Smart Home Technology Solutions for Cardiovascular Diseases: A Systematic Review
Abstract
:1. Introduction
- A systematic review of technological solutions for CVD in smart home settings.
- Highlight the paucity in SHT for CVD management.
- Underline the imperative need for remote health monitoring systems integrated with SHT for CVD management.
- Future directions for developing a real-time CVD monitoring system in smart home settings integrating the Internet of Things (IoT), cloud computing, and big data analytics.
2. Materials and Methods
2.1. Protocol
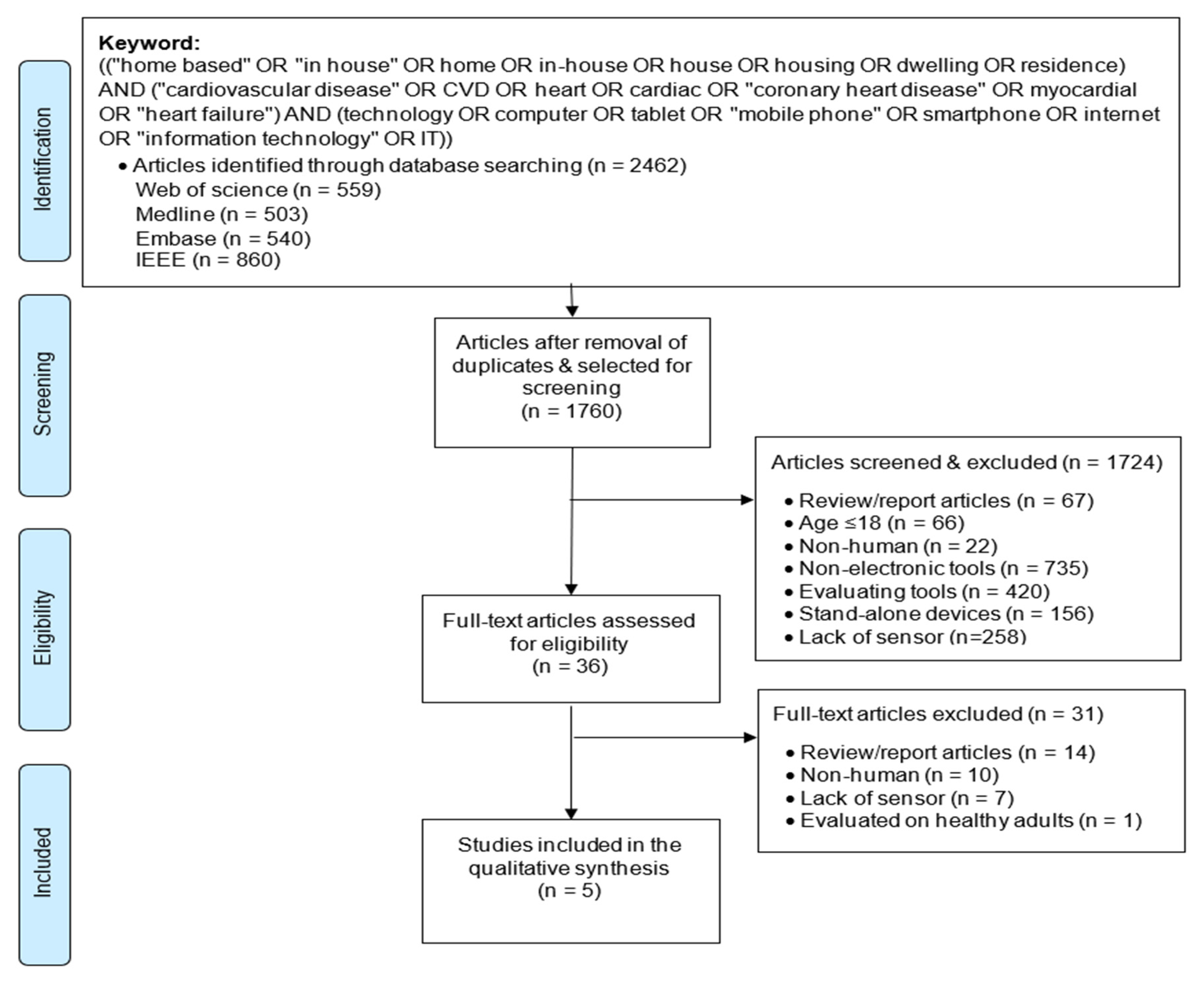
2.2. Data Sources and Search Strategy
2.3. Study Selection Criteria
2.4. Study Selection Process
2.5. Data Extraction
3. Results
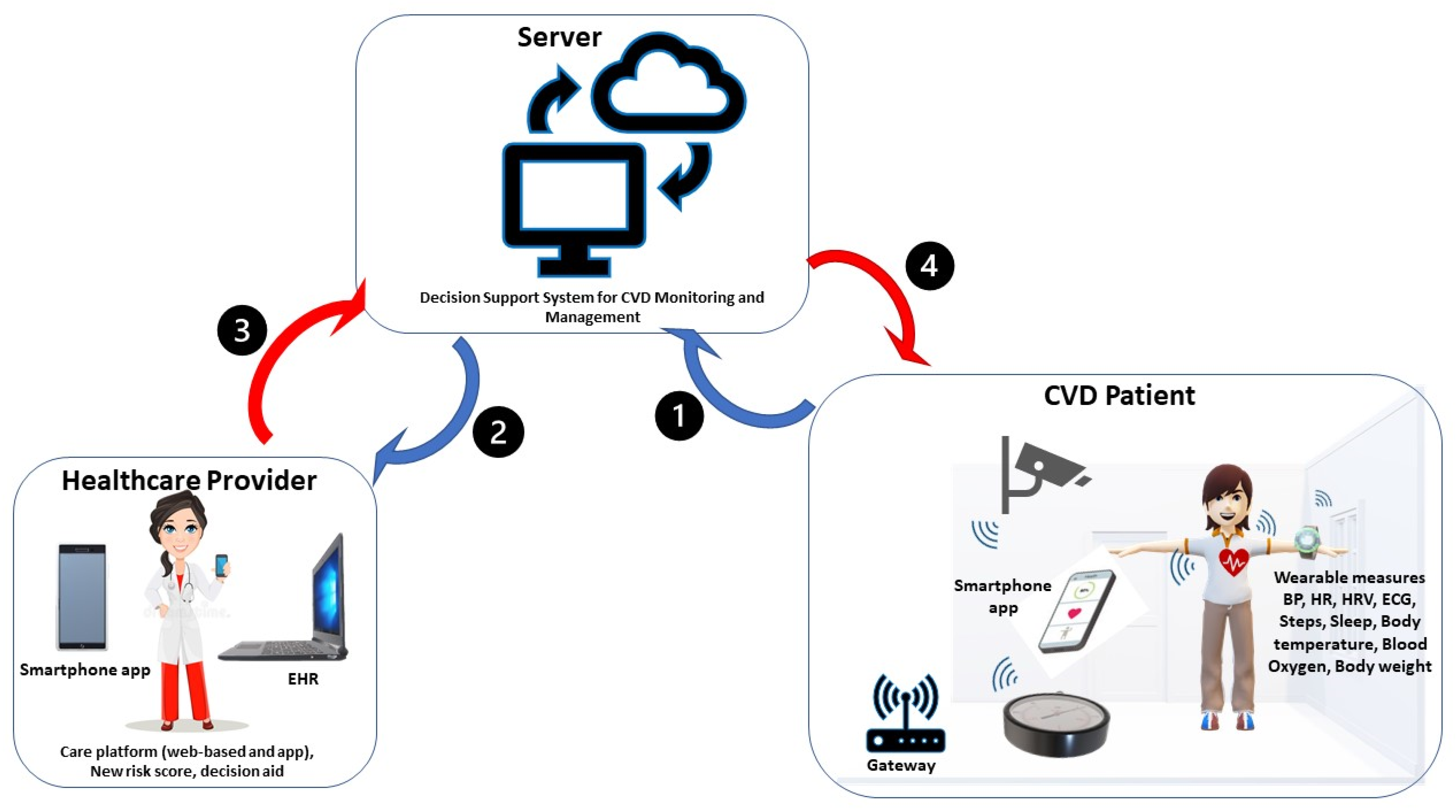
3.1. Available Smart Home Technologies for CVD Management
3.1.1. Sensor and Monitored Parameters
3.1.2. Communication Systems
3.1.3. End-User Applications
3.2. User Acceptance
3.3. Role of Regulatory Agency
4. Discussion
4.1. Limitations of this Study
4.2. Future Directions
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 28 January 2021).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M. Lifestyle Strategies for Risk Factor Reduction, Prevention, and Treatment of Cardiovascular Disease. Am. J. Lifestyle Med. 2019, 13, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cash, R.E.; Bower, J.K.; Focht, B.C.; Paskett, E.D. Physical activity and risk of cardiovascular disease by weight status among U.S adults. PLoS ONE 2020, 15, e0232893. [Google Scholar] [CrossRef]
- Lachman, S.; Boekholdt, S.M.; Luben, R.N.; Sharp, S.J.; Brage, S.; Khaw, K.T.; Peters, R.J.; Wareham, N.J. Impact of physical activity on the risk of cardiovascular disease in middle-aged and older adults: EPIC Norfolk prospective population study. Eur. J. Prev. Cardiol. 2018, 25, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Shariful Islam, S.M.; Farmer, A.J.; Bobrow, K.; Maddison, R.; Whittaker, R.; Pfaeffli Dale, L.A.; Lechner, A.; Lear, S.; Eapen, Z.; Niessen, L.W.; et al. Mobile phone text-messaging interventions aimed to prevent cardiovascular diseases (Text2PreventCVD): Systematic review and individual patient data meta-analysis. Open Heart 2019, 6, e001017. [Google Scholar] [CrossRef] [PubMed]
- Rehan, F.; Qadeer, A.; Bashir, I.; Jamshaid, M. Risk Factors of Cardiovascular Disease in Developing Countries. Int. Curr. Pharm. J. 2016, 5, 69–72. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.R.; Kim, M.N.; Shim, W.J.; Park, S.M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart 2021, 107, 373–380. [Google Scholar] [CrossRef]
- Puccinelli, P.J.; da Costa, T.S.; Seffrin, A.; de Lira, C.A.B.; Vancini, R.L.; Nikolaidis, P.T.; Knechtle, B.; Rosemann, T.; Hill, L.; Andrade, M.S. Reduced level of physical activity during COVID-19 pandemic is associated with depression and anxiety levels: An internet-based survey. BMC Public Health 2021, 21, 425. [Google Scholar] [CrossRef]
- Pecanha, T.; Goessler, K.F.; Roschel, H.; Gualano, B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1441–H1446. [Google Scholar] [CrossRef]
- Marikyan, D.; Papagiannidis, S.; Alamanos, E. A systematic review of the smart home literature: A user perspective. Technol. Forecast. Soc. Change 2019, 138, 139–154. [Google Scholar] [CrossRef]
- Darby, S.J. Smart technology in the home: Time for more clarity. Build. Res. Inf. 2017, 46, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Cho, M.E.; Jun, H.J. Developing Design Solutions for Smart Homes Through User-Centered Scenarios. Front. Psychol. 2020, 11, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mshali, H.; Lemlouma, T.; Moloney, M.; Magoni, D. A survey on health monitoring systems for health smart homes. Int. J. Ind. Ergon. 2018, 66, 26–56. [Google Scholar] [CrossRef] [Green Version]
- Helal, A.; Cook, D.J.; Schmalz, M. Smart Home-Based Health Platform for Behavioral Monitoring and Alteration of Diabetes Patients. J. Diabetes Sci. Technol. 2009, 3, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Constantino, M.; Orr, C.; Synnott, J.; Shewell, C.; Ennis, A.; Cleland, I.; Nugent, C.; Rafferty, J.; Morrison, G.; Larkham, L.; et al. Design and Implementation of a Smart Home in a Box to Monitor the Wellbeing of Residents with Dementia in Care Homes. Front. Digit. Health 2021, 3, 798889. [Google Scholar] [CrossRef] [PubMed]
