A Case Report of a Solitary Fibrous Tumor of the Maxillary Sinus
Abstract
:1. Introduction
2. Case Presentation
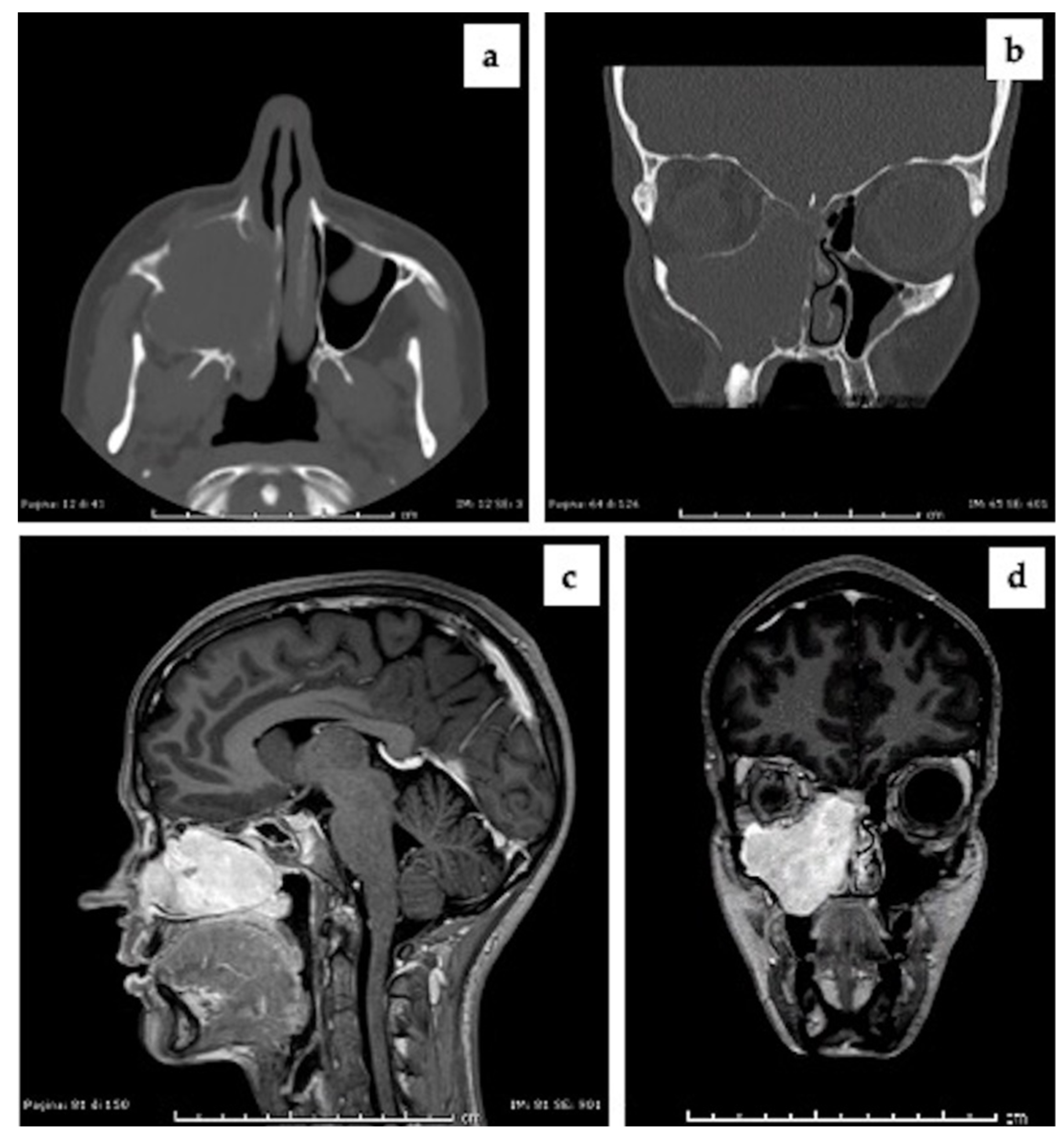
2.1. Clinical Presentation and Preoperative Imaging
2.2. Incisional Biopsy Findings
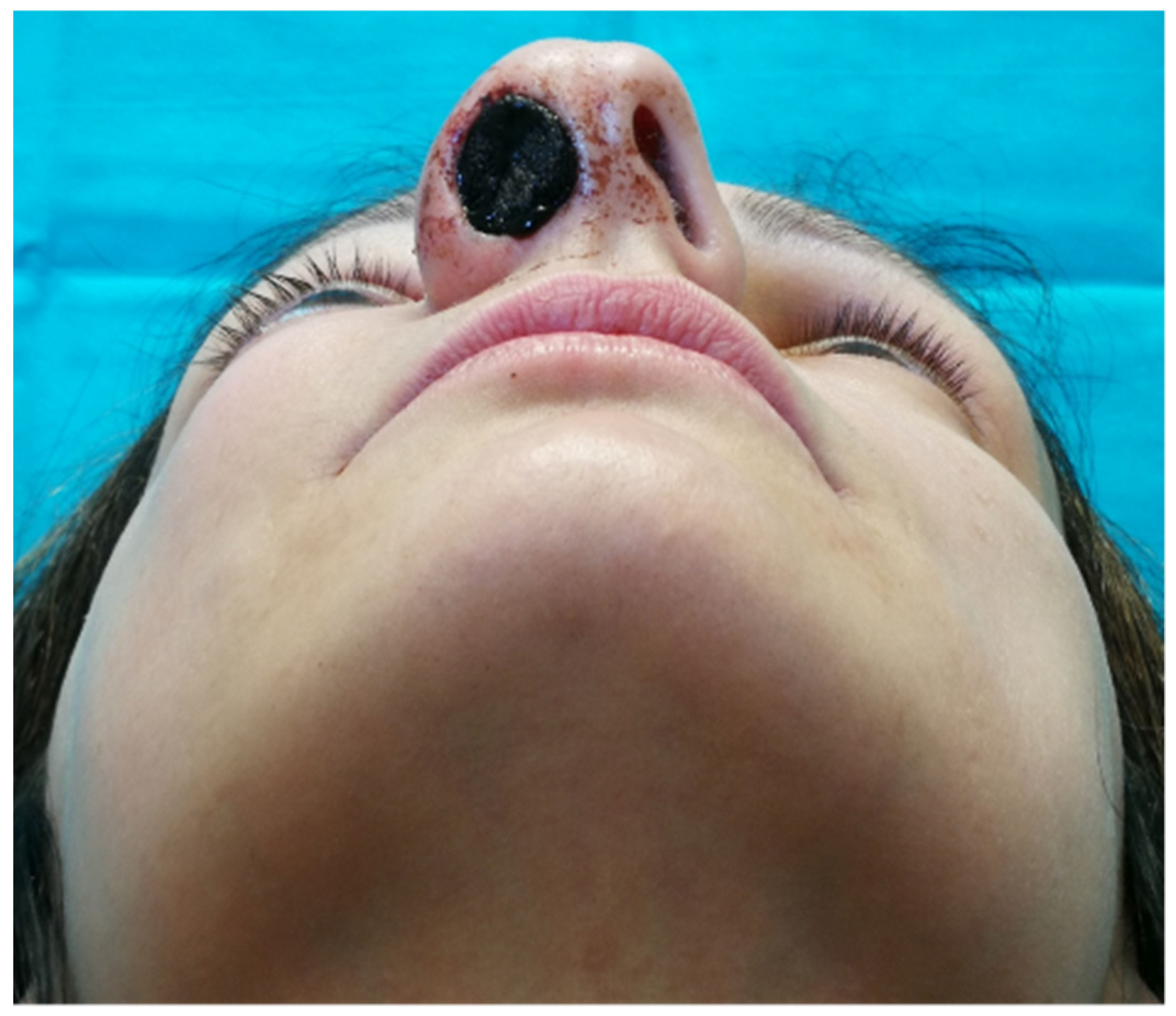
2.3. Intraoperative Imaging and Embolization Procedure
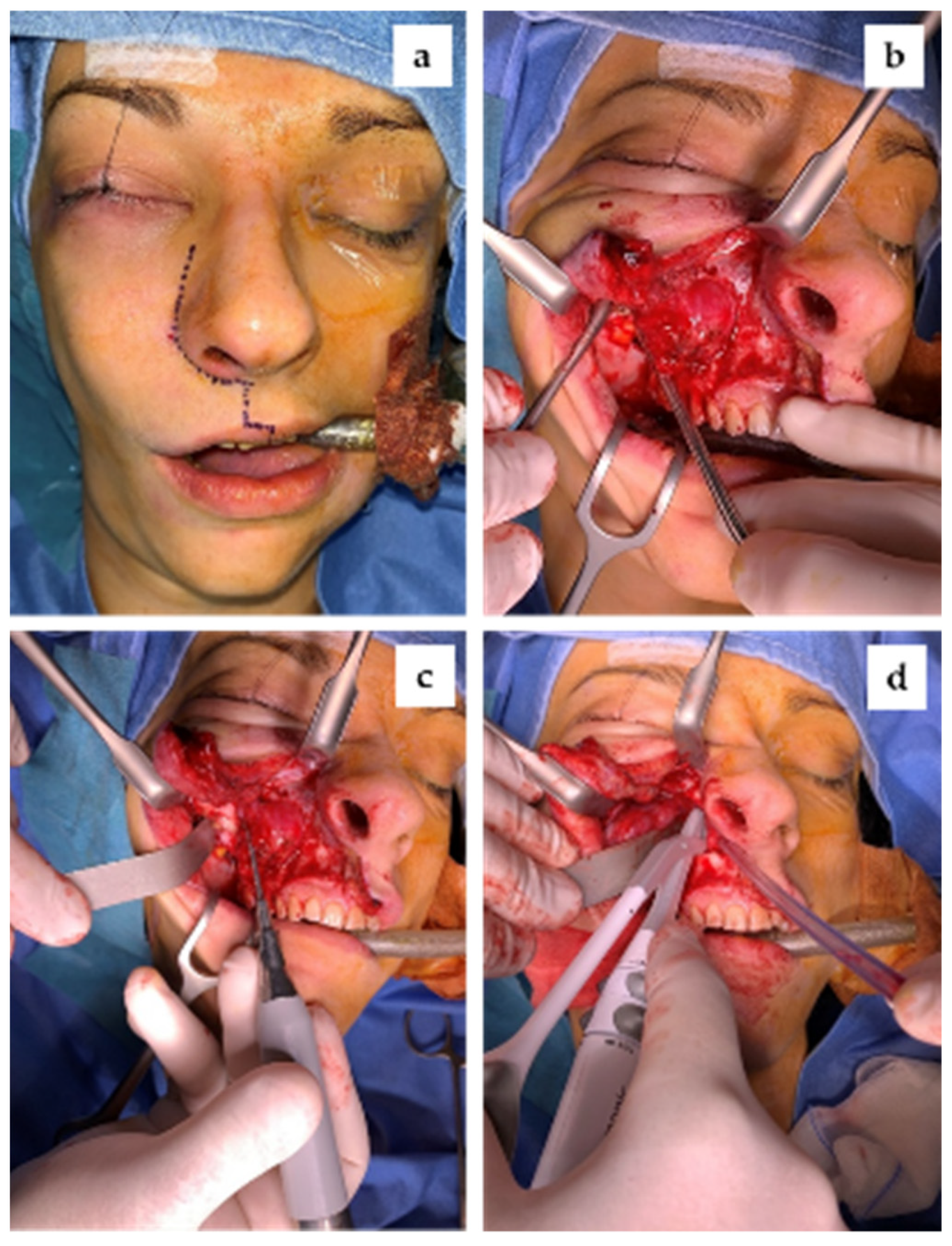
2.4. Surgical Management
2.5. Solitary Fibrous Tumor Diagnosis
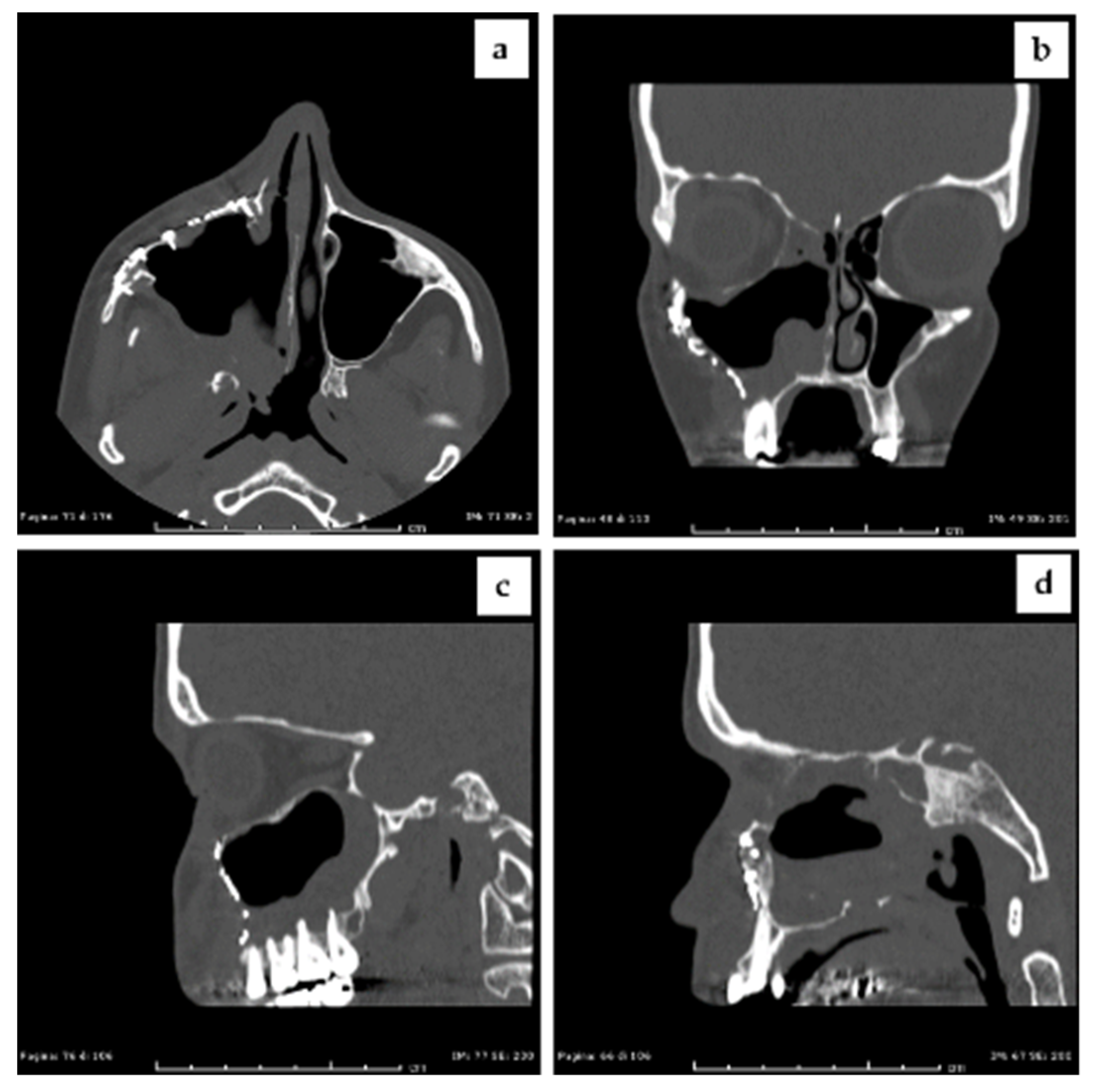
2.6. Follow Up
2.7. Patient Consent
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Klemperer, P.; Rabin, C.B. Primary Neoplasms of the pleura. A report of five cases. Am. J. Ind. Med. 1992, 22, 4–31. [Google Scholar] [CrossRef]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S.; Bassett, R.L.; Pollock, R.E.; Lazar, A.J.; Wang, W.-L. Solitary fibrous tumor: A clinicopathological study of 110 cases and proposed risk assessment model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Gold, J.S.; Antonescu, C.R.; Hajdu, C.; Ferrone, C.R.; Hussain, M.; Lewis, J.J.; Brennan, M.F.; Coit, D.G. Clinicopathologic correlates of solitary fibrous tumors. Cancer 2002, 94, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Gooding, W.E.; Elkins, M.; Patel, R.M.; Harms, P.W.; McDaniel, A.S.; Palanisamy, N.; Uram-Tuculescu, C.; Balzer, B.B.; Lucas, D.R.; et al. Solitary Fibrous Tumors of the Head and Neck: A Multi-Institutional Clinicopathologic Study. Am. J. Surg. Pathol. 