Social Determinants Contribute to Disparities in Test Positivity, Morbidity and Mortality: Data from a Multi-Ethnic Cohort of 1094 GU Cancer Patients Undergoing Assessment for COVID-19
Abstract
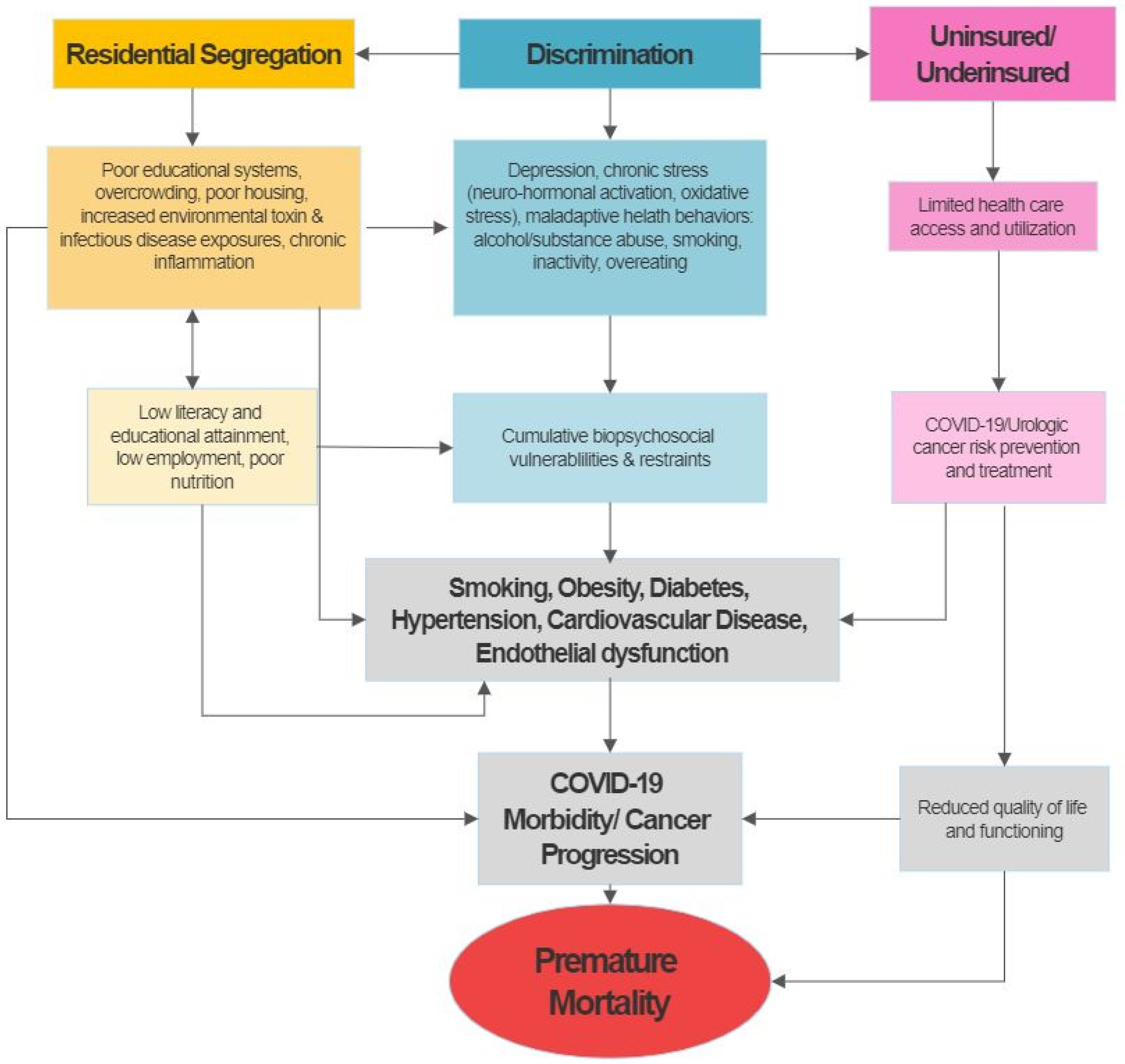
:1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Statistical Analysis
3. Results


4. Discussion
4.1. Urological Cancer Outcomes and Social Determinants
4.2. Social Disparities in COVID-19
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, X.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Kannan, S.; Ali, P.S.S.; Sheeza, A.; Hemalatha, K. COVID-19 (Novel Coronavirus 2019)—Recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2006–2011. [Google Scholar] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Song, H.; Seddighzadeh, B.; Cooperberg, M.R.; Huang, F.W. Expression of ACE2, the SARS-CoV-2 Receptor, and TMPRSS2 in Prostate Epithelial Cells. Eur. Urol. 2020, 78, 296–298. [Google Scholar] [CrossRef]
- Katopodis, P.; Anikin, V.; Randeva, H.S.; Spandidos, D.A.; Chatha, K.; Kyrou, I.; Karteris, E. Pancancer analysis of transmembrane protease serine 2 and cathepsin L that mediate cellular SARSCoV2 infection leading to COVID-19. Int. J. Oncol. 2020, 57, 533–539. [Google Scholar] [CrossRef]
- Norris, K.; Nissenson, A.R. Race, gender, and socioeconomic disparities in CKD in the United States. J. Am. Soc. Nephrol. 2008, 19, 1261–1270. [Google Scholar] [CrossRef]
- Lundon, D.J.; Mohamed, N.; Lantz, A.; Goltz, H.H.; Kelly, B.D.; Tewari, A.K. Social Determinants Predict Outcomes in Data From a Multi-Ethnic Cohort of 20,899 Patients Investigated for COVID-19. Front. Public Health 2020, 8, 571364. [Google Scholar] [CrossRef]
- Nicholas, S.B.; Kalantar-Zadeh, K.; Norris, K.C. Socioeconomic disparities in chronic kidney disease. Adv. Chronic Kidney Dis. 2015, 22, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colla Ruvolo, C.; Stolzenbach, L.F.; Nocera, L.; Deuker, M.; Wenzel, M.; Tian, Z.; La Rocca, R.; Creta, M.; Capece, M.; Saad, F.; et al. Higher Cancer Mortality in Rural Upper Urinary Tract Urothelial Carcinoma Patients. Urol. Int. 2021, 105, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Deuker, M.; Stolzenbach, L.F.; Ruvolo, C.C.; Nocera, L.; Tian, Z.; Roos, F.C.; Becker, A.; Kluth, L.A.; Tilki, D.; Shariat, S.F.; et al. Bladder cancer stage and mortality: Urban vs. rural residency. Cancer Causes Control 2021, 32, 139–145. [Google Scholar] [CrossRef]