- Tiersen, F.; Batey, P.; Harrison, M.J.C.; Naar, L.; Serban, A.I.; Daniels, S.J.C.; Calvo, R.A. Smart Home Sensing and Monitoring in Households With Dementia: User-Centered Design Approach. JMIR Aging 2021, 4, e27047. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mamas, M.A.; Mohamed, M.O.; Kwok, C.S.; Roebuck, C.; Humberstone, B.; Denwood, T.; Luescher, T.; de Belder, M.A.; Deanfield, J.E.; et al. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart 2021, 107, 113–119. [Google Scholar] [CrossRef]
- Gheorghe, A.; Griffiths, U.; Murphy, A.; Legido-Quigley, H.; Lamptey, P.; Perel, P. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: A systematic review. BMC Public Health 2018, 18, 975. [Google Scholar] [CrossRef] [Green Version]
- Conn, N.J.; Schwarz, K.Q.; Borkholder, D.A. In-Home Cardiovascular Monitoring System for Heart Failure: Comparative Study. JMIR mHealth uHealth 2019, 7, e12419. [Google Scholar] [CrossRef]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef]
- Redfern, J.; Coorey, G.; Mulley, J.; Scaria, A.; Neubeck, L.; Hafiz, N.; Pitt, C.; Weir, K.; Forbes, J.; Parker, S.; et al. A digital health intervention for cardiovascular disease management in primary care (CONNECT) randomized controlled trial. NPJ Digit. Med. 2020, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.C.; Adibi, S.; Shariful Islam, S.M.; Wickramasinghe, N.; Nguyen, L. Application of Smartphone Technologies in Disease Monitoring: A Systematic Review. Healthcare 2021, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.C.; Adibi, S.; Wickramasinghe, N.; Nguyen, L.; Angelova, M.; Islam, S.M.S. Smartphone as a Disease Screening Tool: A Systematic Review. Sensors 2022, 22, 3787. [Google Scholar] [CrossRef]
- Vyas, L.; Butakhieo, N. The impact of working from home during COVID-19 on work and life domains: An exploratory study on Hong Kong. Policy Des. Pract. 2021, 4, 59–76. [Google Scholar] [CrossRef]
- Hallman, D.M.; Januario, L.B.; Mathiassen, S.E.; Heiden, M.; Svensson, S.; Bergstrom, G. Working from home during the COVID-19 outbreak in Sweden: Effects on 24-h time-use in office workers. BMC Public Health 2021, 21, 528. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, R.; Warren, J. The role of home-based information and communications technology interventions in chronic disease management: A systematic literature review. Health Inform. J. 2009, 15, 122–146. [Google Scholar] [CrossRef]
- Hamilton, S.J.; Mills, B.; Birch, E.M.; Thompson, S.C. Smartphones in the secondary prevention of cardiovascular disease: A systematic review. BMC Cardiovasc. Disord. 2018, 18, 25. [Google Scholar] [CrossRef]
- Karunanithi, M.; Varnfield, M. Information and communication technology-based cardiac rehabilitation homecare programs. Smart Homecare Technol. Telehealth 2015, 3, 69. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Izudi, J.; Semakula, D.; Sennono, R.; Tamwesigire, I.K.; Bajunirwe, F. Protocol for systematic review and meta-analysis of treatment success rate among adult patients with tuberculosis in sub-Saharan Africa. BMJ Open 2018, 8, e024559. [Google Scholar] [CrossRef] [Green Version]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Do, N.T.; Bellingham, K.; Newton, P.N.; Caillet, C. The quality of medical products for cardiovascular diseases: A gap in global cardiac care. BMJ Glob. Health 2021, 6, e006523. [Google Scholar] [CrossRef] [PubMed]
- Desai, F.; Chowdhury, D.; Kaur, R.; Peeters, M.; Arya, R.C.; Wander, G.S.; Gill, S.S.; Buyya, R. HealthCloud: A system for monitoring health status of heart patients using machine learning and cloud computing. Internet Things 2022, 17, 100485. [Google Scholar] [CrossRef]