2017, 41, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, F.P.; de Conciliis, C.; Schneider, S.; Kersten, R.C.; Kulwin, D.R. Solitary fibrous tumor of the orbit: Is it rare? Report of a case series and review of the literature. Ophthalmology 2003, 110, 1442–1448. [Google Scholar] [CrossRef]
- Fletcher, C.D.M.; World Health Organization. International Agency for Research on Cancer. In WHO Classification of Tumours of Soft Tissue and Bone; International Agency for Research on Cancer: Lyon, France, 2013; ISBN 9789283224341. [Google Scholar]
- Martin-Broto, J.; Mondaza-Hernandez, J.L.; Moura, D.S.; Hindi, N. A Comprehensive Review on Solitary Fibrous Tumor: New Insights for New Horizons. Cancers 2021, 13, 2913. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.U.; Din, N.U.; Abdul-Ghafar, J.; Park, Y.-K. The many faces of solitary fibrous tumor; diversity of histological features, differential diagnosis and role of molecular studies and surrogate markers in avoiding misdiagnosis and predicting the behavior. Diagn. Pathol. 2021, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R.; Lau, S.K. Sinonasal Tract Solitary Fibrous Tumor: A Clinicopathologic Study of Six Cases with a Comprehensive Review of the Literature. Head Neck Pathol. 2018, 12, 471–480. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Pellacani, A.; Negrello, S.; Chiarini, L.; Anesi, A. Emerging challenges and possible strategies in maxillo-facial and oral surgery during the COVID-19 pandemic. J. Oral Sci. 2020, 62, 452–454. [Google Scholar] [CrossRef]
