroy I [
82]. Abbreviations: H, Humerus; F, Femur; R, Radius; T, Tibia; MC, Metacarpal; MT, Metatarsal; PH1A, First Anterior Phalanx; PH1P, First Posterior Phalanx; PH3A, Third Anterior Phalanx.
Figure 23.
Robustness index in relation to the length of metacarpal III. Robustness index = width of diaphysis in the middle (
3)/length (
1) × 1000. Comparative data: Solutré Aurignacian/Perigordian [
89]; Solutré Gravettian [
86]. Measurement system according to Eisenmann [
79]. Abbreviation: T, “Tursac” (GI 3).
Figure 23.
Robustness index in relation to the length of metacarpal III. Robustness index = width of diaphysis in the middle (
3)/length (
1) × 1000. Comparative data: Solutré Aurignacian/Perigordian [
89]; Solutré Gravettian [
86]. Measurement system according to Eisenmann [
79]. Abbreviation: T, “Tursac” (GI 3).
Figure 24.
Capra ibex. Female N6 362-1. Radiocarbon date: 18,350 ± 70 cal BP. Scale: 30 cm. Photo: E. Crégut-Bonnoure.
Figure 24.
Capra ibex. Female N6 362-1. Radiocarbon date: 18,350 ± 70 cal BP. Scale: 30 cm. Photo: E. Crégut-Bonnoure.
Figure 25.
Capra ibex: (A) male N5 283, radiocarbon date: 16,380 ± 50 cal BP; (B) female N6 362-1, radiocarbon date: 18,350 ± 70 cal BP; and (C) young N5 580, 8–9 months old, radiocarbon date: 19,460 ± 60. Scale: 1 cm. Photos: C. Triat.
Figure 25.
Capra ibex: (A) male N5 283, radiocarbon date: 16,380 ± 50 cal BP; (B) female N6 362-1, radiocarbon date: 18,350 ± 70 cal BP; and (C) young N5 580, 8–9 months old, radiocarbon date: 19,460 ± 60. Scale: 1 cm. Photos: C. Triat.
Figure 26.
Dicrostonyx torquatus of Coulet des Roches: (A) M4 R788 z = 4.36 m1 left; (B) M5 R34 z = −4.60 m1 left; (C) M4 R493 z = −4.36 m1 right; (D) N6 R249 z = −4.50 m1 1 left; (E) M4 R957 z = −4.45 m1–m3 right; (F) M4 R468 z = −4.47 M/1 right; (G) M4 R493 z = −4.36 m1 right; (H) N5-N6 R170 z = −4.85 m1 left; and (I) M5 R34 z = −4.60 m1 right. Scale: 1 mm. Abbreviation: z, altitudinal level.
Figure 26.
Dicrostonyx torquatus of Coulet des Roches: (A) M4 R788 z = 4.36 m1 left; (B) M5 R34 z = −4.60 m1 left; (C) M4 R493 z = −4.36 m1 right; (D) N6 R249 z = −4.50 m1 1 left; (E) M4 R957 z = −4.45 m1–m3 right; (F) M4 R468 z = −4.47 M/1 right; (G) M4 R493 z = −4.36 m1 right; (H) N5-N6 R170 z = −4.85 m1 left; and (I) M5 R34 z = −4.60 m1 right. Scale: 1 mm. Abbreviation: z, altitudinal level.
Figure 27.
Chronology of the last expansions of D. torquatus at the end of the Upper Pleistocene.
Figure 27.
Chronology of the last expansions of D. torquatus at the end of the Upper Pleistocene.
Figure 28.
Taphonomy: rodent teeth displaying digestion induced by avian raptors. Photos: E. Desclaux and L. Woodthorpe.
Figure 28.
Taphonomy: rodent teeth displaying digestion induced by avian raptors. Photos: E. Desclaux and L. Woodthorpe.
Figure 29.
Climatogram of the LGM based on the MNI of rodent species identified in the Coulet des Roches levels. ARC, cold arctic steppes; BOR, forested and humid boreal areas; OPEN, continental steppes; MOUNT, open mountain areas; WAT, riverbanks; HUM, meadows; DECID, deciduous forests; MED, Mediterranean region.
Figure 29.
Climatogram of the LGM based on the MNI of rodent species identified in the Coulet des Roches levels. ARC, cold arctic steppes; BOR, forested and humid boreal areas; OPEN, continental steppes; MOUNT, open mountain areas; WAT, riverbanks; HUM, meadows; DECID, deciduous forests; MED, Mediterranean region.
Figure 30.
Climatogram of all the levels of Coulet des Roches based on the MNI of the identified rodent species.
Figure 30.
Climatogram of all the levels of Coulet des Roches based on the MNI of the identified rodent species.
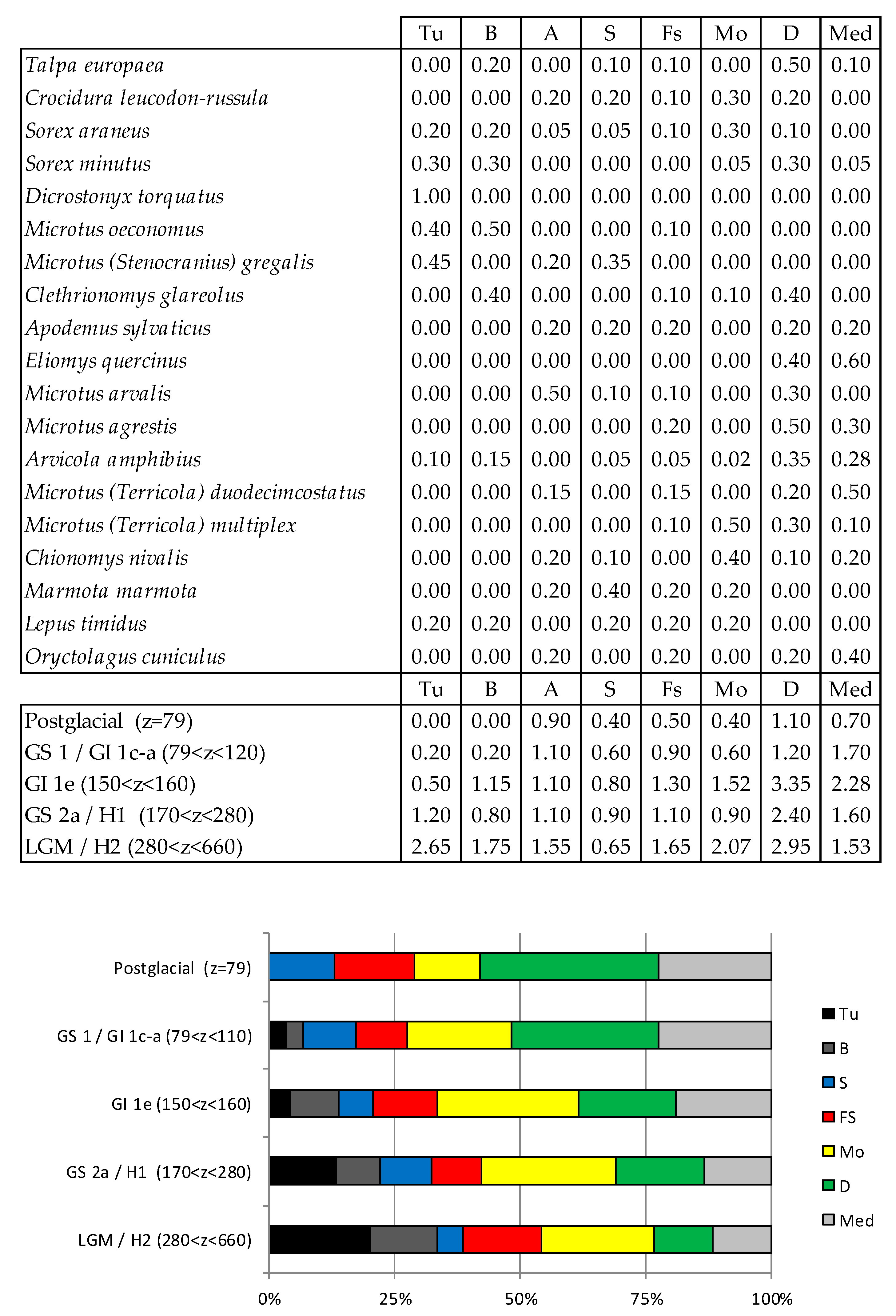
Figure 31.
Taxonomic Habitat Index (THI) values observed at Coulet des Roches. Tu, tundra; B, boreal forest; A, arid area; S, steppe; Fs, forest-steppe; Mo, mountain; D, deciduous forest; Med, Mediterranean biome.
Figure 31.
Taxonomic Habitat Index (THI) values observed at Coulet des Roches. Tu, tundra; B, boreal forest; A, arid area; S, steppe; Fs, forest-steppe; Mo, mountain; D, deciduous forest; Med, Mediterranean biome.
Figure 32.
Cenograms of LGM, GS 2a and GI 1e levels of Coulet des Roches, showing an open and arid environment. Ln(weight), Neperian logarithm of weight.
Figure 32.
Cenograms of LGM, GS 2a and GI 1e levels of Coulet des Roches, showing an open and arid environment. Ln(weight), Neperian logarithm of weight.
Table 1.
Distribution of the species during GI 10-11, GI 3, Last Glacial Maximum (LGM) and Last Glacial-GS 2a.
Table 1.
