An Analysis of Volume, Length and Segmentation of Free Fibula Flap in Reconstruction of the Jaws: Investigation of Their Role on Flap Failure
Abstract
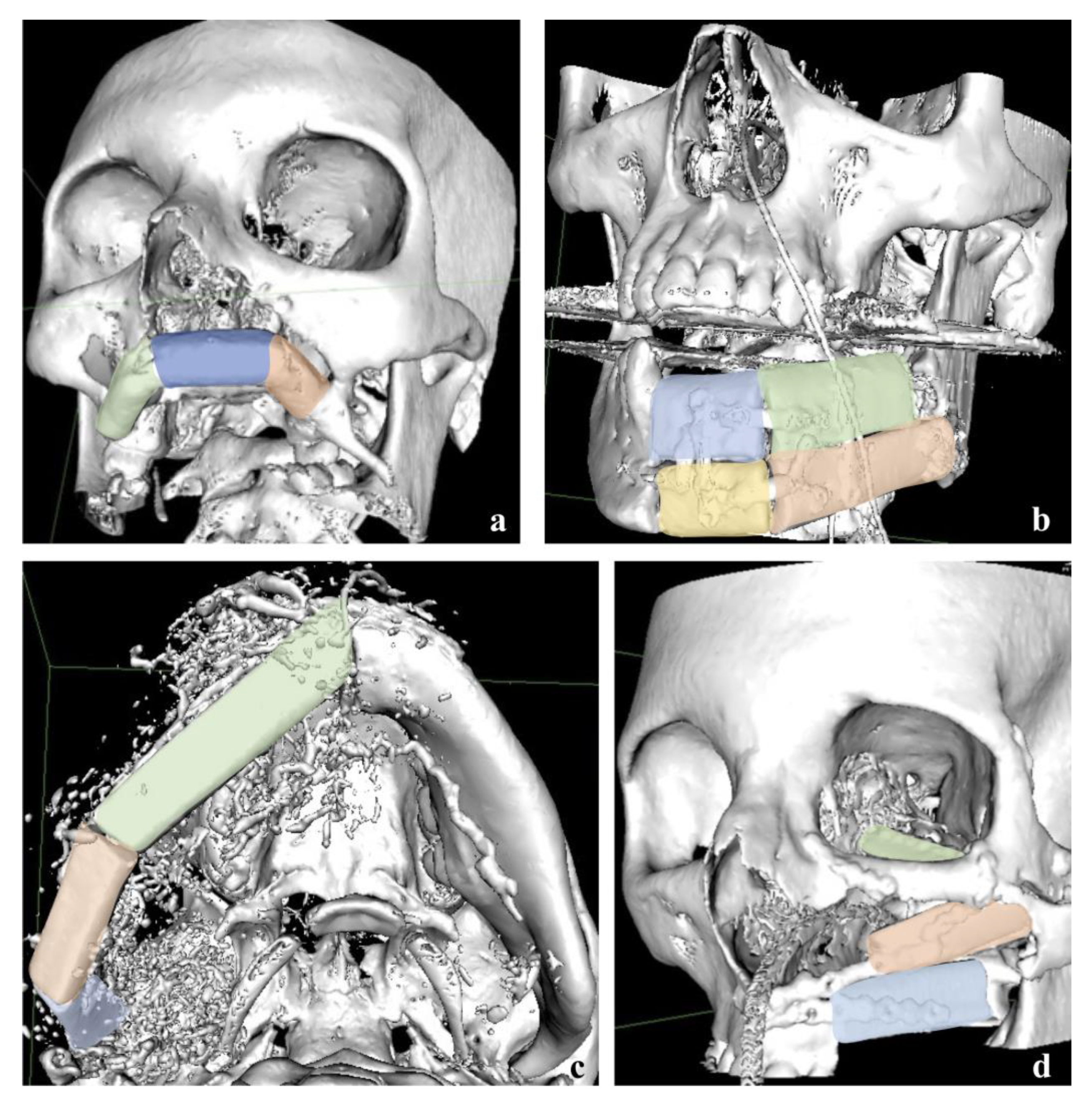
:1. Introduction
- A quite standardized harvesting technique;
- A constant and regular vascular anatomy;
- The large amount of bone, that allows the reconstruction of large deficits;
- The possibility to perform many osteotomies in order to model the flap and to reach a more morphologic/aesthetical and functional result;
- The possibility to position endosseous dental implants, that will subsequently allow a prosthetic reconstruction;
- The possibility to harvest an osteo-myo-cutaneous flap, thus allowing to reconstruct also the soft tissues;
- The reduced morbidity of the donor site [1].