- Thobejane, O.; Maharaj, S. Nasal Septal Angiofibroma. Ear Nose Throat J. 2021, 1455613211026517. [Google Scholar] [CrossRef]
- Ghaloo, S.K.; Dhanani, R.; Pasha, H.A.; Wasif, M.; Fatima, S.; Ikram, M. Glomangiopericytoma: A rare tumour of sinonasal cavity. J. Pak. Med. Assoc. 2020, 70, 2469–2471. [Google Scholar] [CrossRef] [PubMed]
- Assiri, K.S.; Al-Ahmari, M.S.; Alshahrani, M.S.; Mastor, A.; Elhawary, R. Clinical and Pathological Features of Angiomatous Nasal Polyps: A Report of Four Cases and Review of Literature. Cureus 2020, 12, e7642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katre, M.I. Neurofibroma of Nasal Cavity and Nasopharynx. Adv. Case Stud. 2017, 1. [Google Scholar] [CrossRef]
- Bowe, S.N.; Wakely, P.E.; Ozer, E. Head and neck solitary fibrous tumors: Diagnostic and therapeutic challenges. Laryngoscope 2012, 122, 1748–1755. [Google Scholar] [CrossRef]
- Ganly, I.; Patel, S.G.; Stambuk, H.E.; Coleman, M.; Ghossein, R.; Carlson, D.; Edgar, M.; Shah, J.P. Solitary fibrous tumors of the head and neck: A clinicopathologic and radiologic review. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.T.; Song, Z.L.; Wang, Y.Z.; Dong, J.Y.; Wang, Z.C. Solitary fibrous tumor of the sinonasal cavity: CT and MR imaging findings. AJNR Am. J. Neuroradiol. 2013, 34, 1248–1251. [Google Scholar] [CrossRef] [Green Version]