- Raghavan, A.; Demircioglu, M.A.; Taeihagh, A. Public Health Innovation through Cloud Adoption: A Comparative Analysis of Drivers and Barriers in Japan, South Korea, and Singapore. Int. J. Environ. Res. Public Health 2021, 18, 334. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswari, V.; Subramaniyaswamy, V.; Logesh, R.; Vijayakumar, V. A study on medical Internet of Things and Big Data in personalized healthcare system. Health Inf. Sci. Syst. 2018, 6, 14. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F. The Benefits of Telemedicine in Personalized Prevention of Cardiovascular Diseases (CVD): A Systematic Review. J. Pers. Med. 2021, 11, 658. [Google Scholar] [CrossRef]
- Kedzierski, K.; Radziejewska, J.; Slawuta, A.; Wawrzynska, M.; Arkowski, J. Telemedicine in Cardiology: Modern Technologies to Improve Cardiovascular Patients’ Outcomes-A Narrative Review. Medicina 2022, 58, 210. [Google Scholar] [CrossRef]
- Nick, J.M.; Roberts, L.R.; Petersen, A.B. Effectiveness of telemonitoring on self-care behaviors among community-dwelling adults with heart failure: A quantitative systematic review. JBI Evid. Synth. 2021, 19, 2659–2694. [Google Scholar] [CrossRef]
- Ding, H.; Chen, S.H.; Edwards, I.; Jayasena, R.; Doecke, J.; Layland, J.; Yang, I.A.; Maiorana, A. Effects of Different Telemonitoring Strategies on Chronic Heart Failure Care: Systematic Review and Subgroup Meta-Analysis. J. Med. Internet Res. 2020, 22, e20032. [Google Scholar] [CrossRef]
- Yanicelli, L.M.; Goy, C.B.; Martinez, E.C.; Herrera, M.C. Heart failure non-invasive home telemonitoring systems: A systematic review. Comput. Methods Programs Biomed. 2021, 201, 105950. [Google Scholar] [CrossRef]
- Kotb, A.; Cameron, C.; Hsieh, S.; Wells, G. Comparative effectiveness of different forms of telemedicine for individuals with heart failure (HF): A systematic review and network meta-analysis. PLoS ONE 2015, 10, e0118681. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Cameron, A.-F. IT-Enabled Self-Monitoring for Chronic Disease Self-Management: An Interdisciplinary Review. MIS Q. 2020, 44, 451–508. [Google Scholar] [CrossRef]
- Waffenschmidt, S.; Knelangen, M.; Sieben, W.; Buhn, S.; Pieper, D. Single screening versus conventional double screening for study selection in systematic reviews: A methodological systematic review. BMC Med. Res. Methodol. 2019, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, F.; Zhou, C.; Li, J.; Hong, C.; Tong, Q. The effect of mobile applications for improving adherence in cardiac rehabilitation: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2019, 19, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michard, F. A sneak peek into digital innovations and wearable sensors for cardiac monitoring. J. Clin. Monit. Comput. 2017, 31, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.; Boyne, J.; Brandts, J.; Brunner-La Rocca, H.P.; De Maesschalck, L.; De Wit, K.; Dixon, L.; Eurlings, C.; Fitzsimons, D.; Golubnitschaja, O.; et al. Artificial intelligence supported patient self-care in chronic heart failure: A paradigm shift from reactive to predictive, preventive and personalised care. EPMA J. 2019, 10, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Aamodt, I.T.; Lycholip, E.; Celutkiene, J.; Stromberg, A.; Atar, D.; Falk, R.S.; von Lueder, T.; Helleso, R.; Jaarsma, T.; Lie, I. Health Care Professionals’ Perceptions of Home Telemonitoring in Heart Failure Care: Cross-Sectional Survey. J. Med. Internet Res. 2019, 21, e10362. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, R.W.; Kraal, J.J.; Traa, S.C.; Spee, R.F.; Oostveen, L.M.; Kemps, H.M. Effects of cardiac telerehabilitation in patients with coronary artery disease using a personalised patient-centred web application: Protocol for the SmartCare-CAD randomised controlled trial. BMC Cardiovasc. Disord. 2017, 17, 46. [Google Scholar] [CrossRef] [Green Version]