- Stolzenbach, L.F.; Deuker, M.; Collà-Ruvolo, C.; Nocera, L.; Tian, Z.; Maurer, T.; Tilki, D.; Briganti, A.; Saad, F.; Mirone, V.; et al. Differences between rural and urban prostate cancer patients. World J. Urol. 2021, 39, 2507–2514. [Google Scholar] [CrossRef]
- Calderon, J.L.; Zadshir, A.; Norris, K. A survey of kidney disease and risk-factor information on the World Wide Web. MedGenMed 2004, 6, 3. [Google Scholar] [PubMed]
- Neumann, U.; Genze, N.; Heider, D. EFS: An ensemble feature selection tool implemented as R-package and web-application. BioData Min. 2017, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Polikar, R. Ensemble based systems in decision making. IEEE Circ. Syst. Mag. 2006, 6, 21–45. [Google Scholar] [CrossRef]
- Buac, N.P.; Khusid, J.A.; Sturgis, M.R.; Gupta, M.; Lundon, D.J.; Chow, A.K.; Becerra, A.Z. Disparities in patient and system factors explain racial/ethnic disparities in delayed time to treatment in muscle invasive bladder cancer. Urol. Oncol. 2022, 40, 343.e15–343.e20. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Mouw, T.; Koster, A.; Wright, M.; Blank, M.M.; Moore, S.C.; Hollenbeck, A.; Schatzkin, A. Education and risk of cancer in a large cohort of men and women in the United States. PLoS ONE 2008, 3, e3639. [Google Scholar] [CrossRef]
- Greenlee, R.T.; Howe, H.L. County-level poverty and distant stage cancer in the United States. Cancer Causes Control 2009, 20, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Vagero, D.; Persson, G. Cancer survival and social class in Sweden. J. Epidemiol. Commun. Health 1987, 41, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weprin, S.A.; Parker, D.C.; Jones, J.D.; Kaplan, J.R.; Giusto, L.L.; Mydlo, J.H.; Yu, S.-J.S.; Lee, D.I.; Eun, D.D.; Reese, A.C. Association of Low Socioeconomic Status With Adverse Prostate Cancer Pathology Among African American Men Who Underwent Radical Prostatectomy. Clin. Genitourin. Cancer 2019, 17, e1054–e1059. [Google Scholar] [CrossRef]
- Maurice, M.J.; Zhu, H.; Kiechle, J.E.; Kim, S.P.; Abouassaly, R. Nonclinical Factors Predict Selection of Initial Observation for Renal Cell Carcinoma. Urology 2015, 86, 892–899. [Google Scholar] [CrossRef]