- Suroyo, I.; Budianto, T. The role of diagnostic and interventional radiology in juvenile nasopharyngeal angiofibroma: A case report and literature review. Radiol. Case Rep. 2020, 15, 812–815. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Chen, Q.; Xian, J. Evaluation of multiparametric MRI differentiating sinonasal angiomatous polyp from malignant tumors. Neuroradiology 2019, 61, 891–896. [Google Scholar] [CrossRef]
- Lin, N.; Liu, X.; Zhang, F.; Pan, Y.; Qi, M.; Sha, Y. Sinonasal synovial sarcoma: Evaluation of the role of radiological and clinicopathological features in diagnosis. Clin. Radiol. 2021, 76, 78. [Google Scholar] [CrossRef]
- Yang, B.T.; Wang, Z.C.; Xian, J.F.; Hao, D.P.; Chen, Q.H. Leiomyoma of the sinonasal cavity: CT and MRI findings. Clin. Radiol. 2009, 64, 1203–1209. [Google Scholar] [CrossRef]
- Suh, C.H.; Lee, J.H.; Lee, M.K.; Cho, S.J.; Chung, S.R.; Choi, Y.J.; Baek, J.H. CT and MRI Findings of Glomangiopericytoma in the Head and Neck: Case Series Study and Systematic Review. AJNR Am. J. Neuroradiol. 2020, 41, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Lin, P.-C.; Yen, S.-L.; Huang, S.-C.; Tsai, J.-W.; Li, C.-F.; Tai, H.-C.; Lan, J.; Chuang, I.-C.; Yu, S.-C.; et al. Clinicopathological and genetic heterogeneity of the head and neck solitary fibrous tumours: A comparative histological, immunohistochemical and molecular study of 36 cases. Histopathology 2016, 68, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, Q.; Yu, X.; Han, X.; Wang, H.; Xu, Y.; Qiu, X.; Jin, F. Immunohistochemical detection of STAT6, CD34, CD99 and BCL-2 for diagnosing solitary fibrous tumors/hemangiopericytomas. Int. J. Clin. Exp. Pathol. 2015, 8, 13166–13175. [Google Scholar] [PubMed]