Distribution of the species during GI 10-11, GI 3, Last Glacial Maximum (LGM) and Last Glacial-GS 2a.
| | GI 10-11 | GI 3 | LGM | LG/GS 2a |
|---|
| Mammals | | | | |
| Vulpes vulpes | - | - | X | X |
| Alopex lagopus | - | - | X | X |
| Mustela putorius | - | - | X | X |
| Mustela erminea | - | X | X | X |
| Mustela nivalis | X | X | X | X |
| Equus ferus gallicus | - | X | X | X |
| Cervus elaphus | X | - | - | - |
| Rangifer tarandus | - | - | X | X |
| Capra ibex | - | - | X | X |
| Rupicapra rupicapra | - | - | X | X |
| Rupicapra sp. | - | - | X | - |
| Lepus timidus | - | X | X | X |
| Oryctolagus cuniculus | - | - | X | X |
| Talpa europaea | - | - | X | X |
| Crocidura leucodon/russula | - | - | X | X |
| cf. Sorex sp. | - | - | - | X |
| Sorex minutus | - | - | X | - |
| Sorex gr. araneus/coronatus | - | - | X | - |
| Marmota marmota primigenia | - | - | X | X |
| Eliomys quercinus | - | - | X | X |
| Apodemus sylvaticus | - | - | X | - |
| Arvicola amphibius | - | - | X | - |
| Microtus (Stenocranius) gregalis | - | - | X | - |
| Microtus arvalis | - | - | X | X |
| Microtus arvalis/agrestis | - | - | - | X |
| Chionomys nivalis | - | - | X | X |
| Clethrionomys glareolus | - | - | X | X |
| Microtus (Terricola) cf. multiplex | - | - | X | - |
| Microtus oeconomus | - | - | X | X |
| Dicrostonyx torquatus | - | - | X | X |
| Birds | | | | |
| Aquila chrysaetos | - | - | X | X |
| Falco cf. peregrinus | - | - | X | X |
| Falco tinnunculus | - | - | X | - |
| Circus sp. | - | - | X | - |
| Bubo scandiacus | - | - | X | X |
| Lagopus mutus | - | - | X | - |
| Pyrhocorax pyrhocorax | - | - | X | X |
| Pyrhocorax graculus | - | - | X | X |
| Corvus monedula | - | - | X | X |
| Pyrrhocorax/Corvus | - | - | X | X |
| Garrulus glandarius | - | - | X | - |
| Columba oenas | - | - | - | X |
| Columba livia | - | - | X | X |
| Columba livia-oenas | - | - | X | X |
| cf. Nucifraga caryocatactes | - | - | X | - |
| Rallus aquaticus | - | - | - | X |
| Hirundo sp. | - | - | - | X |
| Reptile | | | | |
| Emys orbicularis | X | - | - | - |
Table 2.
Dimensions of
Mustela putorius and
Mustela erminea skulls and upper teeth from Coulet des Roches (in mm). References of skull measurements in
Figure 3. Abbreviations: P, premolar; M, molar; L, length; B, breadth; a, anterior; p, posterior; pr, protocone.
Table 2.
Dimensions of
Mustela putorius and
Mustela erminea skulls and upper teeth from Coulet des Roches (in mm). References of skull measurements in
Figure 3. Abbreviations: P, premolar; M, molar; L, length; B, breadth; a, anterior; p, posterior; pr, protocone.
| | Mustela putorius | Mustela erminea |
|---|
| LGM | LG-GS 2a | LG-GS 2a | LGM |
|---|
| FdN | Planes | R | R | N4 | J3 | F5 | M5 | M6 |
|---|
| R53 | 8092 | 7056 | 164 | 23 | 1 | 512 | 320 |
|---|
| ♂ | ♀ | ♀ | ♂ | ♂ | ♂ | ♀ | ♂ | ♀ |
|---|
| 1 | - | | | | 45.4 | 43.3 | 42.0 | 46.1 | - |
| 2 | 70.0 | - | - | - | 46.3 | 44.2 | 42.5 | 46.5 | - |
| 3 | 66.3 | 59.2 | - | - | 43.1 | 41.2 | 39.5 | 43.8 | - |
| 4 | - | - | - | - | 10.2 | 7.7 | - | 20.1 | - |
| 5 | - | - | - | - | 27.2 | 28.6 | - | 28.2 | - |
| 9 | - | - | 16.3 | - | 20.1 | 19.4 | 17.6 | 20.3 | - |
| 10 | - | - | - | - | 16.3 | 15.7 | 14.9 | 12.3 | - |
| 11 | 12.5 | 12.7 | 7.7 | - | 10.1 | 9.9 | 9.2 | 10.0 | - |
| 12 | - | - | - | - | 26.4 | 26.0 | 25.3 | - | - |
| 13 | 7.4 | 6.9 | 3.9 | - | 4.5 | 4.4 | 4.9 | 4.4 | - |
| 14 | - | - | 9.5 | - | 12.8 | 11.2 | 10.1 | 11.8 | - |
| 15 | - | - | - | - | 15.0 | 14.8 | 14.3 | - | - |
| 16 | - | - | - | - | 15.9 | 15.9 | 14.5 | - | - |
| 17 | - | - | 4.5 | - | 5.9 | 5.4 | 5.5 | 4.6 | - |
| 18 | - | - | 10.9 | - | 13.9 | 13.3 | 12.0 | 12.5 | - |
| 20 | 24.3 | 19.6 | 11.3 | 13.6 | 13.9 | 14.8 | 13.7 | 13.6 | 13.2 |
| 21 | 15.3 | 14.7 | - | 12.6 | 11.5 | 12.9 | 12.4 | 9.2 | 12.7 |
| 22 | - | - | - | 21.9 | 21.2 | 22.4 | 21.8 | 20.0 | 22.6 |
| 23 | 39.1 | 31.4 | - | 20.9 | 22.0 | 21.8 | 21.1 | 22.3 | 22.5 |
| 24 | 17.7 | 15.4 | - | 11.0 | 12.3 | 11.6 | 11.5 | 12.6 | 11.8 |
| 25 | 9.7 | 6.7 | 4.1 | - | 4.4 | 5.1 | 4.1 | 4.8 | - |
| 26 | 7.7 | 6.8 | 4.0 | - | 4.9 | 4.3 | 4.3 | 4.9 | - |
| 27 | 10.0 | 8.7 | - | 6.2 | 6.9 | 6.6 | 6.8 | 6.5 | 5.1 |
| 28 | 7.6 | - | - | 5.6 | 6.1 | | 5.3 | 6.0 | 6.3 |
| 29 | - | - | - | 13.1 | 14.9 | 13.4 | 13.8 | 14.7 | 13.1 |
| 30 | - | - | - | 7.5 | 8.4 | 8.6 | 7.6 | 8.1 | 8.1 |
| 32 | 19.8 | 16.2 | - | - | - | - | - | - | - |
| P3 L | 4.9 | 4.2 | - | - | - | - | - | - | - |
| P3 B | 2.4 | 2.0 | - | - | - | - | - | - | - |
| P4 L | 7.2 | 7.1 | 4.4 | - | 5.2 | 5.0 | 4.9 | 5.2 | - |
| P4 L pr | - | | 1.1 | - | 0.9 | 1.1 | 1.4 | 3.0 | - |
| P4 Ba | 4.4 | 4.1 | 2.1 | - | 2.6 | 2.6 | 2.3 | 2.7 | - |
| P4 Bp | - | - | 1.4 | - | 1.8 | 1.6 | 1.6 | 1.9 | - |
| M1 L | - | - | - | - | 4.2 | 4.3 | 3.9 | 4.5 | - |
| M1 B1 | - | - | - | - | 1.6 | 2.0 | 1.8 | 2.0 | - |
| M1 B2 | - | - | - | - | 1.7 | 1.7 | 1.7 | 1.5 | - |
| M1 B3 | - | - | - | - | 2.0 | 2.0 | 2.2 | 2.3 | - |
Table 3.
Dimensions of
Mustela putorius and
Mustela erminea mandible from Coulet des Roches (in mm). References of mandible measurements in
Figure 4 and
Figure 5. Abbreviations: L, left; R, right; p, premolar; m, molar; L, length; B, breadth; a, anterior; p, posterior; tri, trigonid; tal, talonid.
Table 3.