- Izadmehr, S.; Lundon, D.J.; Mohamed, N.; Katims, A.; Patel, V.; Eilender, B.; Mehrazin, R.; Badani, K.K.; Sfakianos, J.P.; Tsao, C.-K.; et al. The Evolving Clinical Management of Genitourinary Cancers Amid the COVID-19 Pandemic. Front. Oncol. 2021, 11, 734963. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Jemal, A.; Cokkinides, V.; Singh, G.K.; Cardinez, C.; Ghafoor, A.; Thun, M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J. Clin. 2004, 54, 78–93. [Google Scholar] [CrossRef]
- Ko, K.; Park, Y.H.; Lee, J.W.; Ku, J.H.; Kwak, C.; Kim, H.H. Influence of nutritional deficiency on prognosis of renal cell carcinoma (RCC). BJU Int. 2013, 112, 775–780. [Google Scholar] [CrossRef]
- Michalek, I.M.; Martinsen, J.I.; Weiderpass, E.; Kjaerheim, K.; Lynge, E.; Sparen, P.; Tryggvadottir, L.; Pukkala, E. Occupation and risk of cancer of the renal pelvis in Nordic countries. BJU Int. 2019, 123, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, J.S.; Alexander, D.D.; Perez, V.; Mink, P.J. Arsenic exposure and bladder cancer: Quantitative assessment of studies in human populations to detect risks at low doses. Toxicology 2014, 317, 17–30. [Google Scholar] [CrossRef]
- Bulka, C.M.; Jones, R.M.; Turyk, M.E.; Stayner, L.T.; Argos, M. Arsenic in drinking water and prostate cancer in Illinois counties: An ecologic study. Environ. Res. 2016, 148, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Schrag, D.; Hsieh, L.J.; Rabbani, F.; Bach, P.B.; Herr, H.; Begg, C.B. Adherence to surveillance among patients with superficial bladder cancer. J. Natl. Cancer Inst. 2003, 95, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omidele, O.O.; Finkelstein, M.; Omorogbe, A.; Palese, M. Radical Prostatectomy Sociodemographic Disparities Based on Hospital and Physician Volume. Clin. Genitourin. Cancer 2019, 17, e1011–e1019. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W. COVID-19 and African Americans. JAMA 2020, 323, 1891–1892. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Liu, W.; Liu, K.; Fang, Y.-Y.; Shang, J.; Zhou, L.; Wang, K.; Leng, F.; Wei, S.; Chen, L.; et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: A retrospective study. Chin. Med. J. 2020, 133, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H. Racial and Ethnic Disparities in Health Insurance Coverage: Dynamics of Gaining and Losing Coverage over the Life-Course. Popul. Res. Policy Rev. 2017, 36, 181–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Lundon, D.J.; Kelly, B.D.; Nair, S.; Bolton, D.M.; Kyprianou, N.; Wiklund, P.; Tewari, A. Early mortality risk stratification after SARS-CoV-2 infection. Med. Intensiva 2020, 45, e40–e42. [Google Scholar] [CrossRef]