- Chmielecki, J.; Crago, A.M.; Rosenberg, M.; O’Connor, R.; Walker, S.R.; Ambrogio, L.; Auclair, D.; McKenna, A.; Heinrich, M.C.; Frank, D.A.; et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat. Genet. 2013, 45, 131–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.R.; Wu, Y.-M.; Kalyana-Sundaram, S.; Cao, X.; Lonigro, R.J.; Sung, Y.-S.; Chen, C.-L.; Zhang, L.; Wang, R.; Su, F.; et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat. Genet. 2013, 45, 180–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelsche, C.; Schweizer, L.; Renner, M.; Warth, A.; Jones, D.T.W.; Sahm, F.; Reuss, D.E.; Capper, D.; Knösel, T.; Schulz, B.; et al. Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology 2014, 65, 613–622. [Google Scholar] [CrossRef]
- Doyle, L.A.; Vivero, M.; Fletcher, C.D.; Mertens, F.; Hornick, J.L. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod. Pathol. 2014, 27, 390–395. [Google Scholar] [CrossRef] [Green Version]
- van Houdt, W.J.; Westerveld, C.M.A.; Vrijenhoek, J.E.P.; van Gorp, J.; van Coevorden, F.; Verhoef, C.; van Dalen, T. Prognosis of Solitary Fibrous Tumors: A Multicenter Study. Ann. Surg. Oncol. 2013, 20, 4090–4095. [Google Scholar] [CrossRef]
- Anesi, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Bianchi, M.; Russo, A.; Chiarini, L.; Palumbo, C. Structural and ultrastructural analyses of bone regeneration in rabbit cranial osteotomy: Piezosurgery versus traditional osteotomes. J. Cranio-Maxillofac. Surg. 2018, 46, 107–118. [Google Scholar] [CrossRef]