Dimensions of
Mustela putorius and
Mustela erminea mandible from Coulet des Roches (in mm). References of mandible measurements in
Figure 4 and
Figure 5. Abbreviations: L, left; R, right; p, premolar; m, molar; L, length; B, breadth; a, anterior; p, posterior; tri, trigonid; tal, talonid.
| | Mustela putorius | Mustela erminea |
|---|
| Coulet: LGM | LG-GS 2a | LG-GS 2a | LGM |
|---|
| R | R | R | R | Planes | R | R | R | R | M4 | M3-4 | M4 | F4-G4 | F5 G5 |
|---|
| 3994 | 7940 | 3995 | 54 | | | 3037 | 3058 | 8871 | 9309 | 195 | 1.1 | 988-2 | R 1-6 | R 1-4 |
|---|
| ♂ | ♂ | ♂ | ♂ | ♀ (L) | ♀ (R) | ♀ | ♀ | ♂ | ♂ | ♂ | ♂ | ♀ | ♀ | ♀ |
|---|
| 1 | 44.4 | 44.8 | - | 41.7 | 36.0 | - | - | 21.4 | 25.7 | 18.7 | 25.8 | 20.4 | 18.6 | - | - |
| 2 | 43.5 | 44.6 | - | 41.6 | 35.6 | - | - | 20.1 | 23.6 | 17.4 | 23.5 | 19.0 | 17.1 | - | - |
| 3 | 36.3 | 37.1 | - | 35.0 | 31.5 | - | - | 14.0 | 16.2 | 11.8 | 16.0 | 13.1 | 12.7 | - | - |
| 4 | 21.5 | 21.5 | - | 20.0 | 18.7 | 18.4 | 11.2 | 13.4 | 14.9 | 11.8 | 15.5 | 13.1 | 12.5 | - | - |
| 5 | 20.2 | 20.5 | - | 19.0 | 18.4 | 17.8 | 4.9 | 11.7 | 12.1 | 9.7 | 12.2 | 10.8 | 10.2 | - | - |
| 6 | 9.9 | 10.4 | 9.3 | 9.3 | 9.1 | 8.2 | 6.0 | 4.5 | 5.1 | 4.4 | 6.7 | 5.1 | 4.7 | - | - |
| 7 | 10.8 | 10.8 | 10.6 | 10.7 | 10.2 | 9.8 | 1.7 | 6.6 | 7.0 | 5.9 | 6.6 | 5.6 | 5.9 | - | - |
| 8 | 5.1 | 5.0 | 3.5 | 3.4 | 4.4 | 3.3 | - | 1.9 | 2.4 | 2.0 | 2.6 | 2.1 | 1.8 | - | - |
| 10 | - | - | - | - | - | - | - | 10.2 | 13.0 | | 12.7 | 9.6 | 8.0 | - | - |
| 12 | 10.4 | 10.0 | 10.2 | 10.7 | 7.7 | 8.1 | - | 4.9 | 4.5 | 3.3 | 5.1 | 3.6 | 3.4 | - | - |
| 14 | 9.9 | 10.0 | 10.2 | 10.7 | 8.1 | - | 3.7 | 4.2 | 5.6 | 3.9 | 4.5 | 3.9 | 2.7 | - | - |
| 17 | 13.0 | 13.6 | - | 13.1 | 11.4 | - | - | 4.4 | - | 4.7 | 6.7 | 3.9 | 4.5 | - | - |
| c1 L | - | 5.3 | - | 4.8 | 3.5 | 3.9 | - | - | - | - | - | - | - | - | - |
| c1 B | - | 3.9 | - | 3.7 | 2.8 | 3.3 | - | - | - | - | - | - | - | - | - |
| p2 L | - | 2.7 | - | - | - | 2.4 | - | - | - | - | - | - | - | - | - |
| p2 B | - | 2.4 | - | - | - | 1.4 | - | - | - | - | - | - | - | - | - |
| p3 L | 3.6 | 3.5 | 4.0 | - | 3.7 | 4.0 | - | - | - | - | - | - | - | - | - |
| p3 B | 2.4 | 2.1 | 2.4 | 2.1 | 1.9 | 1.8 | - | - | - | - | - | - | - | - | - |
| p4 L | 4.7 | 4.6 | 4.5 | 4.5 | 4.2 | 4.4 | - | - | 2.6 | 3.1 | - | 3.3 | - | - | 2.8 |
| p4 Ba | - | - | - | | - | - | - | - | 1.1 | 1.4 | - | 1.2 | - | - | 1.1 |
| p4 Bp | 2.6 | 2.6 | 2.6 | 2.4 | 2.1 | 2.1 | - | - | 1.4 | 1.7 | - | 1.6 | - | - | 1.3 |
| m1 L | 9.0 | 8.6 | 9.0 | 8.9 | 8.2 | 8.1 | - | 5.2 | 5.5 | 5.7 | 4.6 | 5.6 | 5.0 | 5.3 | - |
| m1 L tri | 6.8 | 6.5 | 6.7 | 6.6 | 5.7 | 5.9 | - | 3.7 | 4.3 | 4.3 | 3.4 | 4.4 | 3.7 | 3.5 | - |
| m1 L tal | - | - | - | - | - | - | - | 1.3 | 1.6 | 1.6 | 1.6 | 1.7 | 1.2 | 1.5 | - |
| m1 B tri | 3.8 | 3.5 | 3.8 | 3.6 | 3.1 | 3.2 | - | 1.8 | 1.9 | 2.1 | 2.1 | 2.4 | 1.8 | 1.7 | - |
| m1 B tal | 2.6 | 2.5 | 2.7 | 2.6 | 2.3 | 2.4 | - | 1.5 | 1.6 | 1.7 | 1.4 | 1.9 | 1.4 | 1.4 | - |
| m2 L | - | 2.2 | - | - | - | - | - | - | - | 1.2 | - | 1.3 | - | - | - |
| m2 B | - | 1.7 | - | - | - | - | - | - | - | 1.2 | - | 0.9 | - | - | - |
Table 4.
Dimensions of mustelid humeri from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8, trochlear breadth.
Table 4.
Dimensions of mustelid humeri from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8, trochlear breadth.
| Species | Collection No. | Sex | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|
| LGM | | | | | | | | | |
| Mustela putorius | FdN R 36 | ♂ | 51.9 | 10.5 | 11.5 | 5.8 | 4.6 | 6.9 | 13.7 | 9.5 |
| Mustela putorius | FdN R 35 | ♂ | 51.6 | 10.3 | 11.4 | 5.7 | 5.7 | 6.8 | 13.5 | 9.4 |
| Mustela putorius | R 7090 | ♂ | 47.8 | 11.0 | 11.6 | 4.8 | 4.8 | 7.7 | 13.4 | 10.7 |
| Mustela putorius | R 7096 | ♂ | 46.6 | 11.5 | 11.4 | 4.6 | 4.6 | 7.3 | 13.5 | 11.4 |
| LG-GS 2a | | | | | | | | | |
| Mustela erminea | R 3043 | ♂ | 34.4 | 5.8 | 5.8 | 2.6 | 2.4 | 3.6 | 6.4 | 5.5 |
| Mustela erminea | R 3044 | ♂/♀ | 33.2 | 6.3 | 6.6 | 2.8 | 2.5 | 3.7 | 6.9 | 5.1 |
| Mustela erminea | R 9295 | ♀ | 26.7 | 5.6 | 5.4 | 2.4 | 2.3 | 3.2 | 5.8 | 4.4 |
| Mustela erminea | M4 181-20 | ♂ | 35.4 | 6.2 | 6.6 | 2.7 | 2.2 | 3.7 | 7.0 | 5.9 |
| Mustela erminea | G4 5-37 | ♂ | - | - | - | 2.7 | 2.4 | 3.8 | 7.0 | 5.4 |
| LGM | | | | | | | | | |
| Mustela erminea | M4 R 449 (L) | ♂ | 22.3 | 6.2 | 4.1 | 1.7 | 1.7 | 2.8 | 4.6 | 4.2 |
| Mustela erminea | M4 R 449 (R) | ♂ | - | - | - | 1.9 | 1.7 | 2.6 | 4.8 | 4.5 |
| GI 3 | | | | | | | | | |
| Mustela erminea | G4 26-26 | ♂ | 31.3 | 6.4 | 5.9 | 2.4 | 2.5 | 3.5 | 6.1 | 4.9 |
| LG-GS 2a | | | | | | | | | |
| Mustela nivalis | R 3016 | ♂ | - | - | - | 2.0 | 2.0 | 3.3 | 5.4 | 4.1 |
| Mustela nivalis | R 3042 | ♀ | 21.7 | 4.1 | 4.6 | 1.9 | 1.5 | 2.6 | 5.1 | 4.1 |
| LGM | | | | | | | | | |
| Mustela nivalis | M4 214 | ♂ | 17.0 | 3.4 | 3.6 | 1.3 | 1.1 | 2.2 | 4.1 | 3.5 |
| Mustela nivalis | N3-N4 R 30 | ♂ | 20.4 | 4.2 | 4.2 | 1.6 | 1.5 | 2.4 | 4.6 | 3.4 |
| Mustela nivalis | M4 217 | ♂ | 16.8 | 3.3 | 3.7 | 1.4 | 1.1 | 2.3 | 4.2 | 3.3 |
| Mustela nivalis | N5-N6 R 115 | ♀ | 20.0 | 4.0 | 4.1 | 1.8 | 1.4 | 2.5 | 4.5 | 3.9 |
| Mustela nivalis | H5 R 2-5 | ♂ | 23.7 | 4.3 | 4.5 | 1.9 | 1.6 | 3.0 | 5.0 | 3.8 |
| Mustela nivalis | H4 7-285 | ♂ | 23.8 | 4.3 | 4.4 | 1.8 | 1.6 | 3.0 | 5.0 | 4.2 |
| Mustela nivalis | G4 6-13 | ♀ | 19.7 | 3.6 | 4.1 | 1.5 | 1.5 | 2.6 | 4.5 | 4.1 |
| Mustela nivalis | G5 7-55 | ♀ | - | - | - | - | - | 2.7 | 4.4 | 3.8 |
| Mustela nivalis | G5 7-56 | ♂ | - | - | - | 1.9 | 1.4 | 2.8 | 4.3 | 3.8 |
| Mustela nivalis | M3-M4 R 71-2 | ♂ | - | - | - | - | - | 2.8 | 4.7 | 3.0 |
| GI 3 | | | | | | | | | |
| Mustela nivalis | L6 R 8-3 | ♂ | 25.4 | 4.5 | 4.4 | 2.0 | 2.1 | 2.8 | 4.9 | 3.6 |
| Mustela nivalis | L6 R 8-3 | ♂ | 25.4 | 4.4 | 4.4 | 1.9 | 2.0 | 3.2 | 5.0 | 4.2 |
| Mustela nivalis | K5 R 76-3 | ♂ | 28.0 | - | - | 2.5 | 2.3 | 3.1 | 5.5 | 4.7 |
| GI 10-11 | | | | | | | | | |
| Mustela nivalis | N5 408-6 | ♂ | 24.3 | 4.3 | 4.4 | 2.0 | 1.9 | 2.8 | 5.3 | 3.9 |
Table 5.