- Smoking habits;
- Alcohol consume (which can act both in a direct and/or indirect manner);
- Pro-thrombotic conditions;
- Diabetes mellitus;
- Previous therapies, such as radiotherapy and/or chemotherapy;
- Malnutrition;
- Body mass index (BMI);
- Male sex;
- Intraoperatory time.
2. Materials and Methods
2.1. Study Design and Data Collected
- A computed tomography angiography, to study the anatomical variations of the peroneal artery.
- The planning of the resection.
- The possible need of a skin paddle.
- The evaluation regarding the localization of the pedicle.
2.2. Statistical Analyses
3. Results
4. Discussion
- In the first group, we had to choose an artery different from the facial one due to oncological radicality (n = 7) and hence the need for a facial artery ligation. Among them, in 5 times a partial or total flap failure occurred.
- In the second group, the choice was made after a thorough intraoperatory evaluation. The indications for surgery were ORN (n = 3) or the ablation of a wide arterio-venous malformation (n = 1). No flap failure occurred in this group.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatani, B.; Fatani, J.A.; Fatani, O.A. Approach for Mandibular Reconstruction Using Vascularized Free Fibula Flap: A Review of the Literature. Cureus 2022, 14, e30161. [Google Scholar] [CrossRef] [PubMed]
- Wilkman, T.; Husso, A.; Lassus, P. Clinical Comparison of Scapular, Fibular, and Iliac Crest Osseal Free Flaps in Maxillofacial Reconstructions. Scand. J. Surg. 2019, 108, 76–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeny, L.; Topf, M.; Wax, M.K.; Rosenthal, E.L.; Greene, B.J.; Heffelfinger, R.; Krein, H.; Luginbuhl, A.; Petrisor, D.; Troob, S.H.; et al. Shift in the timing of microvascular free tissue transfer failures in head and neck reconstruction. Laryngoscope 2020, 130, 347–353. [Google Scholar] [CrossRef]
- Fliss, E.; Yanko, R.; Bracha, G.; Teman, R.; Amir, A.; Horowitz, G.; Muhanna, N.; Fliss, D.M.; Gur, E.; Zaretski, A. The Evolution of the Free Fibula Flap for Head and Neck Reconstruction: 21 Years of Experience with 128 Flaps. J. Reconstr. Microsurg. 2021, 37, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Hernández, J.F.; Martínez-Miramón, A.; Reyes-Vivanco, A. Fibular free flap in mandible reconstruction, a long-term follow-up. Cir. Cir. 2019, 87, 267–271. [Google Scholar] [CrossRef]
- Shpitzer, T.; Neligan, P.C.; Gullane, P.J.; Freeman, J.E.; Boyd, B.J.; Rotstein, L.E.; Brown, D.H.; Irish, J.C.; Gur, E. Oromandibular reconstruction with the fibular free flap. Analysis of 50 consecutive flaps. Arch. Otolaryngol. Head. Neck Surg. 1997, 123, 939–944. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, J.T.M.; Abbink, J.H.; van Es, R.J.J.; Rosenberg, A.J.W.P.; Koole, R.; Van Cann, E.M. Early and late complications in the reconstructed mandible with free fibula flaps. J. Surg. Oncol. 2018, 117, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Parise, G.K.; Guebur, M.I.; Ramos, G.H.A.; Groth, A.K.; da Silva, A.B.D.; Sassi, L.M. Evaluation of complications and flap losses in mandibular reconstruction with microvascularized fibula flap. Oral Maxillofac. Surg. 2018, 22, 281–284. [Google Scholar] [CrossRef]