- Anesi, A.; Di Bartolomeo, M.; Pellacani, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Nocini, R.; Palumbo, C.; Chiarini, L. Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights. Appl. Sci. 2020, 10, 7165. [Google Scholar] [CrossRef]
- Sherman, J.A.; Davies, H.T. Ultracision: The harmonic scalpel and its possible uses in maxillofacial surgery. Br. J. Oral Maxillofac. Surg. 2000, 38, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Ji, Y.; Shan, F.; Guo, W.; Ding, J.; Ge, D. Solitary fibrous tumor of the pleura: An analysis of 13 cases. World J. Surg. 2008, 32, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.; Cassidy, M.R.; Kirane, A.; Kuk, D.; Zanchelli, B.; Antonescu, C.R.; Singer, S.; Brennan, M. Size and Location are the Most Important Risk Factors for Malignant Behavior in Resected Solitary Fibrous Tumors. Ann. Surg. Oncol. 2017, 24, 3865–3871. [Google Scholar] [CrossRef]
- Demicco, E.G.; Wagner, M.J.; Maki, R.G.; Gupta, V.; Iofin, I.; Lazar, A.J.; Wang, W.-L. Risk assessment in solitary fibrous tumors: Validation and refinement of a risk stratification model. Mod. Pathol. 2017, 30, 1433–1442. [Google Scholar] [CrossRef]
- Ghanim, B.; Hess, S.; Bertoglio, P.; Celik, A.; Bas, A.; Oberndorfer, F.; Melfi, F.; Mussi, A.; Klepetko, W.; Pirker, C.; et al. Intrathoracic solitary fibrous tumor—An international multicenter study on clinical outcome and novel circulating biomarkers. Sci. Rep. 2017, 7, 12557. [Google Scholar] [CrossRef] [Green Version]