Dimensions of mustelid radiuses from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8, trochlear breadth.
Table 5.
Dimensions of mustelid radiuses from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8, trochlear breadth.
| Species | Collection No. | Sex | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| LGM | | | | | | | | |
| Mustela putorius | R 9327 | ♂ | 37.2 | 4.4 | 5.9 | 2.7 | 3.5 | 7.4 | 5.5 |
| Mustela putorius | G5 14-4 | ♂ | 38.3 | 4.1 | 5.8 | 2.7 | 3.7 | 7.8 | 5.4 |
| LG-GS 2a | | | | | | | | |
| Mustela erminea | R 3048 | ♂ | 23.5 | 1.8 | 2.8 | 1.6 | 1.9 | 3.2 | 2.5 |
| Mustela erminea | M4 183 | ♂ | 25.2 | 2.2 | 3.4 | 1.5 | 1.5 | 2.9 | 4.1 |
| LGM | | | | | | | | |
| Mustela erminea | F5 R 3-8 | ♀ | 21.2 | 1.9 | 3.0 | 1.4 | 2.0 | 3.7 | 3.7 |
| Mustela erminea | F5-G 5 1 | ♀ | 21.4 | 2.1 | 3.0 | 1.5 | 1.6 | 2.5 | 3.7 |
| Mustela erminea | F5 R 4-11 | ♀ | 22.7 | 1.8 | 3.1 | 1.6 | 1.8 | 2.6 | 3.8 |
| LGM | | | | | | | | |
| Mustela nivalis | M3-M4 R 1-4 | ♂ | - | 1.5 | 2.2 | 0.9 | 1.3 | - | - |
| Mustela nivalis | M4 217 | ♂ | 11.3 | 1.2 | 1.9 | 0.6 | 0.9 | 1.6 | 2.4 |
| GI 3 | | | | | | | | |
| Mustela nivalis | K5 R 76-6 | ♂ | 20.0 | 1.8 | 2.8 | 1.5 | 1.6 | - | - |
| Mustela nivalis | L5-L6 R 7-3 | ♂ | 21.0 | 2.0 | 2.9 | 1.4 | 1.6 | - | - |
Table 6.
Dimensions of mustelid ulnae from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8, trochlear breadth.
Table 6.
Dimensions of mustelid ulnae from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8, trochlear breadth.
| Species | Collection No. | Sex | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| LGM | | | | | | | | |
| Mustela putorius | R 9326 | ♂ | 47.4 | 8.3 | 4.9 | 3.8 | 3.1 | 6.4 | 3.8 |
| LGM | | | | | | | | |
| Mustela erminea | M4 R 449 | ♂ | - | 2.9 | 2.4 | - | - | - | - |
| Mustela erminea | F4 R 1-8 | ♀ | 27.2 | 3.5 | 3.0 | 2.3 | 1.4 | 2.2 | 2.1 |
| Mustela erminea | F5 R 1-3 | ♀ | | | | 2.4 | 1.5 | 3.2 | 2.0 |
| GI 3 | | | | | | | | |
| Mustela erminea | G4 26-27 | ♀ | 26.9 | 3.6 | 3.1 | 2.1 | 1.5 | 3.2 | 2.1 |
| LG-GS 2a | | | | | | | | |
| Mustela nivalis | R 3045 | ♂ | 19.2 | 4.1 | 3.3 | 2.4 | 1.4 | 3.4 | 1.9 |
| Mustela nivalis | R 3046 | ♂ | 21.0 | 3.3 | 3.1 | 1.8 | 1.3 | 2.1 | 1.6 |
| Mustela nivalis | M3 53-57 | ♂ | 19.1 | 2.8 | 2.4 | 1.5 | 0.8 | 2.0 | 1.2 |
| LGM | | | | | | | | |
| Mustela nivalis | M3-M4 R 1-3 | ♂ | 18.1 | 2.8 | 2.4 | 1.5 | 0.7 | 1.8 | 1.5 |
| Mustela nivalis | M4 217 | ♂ | 15.2 | 2.3 | 1.6 | 0.8 | 0.8 | 1.7 | 1.0 |
| Mustela nivalis | M5 R 27 | ♀ | - | 2.8 | 2.5 | 1.8 | 0.8 | - | - |
| Mustela nivalis | L5 R 21-4 | ♂ | - | 3.0 | 2.3 | - | - | - | - |
| GI 3 | | | | | | | | |
| Mustela nivalis | K5 R 76-5 | ♂ | 25.0 | 3.7 | 3.1 | 1.8 | 1.3 | - | - |
Table 7.
Dimensions of mustelid femurs from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8: trochlear breadth.
Table 7.
Dimensions of mustelid femurs from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8: trochlear breadth.
| Species | Collection No. | Sex | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| LGM | | | | | | | | |
| Mustela putorius | R 9328 | ♂ | 54.6 | 13.1 | 6.8 | 3.9 | 4.9 | 9.5 | 12.6 |
| Mustela putorius | R 3288 | ♂ | - | 13.5 | 6.8 | 4.0 | 5.4 | - | - |
| Mustela putorius | G5 16-1 | ♂ | 56.5 | 12.6 | 6.5 | 4.7 | 4.7 | 9.1 | 12.4 |
| Holocene-Atlantic | | | | | | | | |
| Mustela erminea | J3-K3 R 11 | ♂ | 27.9 | 5.2 | 2.9 | 2.1 | 2.2 | 4.2 | 4.9 |
| Mustela erminea | J3-K3 R 11 | ♂ | 27.7 | 5.2 | 2.7 | 2.0 | 2.0 | 4.4 | 4.9 |
| LG-GS 2a | | | | | | | | |
| Mustela erminea | R 3057 | ♀ | 31.1 | 6.4 | 3.1 | 2.3 | 2.4 | 4.9 | 5.6 |
| Mustela erminea | R 9297 | ♂ | 35.5 | 6.7 | 3.5 | 2.7 | 2.8 | 5.7 | 6.3 |
| LGM | | | | | | | | |
| Mustela erminea | F4 R 4-4 | ♀ | 31.9 | 6.2 | 2.9 | 2.6 | 2.4 | 4.5 | 5.9 |
| Mustela erminea | F4 R 4-5 | ♀ | 32.1 | 6.1 | 3.1 | 2.5 | 2.7 | 4.6 | 5.9 |
| LG-GS 2a | | | | | | | | |
| Mustela nivalis | R 3049 | ♂ | 23.9 | 5.6 | 2.9 | 2.0 | 2.2 | 4.2 | 4.9 |
| Mustela nivalis | R 3056 | ♂ | 25.6 | 5.8 | 3.0 | 2.0 | 2.2 | 4.1 | 5.0 |
| Mustela nivalis | M3 53-55 | ♂ | 21.8 | 4.8 | 2.5 | 1.9 | 1.9 | 3.9 | 4.8 |
| LGM | | | | | | | | |
| Mustela nivalis | M4 217 | ♂ | 16.3 | 3.3 | 2.0 | 1.2 | 1.2 | 3.0 | 3.5 |
| Mustela nivalis | M4 217 | ♂ | 16.3 | 3.4 | 1.9 | 1.3 | 1.2 | 3.1 | 3.5 |
| Mustela nivalis | M3-M4 R 56 | ♂ | 19.8 | 4.5 | 2.6 | 1.5 | 1.8 | 3.7 | 4.4 |
| Mustela nivalis | M4 R 329 | ♂ | 20.2 | 5.1 | 2.7 | 1.7 | 1.9 | 3.7 | 4.4 |
| GI 3 | | | | | | | | |
| Mustela nivalis | L6 R 10-2 | ♂ | 27.0 | 5.3 | 2.7 | 2.0 | 2.0 | 4.1 | 4.8 |
| Mustela nivalis | K5 R 76-7 | ♂ | 28.0 | - | - | 2.3 | 2.7 | - | - |
Table 8.
Dimensions of mustelid tibias from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8: trochlear breadth.
Table 8.