- Knitschke, M.; Sonnabend, S.; Bäcker, C.; Schmermund, D.; Böttger, S.; Howaldt, H.-P.; Attia, S. Partial and Total Flap Failure after Fibula Free Flap in Head and Neck Reconstructive Surgery: Retrospective Analysis of 180 Flaps over 19 Years. Cancers 2021, 13, 865. [Google Scholar] [CrossRef]
- Verhelst, P.-J.; Dons, F.; Van Bever, P.-J.; Schoenaers, J.; Nanhekhan, L.; Politis, C. Fibula Free Flap in Head and Neck Reconstruction: Identifying Risk Factors for Flap Failure and Analysis of Postoperative Complications in a Low Volume Setting. Craniomaxillofac. Trauma Reconstr. 2019, 12, 183–192. [Google Scholar] [CrossRef]
- López-Arcas, J.M.; Arias, J.; Del Castillo, J.L.; Burgueño, M.; Navarro, I.; Morán, M.J.; Chamorro, M.; Martorell, V. The fibula osteomyocutaneous flap for mandible reconstruction: A 15-year experience. J. Oral Maxillofac. Surg. 2010, 68, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Colletti, G.; Autelitano, L.; Rabbiosi, D.; Biglioli, F.; Chiapasco, M.; Mandalà, M.; Allevi, F. Technical refinements in mandibular reconstruction with free fibula flaps: Outcome-oriented retrospective review of 99 cases. Acta Otorhinolaryngol. Ital. 2014, 34, 342–348. [Google Scholar] [PubMed]
- Zhang, C.; Sun, J.; Zhu, H.; Xu, L.; Ji, T.; He, Y.; Yang, W.; Hu, Y.; Yang, X.; Zhang, Z. Microsurgical free flap reconstructions of the head and neck region: Shanghai experience of 34 years and 4640 flaps. Int. J. Oral Maxillofac. Surg. 2015, 44, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Shroff, S.S.; Nair, S.C.; Shah, A.; Kumar, B. Versatility of Fibula Free Flap in Reconstruction of Facial Defects: A Center Study. J. Maxillofac. Oral Surg. 2017, 16, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Mücke, T.; Ritschl, L.M.; Roth, M.; Güll, F.D.; Rau, A.; Grill, S.; Kesting, M.R.; Wolff, K.-D.; Loeffelbein, D.J. Predictors of free flap loss in the head and neck region: A four-year retrospective study with 451 microvascular transplants at a single centre. J. Craniomaxillofac. Surg. 2016, 44, 1292–1298. [Google Scholar] [CrossRef]
- Seruya, M.; Fisher, M.; Rodriguez, E.D. Computer-assisted versus conventional free fibula flap technique for craniofacial reconstruction: An outcomes comparison. Plast. Reconstr. Surg. 2013, 132, 1219–1228. [Google Scholar] [CrossRef]
- Eskander, A.; Kang, S.; Tweel, B.; Sitapara, J.; Old, M.; Ozer, E.; Agrawal, A.; Carrau, R.; Rocco, J.W.; Teknos, T.N. Predictors of Complications in Patients Receiving Head and Neck Free Flap Reconstructive Procedures. Otolaryngol. Head. Neck Surg. 2018, 158, 839–847. [Google Scholar] [CrossRef]
- Cannady, S.B.; Hatten, K.M.; Bur, A.M.; Brant, J.; Fischer, J.P.; Newman, J.G.; Chalian, A.A. Use of free tissue transfer in head and neck cancer surgery and risk of overall and serious complication(s): An American College of Surgeons-National Surgical Quality Improvement Project analysis of free tissue transfer to the head and neck. Head Neck 2017, 39, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.D.; Sercarz, J.A.; Abemayor, E.; Calcaterra, T.C.; Rawnsley, J.D.; Alam, D.; Blackwell, K.E. Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch. Otolaryngol. Head. Neck Surg. 2004, 130, 962–966. [Google Scholar] [CrossRef] [Green Version]