- Lahdenoja, O.; Hurnanen, T.; Kaisti, M.; Koskinen, J.; Tuominen, J.; Vaha-Heikkila, M.; Parikka, L.; Wiberg, M.; Koivisto, T.; Pankaala, M. Cardiac monitoring of dogs via smartphone mechanocardiography: A feasibility study. Biomed. Eng. Online 2019, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Millan-Calenti, J.C.; Martinez-Isasi, S.; Lorenzo-Lopez, L.; Maseda, A. Morbidity and medication consumption among users of home telecare services. Health Soc. Care Community 2017, 25, 888–900. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Xu, J.; Wu, L.; Deng, Y.; Wang, J.; An, J. Advances in research on treatment of heart failure with nitrosyl hydrogen. Heart Fail. Rev. 2019, 24, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Forleo, G.B.; Solimene, F.; Pisano, E.C.; Zanotto, G.; Calvi, V.; Pignalberi, C.; Maglia, G.; Iacopino, S.; Quartieri, F.; Biffi, M.; et al. Long-term outcomes after prophylactic ICD and CRT-D implantation in nonischemic patients: Analysis from a nationwide database of daily remote-monitoring transmissions. J. Cardiovasc. Electrophysiol. 2019, 30, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Skobel, E.; Knackstedt, C.; Martinez-Romero, A.; Salvi, D.; Vera-Munoz, C.; Napp, A.; Luprano, J.; Bover, R.; Gloggler, S.; Bjarnason-Wehrens, B.; et al. Internet-based training of coronary artery patients: The Heart Cycle Trial. Heart Vessel. 2017, 32, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Escobar, R.; Gonzalez-Represas, A.; Gomez-Gonzalez, A.M.; Montiel-Trujillo, A.; Aguilar-Jimenez, R.; Carrasco-Ruiz, R.; Salinas-Sanchez, P. Effectiveness and safety of a home-based cardiac rehabilitation programme of mixed surveillance in patients with ischemic heart disease at moderate cardiovascular risk: A randomised, controlled clinical trial. BMC Cardiovasc. Disord. 2017, 17, 66. [Google Scholar] [CrossRef] [Green Version]
- Martin-Lesende, I.; Orruno, E.; Mateos, M.; Recalde, E.; Asua, J.; Reviriego, E.; Bayon, J.C. Telemonitoring in-home complex chronic patients from primary care in routine clinical practice: Impact on healthcare resources use. Eur. J. Gen. Pract. 2017, 23, 135–142. [Google Scholar] [CrossRef]
- Houze de l’Aulnoit, A.; Boudet, S.; Genin, M.; Gautier, P.F.; Schiro, J.; Houze de l’Aulnoit, D.; Beuscart, R. Development of a Smart Mobile Data Module for Fetal Monitoring in E-Healthcare. J. Med. Syst. 2018, 42, 83. [Google Scholar] [CrossRef]
- Davies, R.; Galway, L.; Nugent, C.; Jamison, C.; Gawley, R.; McCullagh, P.; Zhang, H.; Black, N. A Platform for Self-Management Supported by Assistive, Rehabilitation and Telecare Technologies. In Proceedings of the 5th International ICST Conference on Pervasive Computing Technologies for Healthcare, Dublin, Ireland, 23–26 May 2011. [Google Scholar]
- Hagglund, E.; Lynga, P.; Frie, F.; Ullman, B.; Persson, H.; Melin, M.; Hagerman, I. Patient-centred home-based management of heart failure. Findings from a randomised clinical trial evaluating a tablet computer for self-care, quality of life and effects on knowledge. Scand. Cardiovasc. J. 2015, 49, 193–199. [Google Scholar] [CrossRef]
- Melin, M.; Hagglund, E.; Ullman, B.; Persson, H.; Hagerman, I. Effects of a Tablet Computer on Self-care, Quality of Life, and Knowledge: A Randomized Clinical Trial. J. Cardiovasc. Nurs. 2018, 33, 336–343. [Google Scholar] [CrossRef]
- Hagglund, E.; Stromberg, A.; Hagerman, I.; Lynga, P. Theory Testing of Patient Perspectives Using a Mobile Health Technology System in Heart Failure Self-care. J. Cardiovasc. Nurs. 2019, 34, 448–453. [Google Scholar] [CrossRef]
- Bartlett, Y.K.; Haywood, A.; Bentley, C.L.; Parker, J.; Hawley, M.S.; Mountain, G.A.; Mawson, S. The SMART personalised self-management system for congestive heart failure: Results of a realist evaluation. BMC Med. Inform. Decis. Mak. 2014, 14, 109. [Google Scholar] [CrossRef] [Green Version]
- Alluhaidan, A.; Lee, E.; Alnosayan, N.; Chatterjee, S. Designing Patient-Centered mHealth Technology Intervention to Reduce Hospital Readmission for Heart-Failure Patients. In Proceedings of the 48th Hawaii International Conference on System Sciences, Kauai, HI, USA, 5–8 January 2015. [Google Scholar]
- Alnosayan, N.; Lee, E.; Alluhaidan, A.; Chatterjee, S.; Houston-Feenstra, L.; Kagoda, M.; Dysinger, W. MyHeart: An Intelligent mHealth Home Monitoring System Supporting Heart Failure Self-Care. In Proceedings of the 16th International Conference on e-Health Networking, Applications and Services (Healthcom), Natal, Brazil, 15–18 October 2014. [Google Scholar]