Dimensions of mustelid tibias from Coulet des Roches (in mm). References of measurements: 1, greatest length; 2, proximal depth; 3, proximal breadth; 4, midshaft diaphyseal depth; 5, diaphyseal midshaft breadth; 6, distal depth; 7, distal breadth; 8: trochlear breadth.
| Species | Collection No. | Sex | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| LGM | | | | | | | | |
| Mustela putorius | R 8874 | ♂ | 54.4 | 9.7 | 11.7 | 5.2 | 3.3 | 6.8 | 8.7 |
| Mustela putorius | R 8875 | ♂ | 54.0 | 9.8 | 11.8 | 5.1 | 3.4 | 6.4 | 8.6 |
| Mustela putorius | G5 16-2 | ♂ | 60.7 | 9.8 | 11.8 | 5.1 | 4.2 | 6.8 | 8.1 |
| Mustela putorius | G5 28-1 | ♂ | 60.0 | 9.7 | 11.6 | 4.5 | 4.2 | 6.7 | 7.8 |
| LG-GS 2a | | | | | | | | |
| Mustela erminea | Planes 2017 | ♂ | 42.3 | 5.9 | 7.0 | 3.1 | 2.4 | 3.8 | 4.9 |
| LG-GS 2a | | | | | | | | |
| Mustela erminea | R 3051 | ♀ | 34.4 | 5.0 | 5.7 | 2.2 | 1.9 | 3.0 | 4.0 |
| Mustela erminea | M4 191-7 | ♂ | 40.3 | 5.7 | 6.2 | 2.5 | 2.1 | 3.6 | 4.7 |
| LGM | | | | | | | | |
| Mustela erminea | F4 R1-5 | ♀ | 34.3 | 4.9 | 5.8 | 2.4 | 2.2 | 3.1 | 4.2 |
| Mustela erminea | F4 R1-5 | ♀ | 34.4 | 4.6 | 5.8 | 2.3 | 2.1 | 3.2 | 4.1 |
| LG-GS 2a | | | | | | | | |
| Mustela nivalis | R 3050 | ♂ | 28.3 | 4.7 | 5.2 | 2.1 | 1.8 | 3.1 | 3.7 |
| Mustela nivalis | R 3052 | ♂ | 27.8 | 4.3 | 4.9 | 2.0 | 1.6 | 2.6 | 3.4 |
| Mustela nivalis | R 3053 | ♀ | 22.3 | 3.4 | 4.5 | 1.5 | 1.0 | 2.5 | 2.9 |
| Mustela nivalis | R 3054 | ♂ | 24.5 | 3.8 | 4.6 | 1.9 | 1.6 | 2.4 | 3.1 |
| Mustela nivalis | R 3055 | ♂ | 26.3 | 3.8 | 3.8 | 1.6 | 1.5 | 2.6 | 3.4 |
| LGM | | | | | | | | |
| Mustela nivalis | M3 53-56 | ♂ | | 3.9 | 4.5 | 1.6 | 1.4 | | |
| Mustela nivalis | M4 217 | ♂ | 17.6 | 2.8 | 3.5 | 1.2 | 0.9 | 1.7 | 2.5 |
| Mustela nivalis | M4 217 | ♂ | 17.7 | 2.8 | 3.6 | 1.2 | 0.9 | 1.7 | 2.5 |
| Mustela nivalis | M3-M4 R 46 | ♂ | 21.2 | 3.8 | 4.2 | 1.6 | 1.2 | 2.1 | 3.0 |
| Mustela nivalis | M3-M4 R 46 | ♂ | 21.4 | 3.7 | 4.2 | 1.4 | 1.3 | 2.1 | 3.1 |
| Mustela nivalis | N5-N6 R 175 | ♀ | 21.0 | 3.7 | 3.9 | 1.2 | 1.1 | 2.1 | 2.8 |
| Mustela nivalis | L5 53-2 | ♂ | - | - | - | 1.9 | 1.9 | - | - |
| Mustela nivalis | G5 10-2 | ♂ | 26.4 | 4.0 | 4.5 | 1.5 | 1.4 | 2.3 | 3.4 |
| Mustela nivalis | G4 R 15-6 | ♂ | 17.9 | 2.7 | 3.4 | 1.4 | 1.0 | 2.1 | 2.2 |
| GI 10-11 | | | | | | | | |
| Mustela nivalis | M4-N4 R 46-2 | ♂ | - | - | - | 1.7 | 1.5 | - | - |
Table 9.
Skull dimensions of
Mustela nivalis from Coulet des Roches (in mm). References of measurements in
Figure 3. Abbreviations: P, premolar; M, molar; L, length; B, breadth; a, anterior; p, posterior; pr, protocone.
Table 9.
Skull dimensions of
Mustela nivalis from Coulet des Roches (in mm). References of measurements in
Figure 3. Abbreviations: P, premolar; M, molar; L, length; B, breadth; a, anterior; p, posterior; pr, protocone.
| | LG-GS 2a | LGM | GI 10–11 |
|---|
| R | R | R | R | R | R | R | R | N4 | N3–N4 | M3 | M4 | M4 | G5 | G4 | F5 | N5 |
|---|
| 2878 | 7344 | 8857 | 8555 | 7057 | 8856 | 8858 | 8870 | 35 | 2 | 188 | 217 | 548 | 10-janv | 11 | R1-1 | 163-1 |
|---|
| ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♀ | ♀ | ♂ | ♀ | ♂ | ♂ | ♂ | ♂ | ♀ | ♂ | ♂ |
|---|
| 1 | 38.9 | - | 34.5 | - | 36.0 | 34.0 | 30.2 | - | 36.2 | 33.2 | - | 31.0 | 34.3 | 36.4 | - | 32.8 | - |
| 2 | 40.3 | - | 35.2 | - | 37.6 | 34.2 | 30.7 | - | 37.3 | 33.4 | - | 31.0 | 35.8 | 37.2 | 30.1 | 33.9 | - |
| 3 | 37.9 | - | 33.0 | - | 34.8 | 31.8 | 28.9 | - | 35.1 | 31.4 | - | 28.9 | 33.1 | 34.8 | 28.2 | 31.9 | - |
| 4 | 15.5 | - | 10.5 | - | 15.7 | 14.5 | 21.6 | - | 20.5 | 15.3 | - | - | 18.3 | 21.6 | - | - | - |
| 5 | 23.6 | - | 24.5 | - | 20.4 | 20.9 | 11.0 | - | 8.5 | 20.3 | - | - | 18.1 | 18.2 | - | - | - |
| 9 | 17.2 | - | 14.6 | 16.8 | 15.5 | 13.5 | 12.5 | - | 16.2 | 14.0 | 14.8 | 12.8 | 15.1 | 16.2 | 11.9 | 13.9 | - |
| 10 | 13.5 | 13.4 | 10.5 | 12.0 | 12.7 | 11.5 | 11.0 | - | 12.6 | 11.6 | 12.0 | 11.0 | 12.3 | 12.3 | 9.7 | 11.2 | - |
| 11 | 7.8 | 7.9 | 7.1 | - | 7.6 | 6.8 | 7.5 | 6.2 | 6.2 | 7.1 | 7.1 | 6.5 | 7.5 | 7.9 | 6.0 | 6.6 | 7.7 |
| 12 | 21.2 | - | - | - | 19.7 | - | - | - | - | 17.4 | - | 15.2 | 18.8 | - | - | - | - |
| 13 | 3.6 | 8.6 | 7.7 | 3.5 | 3.4 | 2.8 | 3.3 | 3.2 | 3.4 | 3.7 | 3.6 | 2.9 | 2.9 | 3.2 | 2.6 | 3.2 | - |
| 14 | 10.1 | 9.1 | 8.4 | 8.2 | 9.3 | 8.1 | 6.6 | 6.9 | 7.6 | 7.6 | 9.0 | 6.9 | 8.4 | 8.7 | 6.0 | 8.7 | - |
| 15 | 12.2 | 13.2 | - | - | 12.3 | 10.6 | - | - | 11.0 | | 12.7 | 10.3 | 10.7 | - | 10.5 | 10.9 | - |
| 16 | 12.7 | 14.0 | 12.2 | - | 12.9 | 11.2 | - | - | 11.6 | 11.7 | 12.6 | 10.4 | 11.8 | 12.3 | 10.8 | 10.9 | - |
| 17 | 4.3 | - | 4.0 | 3.7 | 4.1 | 3.1 | 3.0 | - | 4.0 | 3.6 | 4.2 | 3.5 | 3.8 | 4.1 | 3.1 | 3.8 | - |
| 18 | 10.5 | 10.6 | 9.1 | 9.9 | 10.6 | 8.4 | 7.1 | 8.5 | 8.7 | 9.0 | 9.8 | 8.1 | - | 10.3 | 8.7 | 9.4 | - |
| 20 | 10.7 | - | 9.6 | 9.4 | | 9.5 | 8.6 | - | 9.6 | 8.9 | 9.0 | 8.4 | 9.8 | 9.7 | 8.5 | 9.0 | 10.4 |
| 21 | 8.7 | - | 8.7 | 8.1 | 7.6 | 7.7 | 8.1 | - | 8.2 | 8.4 | 7.6 | 7.0 | 7.5 | 7.7 | 8.0 | 7.1 | 8.0 |
| 22 | 17.9 | - | 16.5 | - | 16.4 | 15.1 | 14.7 | - | 16.3 | 16.3 | - | 13.5 | 15.6 | 16.6 | 15.5 | 14.4 | - |
| 23 | 18.8 | - | 17.0 | - | 18.4 | 15.6 | 14.6 | - | 16.9 | 16.5 | - | 13.8 | 16.7 | 17.8 | 14.0 | 15.6 | - |
| 24 | 10.9 | - | 10.2 | - | 10.0 | 9.4 | 8.3 | - | | 9.3 | - | 9.0 | 9.4 | 9.8 | 8.4 | 8.8 | 9.8 |
| 25 | 3.8 | 3.6 | 3.3 | 3.5 | 3.1 | 3.2 | 2.7 | 2.7 | 3.3 | 2.9 | 3.0 | 2.9 | 3.0 | 3.9 | 3.2 | 3.1 | 3.6 |
| 26 | 3.9 | 3.4 | 3.6 | 3.7 | 3.4 | 3.5 | 2.8 | 3.4 | 3.6 | 3.1 | 3.6 | 2.6 | 3.6 | 3.5 | 2.9 | 2.6 | 3.7 |
| 27 | 6.2 | - | 6.1 | - | 5.7 | 4.7 | 4.6 | - | | 5.4 | - | 5.0 | 5.1 | 5.1 | 4.6 | 5.0 | 5.8 |
| 28 | 4.9 | - | 4.7 | - | 4.5 | 5.0 | 4.5 | - | | 4.8 | - | 4.0 | 4.9 | 4.1 | 4.0 | 4.2 | 4.8 |
| 29 | 13.3 | - | 11.8 | - | 13.0 | 11.5 | 10.5 | - | 12.3 | 11.5 | - | 10.6 | 12.1 | 10.7 | 9.9 | 11.4 | - |
| 30 | 7.8 | - | 6.8 | - | 6.9 | 6.3 | 6.1 | - | 7.2 | 6.7 | - | 5.4 | 6.4 | 7.7 | 5.1 | 6.4 | - |
| P3 L | - | 2.5 | - | - | 1.9 | - | 2.2 | 2.1 | - | - | - | 1.8 | - | 2.5 | - | 1.9 | - |
| P3 B | - | 1.1 | - | - | 1.0 | - | 1.0 | 1.0 | - | - | - | 1.1 | - | 1.3 | - | 0.9 | - |
| P4 L | 4.3 | 4.5 | 3.8 | - | 3.9 | 3.5 | 3.9 | 3.4 | 4.1 | - | 4.0 | 3.7 | 4.0 | 4.1 | - | 4.0 | 4.5 |
| P4 L pr | 1.1 | 1.2 | 0.8 | - | 0.7 | 0.7 | 0.7 | 1.0 | 1.1 | - | 0.9 | 0.7 | 0.9 | 2.4 | - | 1.6 | 0.9 |
| P4 Ba | 2.4 | 2.4 | 2.2 | - | 2.2 | 2.0 | 2.3 | 2.2 | 2.2 | - | 2.2 | 2.1 | 2.3 | 2.5 | - | 2.4 | 2.4 |
| P4 Bp | 1.5 | 1.5 | 1.3 | - | 1.5 | 1.3 | 1.0 | 1.2 | 1.3 | - | 1.5 | 1.3 | 1.4 | 1.2 | - | 1.2 | 1.5 |
| M1 L | 3.2 | 3.5 | 3.0 | - | 3.0 | 3.1 | 3.0 | - | 3.2 | 2.8 | - | 2.7 | 3.3 | - | - | 3.2 | - |
| M1 B1 | 1.3 | 1.2 | 1.1 | - | 1.5 | 1.2 | 1.4 | - | 1.2 | 1.4 | - | 1.5 | 1.5 | - | - | 1.1 | - |
| M1 B2 | 1.5 | 1.5 | 1.2 | - | 1.4 | 1.1 | 1.0 | - | 1.7 | 1.3 | - | 1.2 | 1.2 | - | - | 1.1 | - |
| M1 B3 | 1.8 | 1.8 | 1.4 | - | 1.4 | 1.4 | 1.1 | - | 1.4 | 1.5 | - | 1.4 | 1.8 | - | - | 1.5 | - |
Table 10.