- Baldi, G.G.; Stacchiotti, S.; Mauro, V.; Dei Tos, A.P.; Gronchi, A.; Pastorino, U.; Duranti, L.; Provenzano, S.; Marrari, A.; Libertini, M.; et al. Solitary fibrous tumor of all sites: Outcome of late recurrences in 14 patients. Clin. Sarcoma Res. 2013, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.K.; Lee, D.H.; Park, J.Y.; Park, S.H.; Kwon, K.Y. Multiple recurrent malignant solitary fibrous tumors: Long-term follow-up of 24 years. Ann. Thorac. Surg. 2011, 91, 1285–1288. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bartolomeo, M.; Negrello, S.; Pellacani, A.; Cesinaro, A.M.; Vallone, S.; Presutti, L.; Chiarini, L.; Anesi, A. A Case Report of a Solitary Fibrous Tumor of the Maxillary Sinus. Reports 2021, 4, 33. https://doi.org/10.3390/reports4040033
Di Bartolomeo M, Negrello S, Pellacani A, Cesinaro AM, Vallone S, Presutti L, Chiarini L, Anesi A. A Case Report of a Solitary Fibrous Tumor of the Maxillary Sinus. Reports. 2021; 4(4):33. https://doi.org/10.3390/reports4040033
Chicago/Turabian StyleDi Bartolomeo, Mattia, Sara Negrello, Arrigo Pellacani, Anna Maria Cesinaro, Stefano Vallone, Livio Presutti, Luigi Chiarini, and Alexandre Anesi. 2021. "A Case Report of a Solitary Fibrous Tumor of the Maxillary Sinus" Reports 4, no. 4: 33. https://doi.org/10.3390/reports4040033
APA StyleDi Bartolomeo, M., Negrello, S., Pellacani, A., Cesinaro, A. M., Vallone, S., Presutti, L., Chiarini, L., & Anesi, A. (2021). A Case Report of a Solitary Fibrous Tumor of the Maxillary Sinus. Reports, 4(4), 33. https://doi.org/10.3390/reports4040033