- Lundon, D.J.; Kelly, B.D.; Nair, S.; Bolton, D.M.; Patel, G.; Reich, D.; Tewari, A. A COVID-19 Test Triage Tool, Predicting Negative Results and Reducing the Testing Burden on Healthcare Systems During a Pandemic. Front. Med. 2021, 8, 563465. [Google Scholar] [CrossRef]
- Lundon, D.J.; Kelly, B.D.; Shukla, D.; Bolton, D.M.; Wiklund, P.; Tewari, A. A Decision Aide for the Risk Stratification of GU Cancer Patients at Risk of SARS-CoV-2 Infection, COVID-19 Related Hospitalization, Intubation, and Mortality. J. Clin. Med. 2020, 9, 2799. [Google Scholar] [CrossRef]
- Gross, O.; Moerer, O.; Weber, M.; Huber, T.B.; Scheithauer, S. COVID-19-associated nephritis: Early warning for disease severity and complications? Lancet 2020, 395, e87–e88. [Google Scholar] [CrossRef]
- Nakanishi, N.; Liu, K.; Kawakami, D.; Kawai, Y.; Morisawa, T.; Nishida, T.; Sumita, H.; Unoki, T.; Hifumi, T.; Iida, Y.; et al. Post-Intensive Care Syndrome and Its New Challenges in Coronavirus Disease 2019 (COVID-19) Pandemic: A Review of Recent Advances and Perspectives. J. Clin. Med. 2021, 10, 3870. [Google Scholar] [CrossRef] [PubMed]
| COVID-19 Test | Hospitalisation | Intubation | Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Negative | Positive | No | Yes | No | Yes | Alive | Deceased |
| (n = 902) | (n = 192) | (n = 64) | (n = 128) | (n = 173) | (n = 19) | (n = 153) | (n = 39) | |
| Age (years) | * | * | * | |||||
| Mean (SD) | 69.3 (10.9) | 71.9 (11.0) | 68.8 (12.9) | 73.5 (9.53) | 71.6 (11.4) | 74.5 (6.18) | 71.0 (11.2) | 75.5 (9.40) |
| Median (Min, Max) | 70.0 (26.0, 90.0) | 72.5 (32.0, 90.0) | 69.0 (32.0, 90.0) | 74.0 (39.0, 90.0) | 71.0 (32.0, 90.0) | 74.0 (67.0, 90.0) | 71.0 (32.0, 90.0) | 76.0 (55.0, 90.0) |
| Sex | ||||||||
| Female | 84 (86.6%) | 13 (13.4%) | 3 (23.1%) | 10 (76.9%) | 13 (100.0%) | 0 (0%) | 10 (76.9%) | 3 (23.1%) |
| Male | 818 (82.0%) | 179 (18.0%) | 61 (34.1%) | 118 (65.9%) | 160 (89.4%) | 19 (10.6%) | 143 (79.9%) | 36 (20.1%) |
| Race/Ethnicity | † | |||||||
| African ancestry | 221 (81.0%) | 52 (19.0%) | 13 (25.0%) | 39 (75.0%) | 51 (98.1%) | 1 (1.9%) | 42 (80.8%) | 10 (19.2%) |
| Asian | 35 (85.4%) | 6 (14.6%) | 2 (33.3%) | 4 (66.7%) | 6 (100.0%) | 0 (0.0%) | 4 (66.7%) | 2 (33.3%) |
| Hispanic/Latinx | 144 (70.6%) | 60 (29.4%) | 23 (38.3%) | 37 (61.7%) | 53 (88.3%) | 7 (11.7%) | 52 (86.7%) | 8 (13.3%) |