- Sanati-Mehrizy, P.; Massenburg, B.B.; Rozehnal, J.M.; Ingargiola, M.J.; Hernandez Rosa, J.; Taub, P.J. Risk Factors Leading to Free Flap Failure: Analysis From the National Surgical Quality Improvement Program Database. J. Craniofac. Surg. 2016, 27, 1956–1964. [Google Scholar] [CrossRef]
- Fichter, A.M.; Ritschl, L.M.; Georg, R.; Kolk, A.; Kesting, M.R.; Wolff, K.-D.; Mücke, T. Effect of Segment Length and Number of Osteotomy Sites on Cancellous Bone Perfusion in Free Fibula Flaps. J. Reconstr. Microsurg. 2019, 35, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Modabber, A.; Legros, C.; Rana, M.; Gerressen, M.; Riediger, D.; Ghassemi, A. Evaluation of computer-assisted jaw reconstruction with free vascularized fibular flap compared to conventional surgery: A clinical pilot study. Int. J. Med. Robot. 2012, 8, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, M.; Allegretti, S.; Bolelli, F.; Di Bartolomeo, M.; Pollastri, F.; Pellacani, A.; Minafra, P.; Anesi, A.; Grana, C. Deep Segmentation of the Mandibular Canal: A New 3D Annotated Dataset of CBCT Volumes. IEEE Access 2022, 10, 11500–11510. [Google Scholar] [CrossRef]
- Mercadante, C.; Cipriano, M.; Bolelli, F.; Pollastri, F.; Di Bartolomeo, M.; Anesi, A.; Grana, C. A Cone Beam Computed Tomography Annotation Tool for Automatic Detection of the Inferior Alveolar Nerve Canal. In Proceedings of the 16th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications, SCITEPRESS—Science and Technology Publications, Virtual, 8–10 February 2021; Volume 4, pp. 724–731. [Google Scholar]
- Zhang, W.B.; Wang, Y.; Liu, X.J.; Mao, C.; Guo, C.B.; Yu, G.Y.; Peng, X. Reconstruction of maxillary defects with free fibula flap assisted by computer techniques. J. Craniomaxillofac. Surg. 2015, 43, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-H.; Deng, J.; Liu, X.-J.; Wang, J.; Guo, Y.-X.; Guo, C.-B. Prospects of Robot-Assisted Mandibular Reconstruction with Fibula Flap: Comparison with a Computer-Assisted Navigation System and Freehand Technique. J. Reconstr. Microsurg. 2016, 32, 661–669. [Google Scholar] [CrossRef] [Green Version]
- De Santis, G.; Cordeiro, P.G.; Chiarini, L. (Eds.) Atlas of Mandibular and Maxillary Reconstruction with the Fibula Flap: A Step-by-Step Approach, 1st ed.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 3030106845. [Google Scholar]