- Mukai, K.; Yonezawa, Y.; Ogawa, H.; Maki, H.; Caldwell, W.M. A remote monitor of bed patient cardiac vibration, respiration and movement. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 5191–5194. [Google Scholar]
- Burns, W.; Davies, R.; Nugent, C.; McCullagh, P.; Zheng, H.; Black, N.; Mountain, G. A Personalised Self-Management System for Chronic Heart Failure. In Proceedings of the 2010 Computing in Cardiology, Belfast, UK, 26–29 September 2010. [Google Scholar]
- Sciacqua, A.; Valentini, M.; Gualtieri, A.; Perticone, F.; Faini, A.; Zacharioudakis, G.; Karatzanis, I.; Chiarugi, F.; Assimakopoulou, C.; Meriggi, P.; et al. Validation of a flexible and innovative platform for the home monitoring of heart failure patients: Preliminary results. In Proceedings of the the 2009 36th Annual Computers in Cardiology Conference (CinC), Park City, UT, USA, 13–16 September 2009; pp. 97–100. [Google Scholar]
- Katra, R.P.; Chakravarthy, N.; Libbus, I. Remote At-Home Detection and Monitoring of Functional Chronotropic Incompetence in Heart Failure Patients. J. Cardiovasc. Transl. Res. 2011, 4, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fanucci, L.; Saponara, S.; Bacchillone, T.; Donati, M.; Barba, P.; Sanchez-Tato, I.; Carmona, C. Sensing Devices and Sensor Signal Processing for Remote Monitoring of Vital Signs in CHF Patients. IEEE Trans. Instrum. Meas. 2013, 62, 553–569. [Google Scholar] [CrossRef]
- Alnosayan, N.; Chatterjee, S.; Alluhaidan, A.; Lee, E.; Houston Feenstra, L. Design and Usability of a Heart Failure mHealth System: A Pilot Study. JMIR Hum. Factors 2017, 4, e9. [Google Scholar] [CrossRef] [PubMed]
- Kotooka, N.; Kitakaze, M.; Nagashima, K.; Asaka, M.; Kinugasa, Y.; Nochioka, K.; Mizuno, A.; Nagatomo, D.; Mine, D.; Yamada, Y.; et al. The first multicenter, randomized, controlled trial of home telemonitoring for Japanese patients with heart failure: Home telemonitoring study for patients with heart failure (HOMES-HF). Heart Vessel. 2018, 33, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Rokas, O.; Chen, L. Healthcare in the Smart Home: A Study of Past, Present and Future. Sustainability 2017, 9, 840. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.; Paulo Silva Cunha, J. Wearable Health Devices-Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [Green Version]
- Gerdes, M.; Trinugroho, Y.B.D.; Næss, M.; Fensli, R. Security, Reliability and Usability of mHealth Environments. In Mobile Health; Adibi, S., Ed.; Springer: Cham, Switzerland, 2015; pp. 1043–1066. [Google Scholar] [CrossRef]
- Rothstein, M.A.; Wilbanks, J.T.; Beskow, L.M.; Brelsford, K.M.; Brothers, K.B.; Doerr, M.; Evans, B.J.; Hammack-Aviran, C.M.; McGowan, M.L.; Tovino, S.A. Unregulated Health Research Using Mobile Devices: Ethical Considerations and Policy Recommendations. J. Law Med. Ethics 2020, 48, 196–226. [Google Scholar] [CrossRef]
- Jogova, M.; Shaw, J.; Jamieson, T. The regulatory challenge of mobile health: Lessons for Canada. Healthc. Policy 2019, 14, 19–28. [Google Scholar] [CrossRef]
- Rodriguez-Villa, E.; Torous, J. Regulating digital health technologies with transparency: The case for dynamic and multi-stakeholder evaluation. BMC Med. 2019, 17, 226. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, X.; Li, X.; Gu, D.; Liang, C.; Li, K.; Zhang, G.; Zhong, J. Exploring emerging IoT technologies in smart health research: A knowledge graph analysis. BMC Med. Inf. Decis. Mak. 2020, 20, 260. [Google Scholar] [CrossRef]
- Dadkhah, M.; Mehraeen, M.; Rahimnia, F.; Kimiafar, K. Use of Internet of Things for Chronic Disease Management: An Overview. J. Med. Signals Sens. 2021, 11, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Campbell, K.L.; Gong, E.; Scuffham, P. The Internet of Things: Impact and Implications for Health Care Delivery. J. Med. Internet Res. 2020, 22, e20135. [Google Scholar] [CrossRef] [PubMed]
- Nasajpour, M.; Pouriyeh, S.; Parizi, R.M.; Dorodchi, M.; Valero, M.; Arabnia, H.R. Internet of Things for Current COVID-19 and Future Pandemics: An Exploratory Study. J. Healthc. Inform. Res. 2020, 4, 325–364. [Google Scholar] [CrossRef] [PubMed]