| White | 409 (89.1%) | 50 (10.9%) | 17 (34.0%) | 33 (66.0%) | 42 (84.0%) | 8 (16.0%) | 37 (74.0%) | 13 (26.0%) |
| Other/Unknown | 93 (79.5%) | 24 (20.5%) | 9 (37.5%) | 15 (62.5%) | 21 (87.5%) | 3 (12.5%) | 18 (75.0%) | 6 (25.0%) |
| Zip code | * | * | ||||||
| Bronx | 53 (81.5%) | 12 (41.7%) | 5 (58.3%) | 7 (5.5%) | 12 (100.0%) | 0 (0.0%) | 9 (75.0%) | 3 (25.0%) |
| Brooklyn | 137 (80.1%) | 34 (23.5%) | 8 (23.5%) | 26 (20.3%) | 26 (76.5%) | 8 (23.5%) | 29 (85.3%) | 5 (14.7%) |
| Manhattan | 405 (78.9%) | 108 (21.1%) | 34 (23.5%) | 74 (68.5%) | 101 (93.5%) | 7 (6.5%) | 90 (83.3%) | 18 (16.7%) |
| Nassau | 24 (96.0%) | 1 (4.0%) | 0 (0.0%) | 1 (100.0%) | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) |
| Queens | 180 (86.5%) | 28 (13.5%) | 13 (46.4%) | 15 (53.6%) | 26 (92.9%) | 2 (7.1%) | 16 (57.1%) | 12 (42.9%) |
| Staten Island | 23 (88.5%) | 3 (11.5%) | 1 (33.3%) | 2 (66.7%) | 3 (100.0%) | 0 (0.0%) | 2 (66.7%) | 1 (33.3%) |
| Suffolk | 21 (95.5%) | 1 (4.5%) | 1 (100%) | 0 (0%) | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) |
| Outside NYS | 19 (82.6%) | 4 (17.4%) | 2 (50.0%) | 2 (50.0%) | 2 (50.0%) | 2 (50.0%) | 4 (100.0%) | 0 (0.0%) |
| English as Preferred 1st Language | ||||||||
| 777 (83.3%) | 156 (16.7%) | 55 (85.9%) | 101 (78.9%) | 143 (91.7%) | 13 (8.3%) | 127 (81.4%) | 29 (18.6%) | |
| Current/Former Smoker | ||||||||
| 559 (85.2%) | 97 (14.8%) | 31 (34.0%) | 66 (68.0%) | 86 (88.7%) | 11 (11.3%) | 74 (76.3%) | 23 (23.7%) | |
| Asthma | 41 (74.5%) | 14 (25.5%) | 6 (42.9%) | 8 (57.1%) | 14 (100.0%) | 0 (0.0%) | 12 (85.6%) | 2 (14.3%) |
| COPD | 59 (76.6%) | 18 (23.4%) | 7 (38.9%) | 11 (61.1%) | 17 (94.4%) | 1 (5.6%) | 15 (83.3%) | 3 (16.7%) |
| Hypertension | 443 (80.3%) | 109 (19.7%) | 31 (28.4%) | 78 (71.6%) | 101 (92.7%) | 8 (7.3%) | 86 (78.9%) | 23 (21.1%) |
| Obesity | 61 (73.5%) | 22 (26.5%) * | 7 (31.8%) | 15 (68.2%) | 22 (100.0%) | 0 (0.0%) | 19 (86.4%) | 3 (13.6%) |
| Diabetes | 166 (74.4%) | 57 (25.6%) † | 17 (29.8%) | 40 (70.2%) | 52 (91.2%) | 5 (8.8%) | 43 (75.4%) | 14 (24.6%) |
| C.K.D | 157 (78.1%) | 44 (21.9%) | 16 (36.4%) | 28 (63.6%) | 42 (95.5%) | 2 (4.5%) | 36 (81.8%) | 8 (18.2%) |
| Prostate Cancer | 525 (79.7%) | 134 (20.3%) * | 39 (29.1%) | 95 (70.9%) | 120 (89.6%) | 14 (10.4%) | 101 (75.4%) | 33 (24.6%) * |