- Starnoni, M.; De Santis, G.; Pinelli, M. Fibula Free Flap Elevation without Tourniquet: Are Harmonic Scalpel Shears Useful? Plast. Reconstr. surgery. Glob. open 2019, 7, e2409. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Pellacani, A.; Negrello, S.; Chiarini, L.; Anesi, A. Emerging challenges and possible strategies in maxillo-facial and oral surgery during the COVID-19 pandemic. J. Oral Sci. 2020, 62, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Negrello, S.; Pellacani, A.; di Bartolomeo, M.; Bernardelli, G.; Nocini, R.; Pinelli, M.; Chiarini, L.; Anesi, A. Primary Intraosseous Squamous Cell Carcinoma of the Anterior Mandible Arising in an Odontogenic Cyst in 34-Year-Old Male. Rep.—Med. Cases Images Videos 2020, 3, 12. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.-B.; Yu, Y.; Wang, Y.; Mao, C.; Guo, C.-B.; Yu, G.-Y.; Peng, X. Risk factors for free flap failure: A retrospective analysis of 881 free flaps for head and neck defect reconstruction. Int. J. Oral Maxillofac. Surg. 2017, 46, 941–945. [Google Scholar] [CrossRef]
- Mijiti, A.; Kuerbantayi, N.; Zhang, Z.Q.; Su, M.Y.; Zhang, X.H.; Huojia, M. Influence of preoperative radiotherapy on head and neck free-flap reconstruction: Systematic review and meta-analysis. Head Neck 2020, 42, 2165–2180. [Google Scholar] [CrossRef]
- Bashar, K.; Healy, D.; Clarke-Moloney, M.; Burke, P.; Kavanagh, E.; Walsh, S.-R. Effects of neck radiation therapy on extra-cranial carotid arteries atherosclerosis disease prevalence: Systematic review and a meta-analysis. PLoS ONE 2014, 9, e110389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianicolo, M.E.; Gianicolo, E.A.L.; Tramacere, F.; Andreassi, M.G.; Portaluri, M. Effects of external irradiation of the neck region on intima media thickness of the common carotid artery. Cardiovasc. Ultrasound 2010, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouland, C.; Albert, N.; Boutremans, E.; Rodriguez, A.; Loeb, I.; Dequanter, D.; Javadian, R. Risk factors assessment in fibular free flap mandibular reconstruction. Ann. Chir. Plast. Esthet. 2021, 66, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, P.G.; Disa, J.J.; Hidalgo, D.A.; Hu, Q.Y. Reconstruction of the mandible with osseous free flaps: A 10-year experience with 150 consecutive patients. Plast. Reconstr. Surg. 1999, 104, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Anesi, A.; Di Bartolomeo, M.; Pellacani, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Nocini, R.; Palumbo, C.; Chiarini, L. Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights. Appl. Sci. 2020, 10, 7165. [Google Scholar] [CrossRef]
- Anesi, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Bianchi, M.; Russo, A.; Chiarini, L.; Palumbo, C. Structural and ultrastructural analyses of bone regeneration in rabbit cranial osteotomy: Piezosurgery versus traditional osteotomes. J. Cranio-Maxillofac. Surg. 2018, 46, 107–118. [Google Scholar] [CrossRef]
- Kumar, S.S.; Thailavathy, V.; Srinivasan, D.; Loganathan, D.; Yamini, J. Comparison of Orthopantomogram and Lateral Cephalogram for Mandibular Measurements. J. Pharm. Bioallied Sci. 2017, 9, S92–S95. [Google Scholar] [CrossRef]
- Chia, H.-L.; Wong, C.-H.; Tan, B.-K.; Tan, K.-C.; Ong, Y.-S. An algorithm for recipient vessel selection in microsurgical head and neck reconstruction. J. Reconstr. Microsurg. 2011, 27, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.-H.; Kim, K.-J.; Jung, K.-Y.; Baek, S.-K.; Park, S.-H.; Yoon, E.-S. Recipient vessel selection for head and neck reconstruction: A 30-year experience in a single institution. Arch. Craniofacial Surg. 2020, 21, 269–275. [Google Scholar] [CrossRef]