Mandible dimensions of
Mustela nivalis from Coulet des Roches (in mm). References of measurements in
Figure 4 and
Figure 5. Abbreviations: L, left; R, right; p, premolar; m, molar; L, length; B, breadth; a, anterior; p, posterior; tri, trigonid; tal, talonid.
Table 10.
Mandible dimensions of
Mustela nivalis from Coulet des Roches (in mm). References of measurements in
Figure 4 and
Figure 5. Abbreviations: L, left; R, right; p, premolar; m, molar; L, length; B, breadth; a, anterior; p, posterior; tri, trigonid; tal, talonid.
| | LG-GS 2a |
| R | R | R | R | R | R | R | R | R | R | R | R | R | R | M3 | M5 | M3–4 | M3–4 |
| 3034 | 3035 | 3036 | 3038 | 3039 | 3719 | 3059 | 8858 | 8858 | 3060 | 9294 | 9295 | 9306 | 9326 | 61-4 | 41-4 | | |
| ♂ | ♂ | ♂ | ♀ | ♀ | ♂ | ♂ | ♀ | ♀ | ♂ | ♂ | ♀ | ♀ | ♂ | ♂ | ♀ | ♂ | ♂ |
| 1 | 17.0 | 14.3 | 16.3 | 16.2 | - | 20.8 | 18.2 | 14.9 | 15.0 | 19.5 | 20.3 | 18.7 | 16.8 | | 19.4 | 17.2 | 15.7 | - |
| 2 | 16.1 | 13.3 | 15.5 | 15.4 | - | 19.9 | 17.0 | 14.3 | 14.3 | 18.4 | 19.3 | 17.2 | 15.8 | 17.4 | 18.4 | 15.8 | 14.7 | - |
| 3 | 11.4 | 9.7 | 10.6 | 10.9 | - | 13.6 | 11.9 | 10.1 | 10.2 | 13.1 | 13.1 | 11.5 | 10.8 | 13.2 | 12.3 | 12.1 | 10.8 | - |
| 4 | 10.3 | - | 9.6 | 11.0 | - | 13.3 | 11.7 | 10.2 | 9.9 | 12.4 | 12.2 | 11.6 | 10.4 | 11.7 | 11.5 | 11.3 | 8.8 | - |
| 5 | 8.9 | - | - | 8.9 | 8.3 | 10.2 | 9.9 | 8.3 | 8.1 | 10.0 | 10.0 | 9.4 | 9.0 | 8.9 | 9.4 | 8.9 | 8.5 | 9.5 |
| 6 | 3.7 | - | 4.4 | 4.4 | 4.0 | 5.3 | 4.2 | 3,4 | 3.5 | 4.6 | 4.8 | 4.3 | 3.4 | 3.8 | 9.7 | 4.2 | 3.9 | 4.8 |
| 7 | 4.5 | 4.9 | - | 4.8 | 4.1 | 5.3 | 5.5 | 4.7 | 5.3 | 4.9 | 5.3 | 4.7 | 4.7 | 5.4 | 5.1 | 4.3 | 4.4 | 5.1 |
| 8 | 1.9 | - | 1.6 | 1.5 | 1.4 | 2.1 | 1.5 | 1.8 | 1.9 | 2.5 | 1.9 | 1.2 | 1.5 | - | 1.6 | 1.7 | 1.2 | - |
| 10 | 8.1 | 8.8 | - | 8.2 | - | - | - | 6.8 | 6.9 | 9.2 | 10.3 | 9.1 | 8.6 | - | 9.1 | 8.3 | 7.6 | - |
| 12 | 3.4 | - | 3.2 | 3.0 | 2.8 | 4.3 | - | 2.5 | 2.7 | 3.5 | 4.3 | 4.0 | 2.9 | 3.6 | 4.6 | 3.6 | 2.9 | - |
| 14 | 3.2 | - | 3.1 | 3.1 | 3.1 | 4.2 | 3.4 | 2.9 | 3.3 | 4.0 | 4.2 | 3.7 | 3.3 | 4.4 | 3.4 | | 2.8 | 3.4 |
| 17 | | 4.7 | 3.4 | 3.5 | 3.3 | - | 4.3 | 3.3 | 3.2 | 4.7 | 4.5 | 4.8 | 4.1 | - | 4.5 | 4.2 | 3.7 | 3.8 |
| p2 L | | - | - | - | 1.0 | - | - | 0.8 | - | 1.2 | - | 1.2 | - | - | - | - | 1.5 | - |
| p2 B | | - | - | - | 0.9 | - | - | 0.5 | - | 0.8 | - | 0.8 | - | - | - | - | 0.7 | - |
| p3 L | | - | 2.3 | - | - | 1.9 | - | 1.3 | - | - | - | 2.1 | 1.4 | - | - | - | - | 1.6 |
| p3 B | | - | 0.9 | - | - | 1.1 | - | 0.9 | - | - | - | 1.1 | 0.9 | - | - | - | - | 1.0 |
| p4 L | 2.2 | - | 2.1 | - | 1.6 | 2.6 | - | 1.9 | 1.9 | 2.4 | - | 2.3 | 2.3 | - | - | 2.0 | 2.0 | 2.3 |
| p4 Ba | 0.7 | - | 0.8 | - | 0.0 | 1.1 | - | 0.9 | 0.7 | 0.9 | - | 1.2 | 1.1 | - | - | 0.9 | 0.9 | 1.0 |
| p4 Bp | 1.1 | - | 0.9 | - | 1.2 | 1.5 | - | 1.1 | 0.8 | 1.2 | - | 1.4 | 1.2 | - | - | 1.1 | 1.0 | 1.4 |
| m1 L | 4.0 | 4.3 | - | 3.9 | 3.3 | 4.4 | 4.5 | 3.7 | 3.6 | 4.4 | 4.3 | 3.8 | 3.9 | 4.6 | 4.2 | 3.9 | 4.5 | 4.2 |
| m1 L tri | 2.7 | 2.9 | - | 2.8 | 2.5 | 3.3 | 3.3 | 2.7 | 2.7 | 3.4 | 3.2 | 3.3 | 2.8 | 3.3 | 2.9 | 2.7 | 2.9 | 3.0 |
| m1 L tal | 1.3 | 1.3 | - | 0.9 | 0.7 | 1.1 | 1.2 | 0.9 | 1.0 | 1.3 | 1.3 | 0.8 | 1.1 | 1.3 | 1.5 | 1.4 | 0.7 | 0.9 |
| m1 B tri | 1.3 | 1.6 | - | 1.6 | 1.5 | 1.7 | 1.9 | 1.4 | 1.3 | 1.5 | 1.6 | 1.7 | 1.5 | 1.9 | 1.4 | 1.5 | 1.5 | 1.5 |
| m1 B tal | 1.2 | 1.5 | - | 1.5 | 1.3 | 1.5 | 1.5 | 1.1 | 1.1 | 1.3 | 1.3 | 1.3 | 1.3 | 1.5 | 1.3 | 1.2 | 1.2 | 1.4 |
| m2 L | 0.8 | - | - | - | - | 1.1 | - | - | - | 0.6 | - | 0.7 | - | - | - | 0.5 | 0.7 | - |
| m2 B | 0.9 | - | - | - | - | 1.2 | - | - | - | 0.7 | - | 0.7 | - | - | - | 0.6 | 1.1 | - |
| | LGM | GI 3 | GI 3? | | | | | | |
| N5-6 | M4 | M4 | M4 | G5 | F5 | F5 | L6 | L6 | L5 | M6-7 | M6-M7 | | | | | | |
| | R1 | 217 | 458 | 458 | | R1-2 | R5-8 | R10-1 | R10-1 | 53-1 | R62-1 | R57-1 | | | | | | |
| | ♂ | ♂ | ♂ | ♂ | | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | ♂ | | | | | | |
| 1 | 17.8 | 15.7 | 18.5 | - | 16.6 | 17.3 | 17.2 | 19.4 | - | 18.8 | 20.7 | 20.8 | | | | | | |