- Morales-Botello, M.L.; Gachet, D.; de Buenaga, M.; Aparicio, F.; Busto, M.J.; Ascanio, J.R. Chronic patient remote monitoring through the application of big data and internet of things. Health Inform. J. 2021, 27, 14604582211030956. [Google Scholar] [CrossRef]
- Whitelaw, S.; Pellegrini, D.M.; Mamas, M.A.; Cowie, M.; Van Spall, H.G.C. Barriers and facilitators of the uptake of digital health technology in cardiovascular care: A systematic scoping review. Eur. Heart J. Digit. Health 2021, 2, 62–74. [Google Scholar] [CrossRef]
- McHorney, C.A.; Mansukhani, S.G.; Anatchkova, M.; Taylor, N.; Wirtz, H.S.; Abbasi, S.; Battle, L.; Desai, N.R.; Globe, G. The impact of heart failure on patients and caregivers: A qualitative study. PLoS ONE 2021, 16, e0248240. [Google Scholar] [CrossRef]
- Umer, M.; Sadiq, S.; Karamti, H.; Karamti, W.; Majeed, R.; Nappi, M. IoT Based Smart Monitoring of Patients’ with Acute Heart Failure. Sensors 2022, 22, 2431. [Google Scholar] [CrossRef]
- Brahmbhatt, D.H.; Cowie, M.R. Remote Management of Heart Failure: An Overview of Telemonitoring Technologies. Card. Fail. Rev. 2019, 5, 86–92. [Google Scholar] [CrossRef]
- Sohn, A.; Speier, W.; Lan, E.; Aoki, K.; Fonarow, G.C.; Ong, M.K.; Arnold, C.W. Integrating remote monitoring into heart failure patients’ care regimen: A pilot study. PLoS ONE 2020, 15, e0242210. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. Quantitative Evaluation of Task-Induced Neurological Outcome after Stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. Prediction of Myoelectric Biomarkers in Post-Stroke Gait. Sensors 2021, 21, 5334. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. Big-ECG: Cardiographic Predictive Cyber-Physical System for Stroke Management. IEEE Access 2021, 9, 123146–123164. [Google Scholar] [CrossRef]
- Almarzouki, H.Z.; Alsulami, H.; Rizwan, A.; Basingab, M.S.; Bukhari, H.; Shabaz, M. An Internet of Medical Things-Based Model for Real-Time Monitoring and Averting Stroke Sensors. J. Healthc. Eng. 2021, 2021, 1233166. [Google Scholar] [CrossRef]
- Boukhennoufa, I.; Zhai, X.; Utti, V.; Jackson, J.; McDonald-Maier, K.D. Wearable sensors and machine learning in post-stroke rehabilitation assessment: A systematic review. Biomed. Signal Process. Control 2022, 71, 103197. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Kario, K. Home Blood Pressure Monitoring: Current Status and New Developments. Am. J. Hypertens. 2021, 34, 783–794. [Google Scholar] [CrossRef]
- Yazdanpanah, M.H.; Homayounfar, R.; Khademi, A.; Zarei, F.; Shahidi, A.; Farjam, M. Short sleep is associated with higher prevalence and increased predicted risk of cardiovascular diseases in an Iranian population: Fasa PERSIAN Cohort Study. Sci. Rep. 2020, 10, 4608. [Google Scholar] [CrossRef]
- Nikbakhtian, S.; Reed, A.B.; Obika, B.D.; Morelli, D.; Cunningham, A.C.; Aral, M.; Plans, D. Accelerometer-derived sleep onset timing and cardiovascular disease incidence: A UK Biobank cohort study. Eur. Heart J.-Digit. Health 2021, 2, 658–666. [Google Scholar] [CrossRef]
- Leopold, J.A.; Antman, E.M. Digital health device measured sleep duration and ideal cardiovascular health: An observational study. BMC Cardiovasc. Disord. 2021, 21, 497. [Google Scholar] [CrossRef]
- Hall, M.H.; Brindle, R.C.; Buysse, D.J. Sleep and cardiovascular disease: Emerging opportunities for psychology. Am. Psychol. 2018, 73, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Bertagnin, E.; Greco, A.; Bottaro, G.; Zappulla, P.; Romanazzi, I.; Russo, M.D.; Lo Presti, M.; Valenti, N.; Sollano, G.; Calvi, V. Remote monitoring for heart failure management during COVID-19 pandemic. Int. J. Cardiol. Heart Vasc. 2021, 32, 100724. [Google Scholar] [CrossRef] [PubMed]
- Savaris, R.F.; Pumi, G.; Dalzochio, J.; Kunst, R. Stay-at-home policy is a case of exception fallacy: An internet-based ecological study. Sci. Rep. 2021, 11, 5313. [Google Scholar] [CrossRef]
- Mohebali, D.; Kittleson, M.M. Remote monitoring in heart failure: Current and emerging technologies in the context of the pandemic. Heart 2021, 107, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Bove, L.A. Increasing Patient Engagement Through the Use of Wearable Technology. J. Nurse Pract. 2019, 15, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Zhang, J.; Xie, Y.; Gao, F.; Xu, S.; Wu, X.; Ye, Z. Wearable Health Devices in Health Care: Narrative Systematic Review. JMIR mHealth uHealth 2020, 8, e18907. [Google Scholar] [CrossRef]