| Bladder Cancer | 246 (86.9%) | 37 (13.1%) | 16 (43.2%) | 21 (64.5%) | 34 (91.9%) | 3 (8.1%) | 32 (86.5%) | 5 (13.5%) |
| Kidney Cancer | 163 (84.0%) | 31 (16.0%) | 11 (35.5%) | 20 (64.5%) | 29 (93.5%) | 2 (6.5%) | 27 (87.1%) | 4 (12.9%) |
| Testis Cancer | 26 (89.7%) | 3 (10.3%) | 2 (66.7%) | 1 (33.3%) | 3 (100.0%) | 0 (0.0%) | 3 (100.0%) | 0 (0.0%) |
| Outcome | Positive COVID-19/SARS-C0V-2 Test | Hospitalisation | Intubation | Death |
|---|---|---|---|---|
| Parameter | Age | Age | Age | Age |
| Race/Ethnicity | Zip code | Zip code | Zip code | |
| Diabetes | Hypertension | Hypertension | Race/Ethnicity | |
| Ever Smoker | Prostate Cancer | English as Preferred 1st Language | Ever Smoker | |
| Prostate Cancer | Bladder Cancer | Obesity | Prostate Cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moorhead, R.A.; O’Brien, J.S.; Kelly, B.D.; Shukla, D.; Bolton, D.M.; Kyprianou, N.; Wiklund, P.; Lantz, A.; Mohamed, N.; Goltz, H.H.; et al. Social Determinants Contribute to Disparities in Test Positivity, Morbidity and Mortality: Data from a Multi-Ethnic Cohort of 1094 GU Cancer Patients Undergoing Assessment for COVID-19. Reports 2022, 5, 29. https://doi.org/10.3390/reports5030029
Moorhead RA, O’Brien JS, Kelly BD, Shukla D, Bolton DM, Kyprianou N, Wiklund P, Lantz A, Mohamed N, Goltz HH, et al. Social Determinants Contribute to Disparities in Test Positivity, Morbidity and Mortality: Data from a Multi-Ethnic Cohort of 1094 GU Cancer Patients Undergoing Assessment for COVID-19. Reports. 2022; 5(3):29. https://doi.org/10.3390/reports5030029
Chicago/Turabian StyleMoorhead, Rebecca A., Jonathan S. O’Brien, Brian D. Kelly, Devki Shukla, Damien M. Bolton, Natasha Kyprianou, Peter Wiklund, Anna Lantz, Nihal Mohamed, Heather H. Goltz, and et al. 2022. "Social Determinants Contribute to Disparities in Test Positivity, Morbidity and Mortality: Data from a Multi-Ethnic Cohort of 1094 GU Cancer Patients Undergoing Assessment for COVID-19" Reports 5, no. 3: 29. https://doi.org/10.3390/reports5030029
APA StyleMoorhead, R. A., O’Brien, J. S., Kelly, B. D., Shukla, D., Bolton, D. M., Kyprianou, N., Wiklund, P., Lantz, A., Mohamed, N., Goltz, H. H., Lundon, D. J., & Tewari, A. (2022). Social Determinants Contribute to Disparities in Test Positivity, Morbidity and Mortality: Data from a Multi-Ethnic Cohort of 1094 GU Cancer Patients Undergoing Assessment for COVID-19. Reports, 5(3), 29. https://doi.org/10.3390/reports5030029