| 2 | 16.6 | 15.0 | 17.0 | - | 15.9 | 16.1 | 16.1 | 18.1 | - | 17.8 | 19.3 | 19.3 | | | | | | |
| 3 | 11.5 | 10.8 | 12.5 | - | 11.3 | 11.4 | 11.0 | 12.0 | - | 13.1 | 13.3 | 13.7 | | | | | | |
| 4 | 10.7 | 10.1 | 11.4 | - | 10.6 | 10.9 | 11.0 | 12.1 | - | 11.7 | 12.6 | 12.9 | | | | | | |
| 5 | 9.5 | 8.6 | 9.3 | - | 8.5 | 8.8 | 9.5 | 9.9 | - | - | 11.1 | 10.2 | | | | | | |
| 6 | 8.8 | 4.3 | 4.3 | - | 3.9 | 4.7 | 4.0 | 4.8 | - | - | 5.1 | 4.4 | | | | | | |
| 7 | 5.0 | 4.6 | 5.0 | - | 4.5 | 4.5 | 4.6 | 5.8 | - | 6.4 | 6.0 | 6.5 | | | | | | |
| 8 | 1.9 | 1.1 | 1.4 | - | 1.6 | 2.4 | 2.1 | 2.2 | - | | 1.9 | 0.9 | | | | | | |
| 10 | 8.5 | 7.6 | 8.8 | - | 8.5 | 8.0 | 8.0 | 10.1 | - | 10.8 | 10.2 | 10.3 | | | | | | |
| 12 | 3.9 | 3.1 | 3.6 | - | 4.0 | 3.3 | 3.9 | 3.7 | - | | 3.3 | 4.5 | | | | | | |
| 14 | 3.4 | 2.9 | 3.9 | - | 3.5 | 3.2 | 3.4 | 3.8 | - | 3.6 | 3.9 | 3.8 | | | | | | |
| 17 | 4.4 | 4.0 | 4.5 | - | 4.0 | 4.1 | 4.0 | 4.5 | - | 5.9 | 4.9 | 5.0 | | | | | | |
| p2 L | - | 0.9 | 0.9 | 1.0 | - | 0.7 | - | - | - | - | - | - | | | | | | |
| p2 B | - | 0.7 | 0.7 | 1.0 | - | 0.7 | - | - | - | - | - | - | | | | | | |
| p3 L | - | - | 1.5 | 1.7 | 1.6 | 1.9 | 2.3 | - | - | - | - | - | | | | | | |
| p3 B | - | - | 1.0 | 1.0 | 1.2 | 1.0 | 1.1 | - | - | - | - | - | | | | | | |
| p4 L | 2.1 | 2.0 | 2.0 | 2.0 | - | 2.0 | 2.3 | 2.2 | - | - | - | 2.6 | | | | | | |
| p4 Ba | 0.8 | 1.0 | 1.1 | 1.1 | - | 0.8 | 1.1 | 1.1 | - | - | - | 1.2 | | | | | | |
| p4 Bp | 1.3 | 1.3 | 1.2 | 1.3 | - | 0.9 | 1.1 | 1.1 | - | - | - | 1.4 | | | | | | |
| m1 L | 4.3 | 4.1 | 4.2 | 4.3 | 4.0 | 4.0 | 4.4 | 4.8 | 5.0 | 5.4 | 5.1 | 5.3 | | | | | | |
| m1 L tri | 3.1 | 2.7 | 3.0 | 3.3 | 2.6 | 2.9 | 3.3 | 3.5 | 3.3 | 4.0 | 3.6 | 3.6 | | | | | | |
| m1 L tal | 1.1 | 0.8 | 1.1 | 1.1 | 1.2 | 1.5 | 1.5 | 1.6 | 1.5 | 1.8 | 1.4 | 1.3 | | | | | | |
| m1 B tri | 1.9 | 1.4 | 1.7 | 1.4 | 1.4 | 1.4 | 1.4 | 1.7 | 1.8 | 1.9 | 1.8 | 2.0 | | | | | | |
| m1 B tal | 1.7 | 1.2 | 1.5 | 1.3 | 1.3 | 1.2 | 1.2 | 1.5 | 1.6 | 1.8 | 1.6 | 1.7 | | | | | | |
| m2 L | 1.0 | - | 1.4 | - | 0.9 | 1.0 | - | 1.0 | 1.1 | 1.0 | - | - | | | | | | |
| m2 B | 0.8 | - | 0.9 | - | 0.7 | 1.0 | - | 0.7 | 1.1 | 0.9 | - | - | | | | | | |
Table 11.
Lower carnassial (m1) size comparison between the weasel
Mustela nivalis from different late Middle Pleistocene, Late Pleistocene and Holocene localities. Abbreviations: H, Holocene; Min, minimum; Max, maximum; m, average; SD, standard deviation;
1 this work;
2 [
36];
3 [
35];
4 [
69];
5 [
34]; and
6 [
67].
Table 11.
Lower carnassial (m1) size comparison between the weasel
Mustela nivalis from different late Middle Pleistocene, Late Pleistocene and Holocene localities. Abbreviations: H, Holocene; Min, minimum; Max, maximum; m, average; SD, standard deviation;
1 this work;
2 [
36];
3 [
35];
4 [
69];
5 [
34]; and
6 [
67].
| Site | Sex | n | Min | Max | m | SD |
|---|
| Coulet des Roches 1 | ♂ | 13 | 4.01 | 4.53 | 4.28 | 0.14 |
| Dolmen Goult (H) 1 | ♂ | 2 | 4.77 | 4.79 | 4.78 | - |
| Les Planes (H) 1 | ♂ | 1 | - | - | 4.51 | - |
| Grotte de Gerde 2 | ♂ | 18 | 4.00 | 4.70 | 4.32 | 0.24 |
| Coudes 3 | ♂ | 2 | 4.23 | 4.49 | 4.36 | - |
| Lourdes Calvaire 2 | ♂ | 3 | 4.00 | 4.00 | 4.00 | - |
| Grotte du bois de Cantet 4 | ♂ | 2 | 4.30 | 4.50 | 4.35 | - |
| La Colombière 3 | ♂ | 23 | 3.84 | 4.49 | 4.14 | 0.20 |
| La Baume de Gigny 5 | ♂ | 24 | 3.78 | 4.42 | 4.10 | 0.18 |
| Abîmes de la Fage 3 | ♂ | 114 | 4.01 | 4.96 | 4.40 | 0.20 |
| France recent (vulgaris) 6 | ♂ | 22 | - | - | 4.21 | 0.20 |
| South Europe (boccamela) 6 | ♂ | 17 | - | - | 4.60 | 0.10 |
| Coulet des Roches 1 | ♀ | 7 | 3.33 | 3.91 | 3.70 | 0.21 |
| Coulet des Roches (H) 1 | ♀ | 2 | 3.82 | 3.93 | 3.88 | - |
| Grotte de Gerde 2 | ♀ | 8 | 3.60 | 3.90 | 3.75 | 0.09 |
| Coudes 3 | ♀ | 2 | 3.46 | 3.84 | 3.65 | - |
| Lourdes Calvaire 2 | ♀ | 1 | - | - | 3.50 | - |
| La Colombière 3 | ♀ | 30 | 3.26 | 3.83 | 3.57 | 0.16 |
| La Baume de Gigny 5 | ♀ | 25 | 3.00 | 3.78 | 3.48 | 0.20 |
| Abîmes de la Fage 3 | ♀ | 69 | 3.24 | 3.98 | 3.69 | 0.21 |
Table 12.
Equus ferus gallicus. Radiocarbon dates. Abbreviation: BA: Beta Analytic.
Table 12.