- Brown, J.S.; Magennis, P.; Rogers, S.N.; Cawood, J.I.; Howell, R.; Vaughan, E.D. Trends in head and neck microvascular reconstructive surgery in Liverpool (1992–2001). Br. J. Oral Maxillofac. Surg. 2006, 44, 364–370. [Google Scholar] [CrossRef]
- Singh, B.; Cordeiro, P.G.; Santamaria, E.; Shaha, A.R.; Pfister, D.G.; Shah, J.P. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast. Reconstr. Surg. 1999, 103, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Urken, M.L.; Weinberg, H.; Buchbinder, D.; Moscoso, J.F.; Lawson, W.; Catalano, P.J.; Biller, H.F. Microvascular free flaps in head and neck reconstruction. Report of 200 cases and review of complications. Arch. Otolaryngol. Head. Neck Surg. 1994, 120, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Langdell, H.C.; Shammas, R.L.; Atia, A.; Chang, E.I.; Matros, E.; Phillips, B.T. Vein Grafts in Free Flap Reconstruction: Review of Indications and Institutional Pearls. Plast. Reconstr. Surg. 2022, 149, 742–749. [Google Scholar] [CrossRef] [PubMed]



| Variable | n = 71 | % | |
|---|---|---|---|
| Sex | Males | 37 | 52.1 |
| Females | 34 | 47.9 | |
| Smoke | Active smokers | 15 | 21.1 |
| Former smokers | 27 | 38 | |
| Non-smokers | 29 | 40.9 | |
| Alcohol consume | Yes | 21 | 29.5 |
| No | 50 | 70.5 | |
| Previous treatments | Previous surgery | 33 | 46.5 |
| Previous chemotherapy | 12 | 17.0 | |
| Previous radiotherapy | 16 | 22.5 | |
| ASA score | ASA I | 9 | 12.7 |
| ASA II | 37 | 52.1 | |
| ASA III | 24 | 33.8 | |
| ASA IV | 1 | 1.4 | |
| Diabetes mellitus | Yes | 4 | 5.7 |
| No | 67 | 94.3 | |
| Autoimmune diseases | Yes | 5 | 7 |
| No | 66 | 93 | |
| Indication for surgery | Malignant oncologic disease | 32 | 45.1 |
| Osteoradionecrosis (ORN) | 6 | 8.5 | |
| Benign oncologic disease | 17 | 23.9 | |
| Others (MRONJ, atrophic jaws, vascular malformations) | 16 | 22.5 | |
| Site of reconstruction | Mandible | 47 | 66.2 |
| Maxilla | 22 | 31.0 | |
| Combined maxillo-mandibular reconstruction | 2 | 2.8 | |
| Simultaneous neck dissection | Yes | 18 | 25.4 |
| No | 53 | 74.6 | |
| Simultaneous tracheotomy | Yes | 47 | 66.2 |
| No | 24 | 33.8 | |
| Recipient vein | Single anastomosis | 26 | 36.6 |
| Double anastomosis | 22 | 31.0 | |
| Recipient artery | Facial artery | 49 | 69.0 |
| Other arteries (external carotid, superior thyroid artery, lingual artery, submandibular artery) | 11 | 15.5 |
| Parameter | Measured Variable | Odds Ratio | CI (LL) | CI (UL) | p-Value |
|---|---|---|---|---|---|
| Age | Continuous variable | 1.010 | 0.975 | 1.047 | 0.575 |
| Sex | Male:Female | 2.148 | 0.650 | 7.094 | 0.210 |
| Smoke | Non smokers vs. Active smoker | 0.717 | 0.167 | 3.073 | 0.655 |
| Non-smoker vs. Ex-smoker | 1.435 | 0.356 | 5.781 | 0.612 | |
| Active smoker vs. Ex-smokers | 2.000 | 0.419 | 9.551 | 0.385 | |
| BMI | Continuous variable | 1.002 | 0.873 | 1.150 | 0.976 |
| Hypertension | No vs. Yes | 0.667 | 0.194 | 2.285 | 0.519 |