- Rens, N.; Gandhi, N.; Mak, J.; Paul, J.; Bent, D.; Liu, S.; Savage, D.; Nielsen-Bowles, H.; Triggs, D.; Ata, G.; et al. Activity data from wearables as an indicator of functional capacity in patients with cardiovascular disease. PLoS ONE 2021, 16, e0247834. [Google Scholar] [CrossRef]
- Naseri Jahfari, A.; Tax, D.; Reinders, M.; van der Bilt, I. Machine Learning for Cardiovascular Outcomes From Wearable Data: Systematic Review From a Technology Readiness Level Point of View. JMIR Med. Inform. 2022, 10, e29434. [Google Scholar] [CrossRef]
- Fletcher, A.C.; Mura, C. Ten quick tips for using a Raspberry Pi. PLoS Comput. Biol. 2019, 15, e1006959. [Google Scholar] [CrossRef]
- Johnston, S.; Cox, S. The Raspberry Pi: A Technology Disrupter, and the Enabler of Dreams. Electronics 2017, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Akinosun, A.S.; Polson, R.; Diaz-Skeete, Y.; De Kock, J.H.; Carragher, L.; Leslie, S.; Grindle, M.; Gorely, T. Digital Technology Interventions for Risk Factor Modification in Patients with Cardiovascular Disease: Systematic Review and Meta-analysis. JMIR mHealth uHealth 2021, 9, e21061. [Google Scholar] [CrossRef] [PubMed]
- Harst, L.; Lantzsch, H.; Scheibe, M. Theories Predicting End-User Acceptance of Telemedicine Use: Systematic Review. J. Med. Internet Res. 2019, 21, e13117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, H.J.; Payne, C.J.; Hughes-Hallett, A.; Marcus, A.P.; Yang, G.Z.; Darzi, A.; Nandi, D. Regulatory approval of new medical devices: Cross sectional study. BMJ 2016, 353, i2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, L.; Materwala, H.; Karduck, A.P.; Adem, A. Requirements of Health Data Management Systems for Biomedical Care and Research: Scoping Review. J. Med. Internet Res. 2020, 22, e17508. [Google Scholar] [CrossRef]
- Umair, M.; Cheema, M.A.; Cheema, O.; Li, H.; Lu, H. Impact of COVID-19 on IoT Adoption in Healthcare, Smart Homes, Smart Buildings, Smart Cities, Transportation and Industrial IoT. Sensors 2021, 21, 3838. [Google Scholar] [CrossRef]
- Krittanawong, C.; Virk, H.U.H.; Bangalore, S.; Wang, Z.; Johnson, K.W.; Pinotti, R.; Zhang, H.; Kaplin, S.; Narasimhan, B.; Kitai, T.; et al. Machine learning prediction in cardiovascular diseases: A meta-analysis. Sci. Rep. 2020, 10, 16057. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Ma, R.; Wang, X.; Liu, J.; Keerman, M.; Yan, Y.; Ma, J.; Song, Y.; Zhang, J.; et al. Cardiovascular Disease Prediction by Machine Learning Algorithms Based on Cytokines in Kazakhs of China. Clin. Epidemiol. 2021, 13, 417–428. [Google Scholar] [CrossRef]
- Chao, H.; Shan, H.; Homayounieh, F.; Singh, R.; Khera, R.D.; Guo, H.; Su, T.; Wang, G.; Kalra, M.K.; Yan, P. Deep learning predicts cardiovascular disease risks from lung cancer screening low dose computed tomography. Nat. Commun. 2021, 12, 2963. [Google Scholar] [CrossRef]
- Miller, J.C.; Skoll, D.; Saxon, L.A. Home Monitoring of Cardiac Devices in the Era of COVID-19. Curr. Cardiol. Rep. 2020, 23, 1. [Google Scholar] [CrossRef]
- Javaid, M.; Khan, I.H. Internet of Things (IoT) enabled healthcare helps to take the challenges of COVID-19 Pandemic. J. Oral Biol. Craniofac. Res. 2021, 11, 209–214. [Google Scholar] [CrossRef]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Plews, D.; Froelicher, V. Heart Rate Variability: An Old Metric with New Meaning in the Era of using mHealth Technologies for Health and Exercise Training Guidance. Part One: Physiology and Methods. Arrhythm. Electrophysiol. Rev. 2018, 7, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.C.; Wu, Y.L.; Tsai, P.S. Heart Rate Variability and Risk of All-Cause Death and Cardiovascular Events in Patients With Cardiovascular Disease: A Meta-Analysis of Cohort Studies. Biol. Res. Nurs. 2020, 22, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Lees, T.; Shad-Kaneez, F.; Simpson, A.M.; Nassif, N.T.; Lin, Y.; Lal, S. Heart Rate Variability as a Biomarker for Predicting Stroke, Post-stroke Complications and Functionality. Biomark. Insights 2018, 13, 1177271918786931. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, L.; Wang, Q.; Zhou, G.; Wang, Y.; Jiang, Q. A new approach to detect congestive heart failure using short-term heart rate variability measures. PLoS ONE 2014, 9, e93399. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Takata, Y.; Maruyama, K.; Eguchi, E.; Kato, T.; Shirahama, R.; Tomooka, K.; Kawamura, R.; Sano, M.; Tabara, Y.; et al. Association Between Heart Rate Variability and Home Blood Pressure: The Toon Health Study. Am. J. Hypertens 2018, 31, 1120–1126. [Google Scholar] [CrossRef] [Green Version]
- Vigier, M.; Vigier, B.; Andritsch, E.; Schwerdtfeger, A.R. Cancer classification using machine learning and HRV analysis: Preliminary evidence from a pilot study. Sci. Rep. 2021, 11, 22292. [Google Scholar] [CrossRef]
- Chiew, C.J.; Liu, N.; Tagami, T.; Wong, T.H.; Koh, Z.X.; Ong, M.E.H. Heart rate variability based machine learning models for risk prediction of suspected sepsis patients in the emergency department. Medicine 2019, 98, e14197. [Google Scholar] [CrossRef]

| Inclusion Criteria: |
|
| Exclusion Criteria: |
|
| Articles Reviewed | Study Type | Study Duration | Country | Participants | Age (Years) | Male (%) | Female (%) |
|---|---|---|---|---|---|---|---|
| (Sciacqua, 2009) [67] | CO | Spot reading/day | Italy | 10 elderly CHF | NS | 90 | 10 |
| (Katra, 2011) [68] | CO | 90 days | Asia | 180 HF | 61 ± 13 | 70 | 30 |
| (Fanucci, 2013) [69] | CO | 1 month | Italy | 30 CHF | μ: 62 | NS | NS |
| (Alnosayan, 2017) [70] | CO | 6 months | USA | 8 HF | 61.5 ± 9.3 | 63 | 37 |
| (Kotooka, 2018) [71] | RCT | 0–31 months | Japan | 181 HF | Tel: 67.1 ± 12.8 Usual: 65.4 ± 15.6 | 59 | 41 |
| Articles Reviewed | Parameters Monitored | System | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Manual | Device | Communication System | Gateway | Interactive User Interface | Report Viewed by | Alarm Situation | |||
| Wearable | Non-Wearable | ||||||||
| (Sciacqua, 2009) [65] | HR, BP, BW, SpO2, Temperature. | RR, ECG, Chest movement. | HR, BP, BW, SpO2. | Device to Gateway: BT, Wi-Fi. | Gateway to App: Internet. | Computer | User: questionnaire, guides in vital measurement. | Health Practitioner | Doctor contacted patients. |
| (Katra, 2011) [66] | - | HR, RR, Body Movement, Posture. | NA | Device to Gateway: BT. | Gateway to App: Internet. | Device | NA | Researcher | NA |
| (Fanucci, 2013) [67] | - | - | RR, ECG, Chest movement, BP, BW, Posture, SpO2. | Device to Gateway: BT. | Gateway to App: Internet. | Computer | User: assist in therapy. Clinician: interact with the system | Health Practitioner | Caregivers or relatives are contacted via SMS. |
| (Alnosayan, 2017) [68] | Symptoms | - | BW, BP, BG | Device to Gateway: BT. | GW to App: Internet. | Device | User: personal health tracking system. Clinician: view patient recordings. | Heart failure nurses | Nurse contacted the patients. |
| (Kotooka, 2018) [69] | - | - | BW, PR, BP. | Device to Gateway W: BT. | Gateway to App: Internet. | Device | NA | Health Practitioner | Nurse notified the patient’s physician. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moses, J.C.; Adibi, S.; Angelova, M.; Islam, S.M.S. Smart Home Technology Solutions for Cardiovascular Diseases: A Systematic Review. Appl. Syst. Innov. 2022, 5, 51. https://doi.org/10.3390/asi5030051
Moses JC, Adibi S, Angelova M, Islam SMS. Smart Home Technology Solutions for Cardiovascular Diseases: A Systematic Review. Applied System Innovation. 2022; 5(3):51. https://doi.org/10.3390/asi5030051
Chicago/Turabian StyleMoses, Jeban Chandir, Sasan Adibi, Maia Angelova, and Sheikh Mohammed Shariful Islam. 2022. "Smart Home Technology Solutions for Cardiovascular Diseases: A Systematic Review" Applied System Innovation 5, no. 3: 51. https://doi.org/10.3390/asi5030051
APA StyleMoses, J. C., Adibi, S., Angelova, M., & Islam, S. M. S. (2022). Smart Home Technology Solutions for Cardiovascular Diseases: A Systematic Review. Applied System Innovation, 5(3), 51. https://doi.org/10.3390/asi5030051