Equus ferus gallicus. Radiocarbon dates. Abbreviation: BA: Beta Analytic.
| Calibrated BP | Datations IntCal09 | References |
|---|
| IntCal09 | Calibrated BP | Calibrated BC | Material | Sedimentary Unit | Laboratory |
|---|
| LG—GS 2a | | | | | |
| 18,300–16,300 | 16,450–15,210 | 14,501–13,261 | Mare; K4 9 | US3 | BA 267379 |
| | | 16,476–15,225 | 14,528–13,276 | Mare; N5 76 | US3 | BA 267380 |
| LGM | | | | | |
| | | 18,623–18,021 | 16,623–16,072 | Mare; K3 111 | US3 | BA 267378 |
| | | 21,520–21,320 | 19,570–19,370 | Mare; N4 289 | US3 | BA 308066 |
| | | 21,670–21,320 | 19,720–19,370 | Stallion; M4 547 | US3 | BA 329249 |
| | | 24,240–23,950 | 22,290–22,000 | Mare; M4 910 | US3 | BA 388485 |
| GI 3 oscillation | | | | | |
| 26,000–27,000 | 26,375–26,020 | 24,425–24,070 | Stallion; N5 680 | US3 | BA 422567 |
| | | 26,700–26,250 | 24,750–24,300 | Stallion; L6 41 | US3/clay | BA 422566 |
| LGM | | | | | |
| | | 27,107–26,610 | 25,155–24,660 | Stallion; M5 244 | US3/scree | BA 388486 |
| | | 27,462–27,075 | 25,513–25,126 | Stallion; M7 97 | US3/scree | BA 476994 |
| | | 27,580–27,375 | 25,630–25,425 | Mare; L5 78 bis | US3/scree | BA 447017 |
| | | 27,783–27,441 | 25,834–25,492 | Mare; M6 437-1 | US3/US5 | BA 476993 |
Table 13.
Muzzle dimensions of
Equus ferus gallicus from Coulet des Roches (in mm). Measurement system according to Eisenmann [
79].
Table 13.
Muzzle dimensions of
Equus ferus gallicus from Coulet des Roches (in mm). Measurement system according to Eisenmann [
79].
| | | Length (5) | Breadth (17) |
|---|
| LG-GS 2a | | | |
| N5-106 | ♀ | 120.6 | 76.0 |
| LGM | | | |
| N4.289.1 | ♀ | 124.0 | 82.5 |
| M4-546 | ♂ | 115.0 | 79.5 |
| L4 78 + M4 878 + 879 | ♀ | 116.5 | 75.0 |
| L6-41 | ♂ | 128.0 | 73.8 |
| N5-680 | ♂ | | 78.0 |
| L5-78 | ♀ | 115.5 | 77.4 |
| M6 437-1 | ♂ | 124.4 | 82.4 |
| M7 97 | ♂ | 123.4 | 73.5 |
Table 14.
Length of the limb bones and third phalanx of Equus ferus gallicus from Coulet des Roches (in mm). Average value and number of specimens. Abbreviations: H, Humerus; F: Femur; R, Radius; T, Tibia; MC, Metacarpal III; MT, Metatarsal III; PH1A, First Anterior Phalanx; PH1P, First Posterior Phalanx; PH3A, Third Anterior Phalanx; (), number of specimens.
Table 14.
Length of the limb bones and third phalanx of Equus ferus gallicus from Coulet des Roches (in mm). Average value and number of specimens. Abbreviations: H, Humerus; F: Femur; R, Radius; T, Tibia; MC, Metacarpal III; MT, Metatarsal III; PH1A, First Anterior Phalanx; PH1P, First Posterior Phalanx; PH3A, Third Anterior Phalanx; (), number of specimens.
| | H | F | R | T | MC | MT | PH1A | PH1P | PH3A |
|---|
| LG-GS 2a | 280.9 (4) | 384.1 (6) | 332.9 (6) | 339.3 (5) | 223.3 (6) | 266.0 (8) | 85.7 (8) | 84.1 (8) | 79.5 (7) |
| LGM | 282.2 (14) | 389.2 (11) | 326.6 (14) | 343.3 (12) | 218.7 (19) | 261.8 (16) | 85.1 (14) | 83.5 (13) | 80.0 (12) |
Table 15.
Metacarpal III of
Equus ferus gallicus from Coulet des Roches (in mm). Abbreviations: L, left; R, right. Measurement system according to Eisenmann [
79].
Table 15.
Metacarpal III of
Equus ferus gallicus from Coulet des Roches (in mm). Abbreviations: L, left; R, right. Measurement system according to Eisenmann [
79].
| | | Length (1) | Breath at Mid-Diaphysis (3) | 3/1 × 1000 |
|---|
| LG-GS 2a | | | | |
| M4-133 | L | 218.0 | 33.0 | 151.4 |
| M4-70 | R | 217.5 | 32.4 | 149.0 |
| M4-135 | L | 225.8 | 33.8 | 149.7 |
| L5-4 | R | 223.9 | 33.9 | 151.4 |
| I4-21 | L | 229.0 | 33.3 | 145.4 |
| J4-11 | R | 225.8 | 32.2 | 142.6 |
| LGM | | | | |
| M4-727 | L | 225.0 | 35.5 | 157.8 |
| M4-814 | R | 224.5 | 34.9 | 155.5 |
| L5-69 | L | 226.9 | 35,0 | 154.3 |
| L5-66 | R | 226.6 | 36,0 | 158.9 |
| L6-104 | L | 209.4 | 35.6 | 170.0 |
| L6-96.1 | R | 209.0 | 35.9 | 171.8 |
| M6-189.1 | L | 221.0 | 36.7 | 166.1 |
| N6-545 | R | 221.7 | 38.0 | 171.4 |
| M4-1157 | L | 216.0 | 33.6 | 155.6 |
| M4-1165 | R | 214.5 | 33.4 | 155.7 |
| M4-677 | L | 220.4 | 35.8 | 162.4 |
| N6-291 | R | 220.9 | 35.9 | 162.5 |
| M4-582 | L | 212.2 | 34.8 | 164.0 |
| M4-575 | R | 214.0 | 35.2 | 164.5 |
| M5-477 | L | 217.0 | 33.6 | 154.8 |
| M5-503 | R | 215.5 | 34.3 | 159.2 |
| M7-92.1 | L | 218.4 | 34.1 | 156.1 |
| M6-443.1 | R | 218.3 | 34.4 | 157.6 |
| M6-405 | R | 224.5 | 36.0 | 160.4 |
Table 16.
Capra ibex. Radiocarbon dates. Abbreviation: BA, Beta Analytic.
Table 16.
Capra ibex. Radiocarbon dates. Abbreviation: BA, Beta Analytic.
| Calibrated BP | Datations IntCal09 | References |
|---|
| IntCal09 | Calibrated BP | Calibrated BC | Material | Sedimentary Unit | Laboratory |
|---|
| LG | | | | | |
| GI 1-e | 14,600–12,900 | 14,947–14,059 | 12,998–12,110 | Young; N3(5) 1 | US3 | BA 267381 |
| GS 2a | 18,300–16,300 | 16,130–15,220 | 14,180–13,280 | Adult; R 6017 | US3 | BA 337294 |
| LGM | | | | | |
| | | 19,382–18,869 | 17,920–17,432 | Female; M3 137-2 | US3 | BA 288279 |
| | | 19,560–19,430 | 17,610–17,480 | Male; N5 283 | US3 | BA 308067 |
| | | 22,250–21,870 | 20,300–19,920 | Female; N6 362-1 | US3 | BA 360688 |
| | | 23,585–23,330 | 21,635–21,380 | Young; N5 580 | US3 | BA 388487 |
Table 17.
Faunal list of the micromammals identified in the Coulet des Roches levels.
Table 17.
Faunal list of the micromammals identified in the Coulet des Roches levels.
| | Pleniglacial | Tardiglacial | Postglacial |
|---|
| LGM | GS 2a (H1) | GI 1e | GI 1ca + GS 1 |
|---|
| 770 < z > 660 | 660 < z > 600 | 600 < z < 560 | 560 < z < 510 | 510 < z < 400 | 400 < z < 320 | 320 < z < 280 | 280 < z < 210 | 180 < z < 170 | 160 < z < 150 | 120 < z < 110 | 110 < z < 79 | z = 79 |
|---|
| Soricomorpha | | | | | | | | | | | | | |
| Talpa europaea | | 1 | 1 | | | | | 2 | | 1 | | | |
| Crocidura leucodon/russula | | | | 1 | | | | | | | | | |
| cf. Sorex sp. | | | | | | | | | | 1 | | | |
| Sorex minutus | | | | | 1 | | | | | | | | |
| Sorex ex gr. araneus/coronatus | | | | | 1 | | | | | | | | |
| Rodentia | | | | | | | | | | | | | |
| Marmota marmota | | | 1 | | 2 | | | 1 | 1 | | | | |
| Eliomys quercinus | | | 1 | | 1 | | | 1 | | 1 | | 2 | |
| Apodemus sylvaticus | 2 | 3 | 7 | 5 | 3 | | | | | 3 | 1 | 6 | 1 |
| Arvicola amphibius | | | | | 1 | | | | | 1 | | | |
| Microtus (Stenocranius) gregalis | | 2 | | | | | | | | | | | |
| Microtus arvalis | 6 | 20 | 35 | 14 | 19 | 6 | 2 | | 1 | 1 | | | |
| Microtus ex gr. arvalis/agrestis | | | | | | | | 5 | 1 | 5 | 3 | 3 | 1 |
| Chionomys nivalis | 11 | 34 | 78 | 35 | 42 | 13 | 3 | 3 | | 1 | 3 | 1 | 1 |
| Clethrionomys glareolus | | 1 | | | 2 | 1 | | 1 | | 2 | | | |
| Microtus (T.) duodecimcostatus | | | | | | | | | | 1 | | | |
| Microtus (T.) cf. multiplex | | | | | 1 | | | | | 1 | | | |
| Microtus oeconomus | | 6 | 3 | 8 | 3 | 5 | | | 1 | | | | |
| Dicrostonyx torquatus | | 2 | 5 | 2 | 20 | 2 | | 1 | | | | | |
| Total MNI | 19 | 69 | 131 | 65 | 96 | 27 | 5 | 14 | 4 | 18 | 7 | 12 | 3 |