| Diabetes mellitus | No vs. Yes | >999 | <0.001 | >999 | 0.979 |
| Autoimmune diseases | No vs. Yes | 0.368 | 0.056 | 2.434 | 0.210 |
| ASA score | I vs. II | <0.001 | <0.001 | >999 | 0.895 |
| I vs. III | <0.001 | <0.001 | >999 | 0.887 | |
| I vs. IV | 1.000 | <0.001 | >999 | 1.000 | |
| II vs. III | 0.467 | 0.143 | 1.522 | 0.206 | |
| II vs. IV | >999 | <0.001 | >999 | 0.965 | |
| III vs. IV | >999 | <0.001 | >999 | 0.962 | |
| Preoperative radiotherapy | No vs. Yes | 0.714 | 0.190 | 2.687 | 0.619 |
| Previous surgery | No vs. Yes | 0.706 | 0.225 | 2.213 | 0.550 |
| Site | Mandible vs. Maxilla | 0.089 | 0.024 | 0.333 | <0.001 * |
| Neck dissection | No vs. Yes | 0.650 | 0.188 | 2.52 | 0.497 |
| Tracheotomy | No vs. Yes | 0.545 | 0.152 | 1.955 | 0.352 |
| Operation time | Continuous variable | 1.002 | 0.998 | 1.006 | 0.425 |
| Recipient artery | Facial artery vs. Other arteries | 0.234 | 0.057 | 0.957 | 0.043 * |
| Venous anastomosis | Single vs. Double | 2.815 | 0.644 | 12.306 | 0.169 |
| Segment length | Continuous variable | 0.680 | 0.329 | 1.405 | 0.297 |
| Segment volume | Continuous variable | 1.001 | 0.772 | 1.298 | 0.994 |
| Number of used segments | 1 segment vs. 2 segments | 0.964 | 0.166 | 5.596 | 0.968 |
| 1 segment vs. 3 segments | 0.750 | 0.118 | 4.773 | 0.761 | |
| 1 segment vs. 4 segments | 1.500 | 0.109 | 20.675 | 0.762 | |
| 2 segments vs. 3 segments | 0.778 | 0.210 | 2.882 | 0.707 | |
| 2 segments vs. 4 segments | 1.556 | 0.160 | 15.123 | 0.703 | |
| 3 segments vs. 4 segments | 2.000 | 0.191 | 20.898 | 0.563 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bartolomeo, M.; Lusetti, I.L.; Pinelli, M.; Negrello, S.; Pellacani, A.; Angelini, S.; Chiarini, L.; Nocini, R.; De Santis, G.; Anesi, A. An Analysis of Volume, Length and Segmentation of Free Fibula Flap in Reconstruction of the Jaws: Investigation of Their Role on Flap Failure. Reports 2023, 6, 4. https://doi.org/10.3390/reports6010004
Di Bartolomeo M, Lusetti IL, Pinelli M, Negrello S, Pellacani A, Angelini S, Chiarini L, Nocini R, De Santis G, Anesi A. An Analysis of Volume, Length and Segmentation of Free Fibula Flap in Reconstruction of the Jaws: Investigation of Their Role on Flap Failure. Reports. 2023; 6(1):4. https://doi.org/10.3390/reports6010004
Chicago/Turabian StyleDi Bartolomeo, Mattia, Irene Laura Lusetti, Massimo Pinelli, Sara Negrello, Arrigo Pellacani, Stefano Angelini, Luigi Chiarini, Riccardo Nocini, Giorgio De Santis, and Alexandre Anesi. 2023. "An Analysis of Volume, Length and Segmentation of Free Fibula Flap in Reconstruction of the Jaws: Investigation of Their Role on Flap Failure" Reports 6, no. 1: 4. https://doi.org/10.3390/reports6010004
APA StyleDi Bartolomeo, M., Lusetti, I. L., Pinelli, M., Negrello, S., Pellacani, A., Angelini, S., Chiarini, L., Nocini, R., De Santis, G., & Anesi, A. (2023). An Analysis of Volume, Length and Segmentation of Free Fibula Flap in Reconstruction of the Jaws: Investigation of Their Role on Flap Failure. Reports, 6(1), 4. https://doi.org/10.3390/reports6010004









