Significant Spontaneous Pneumomediastinum and Extensive Subcutaneous Emphysema in a COVID-19 Patient
Abstract
:1. Background
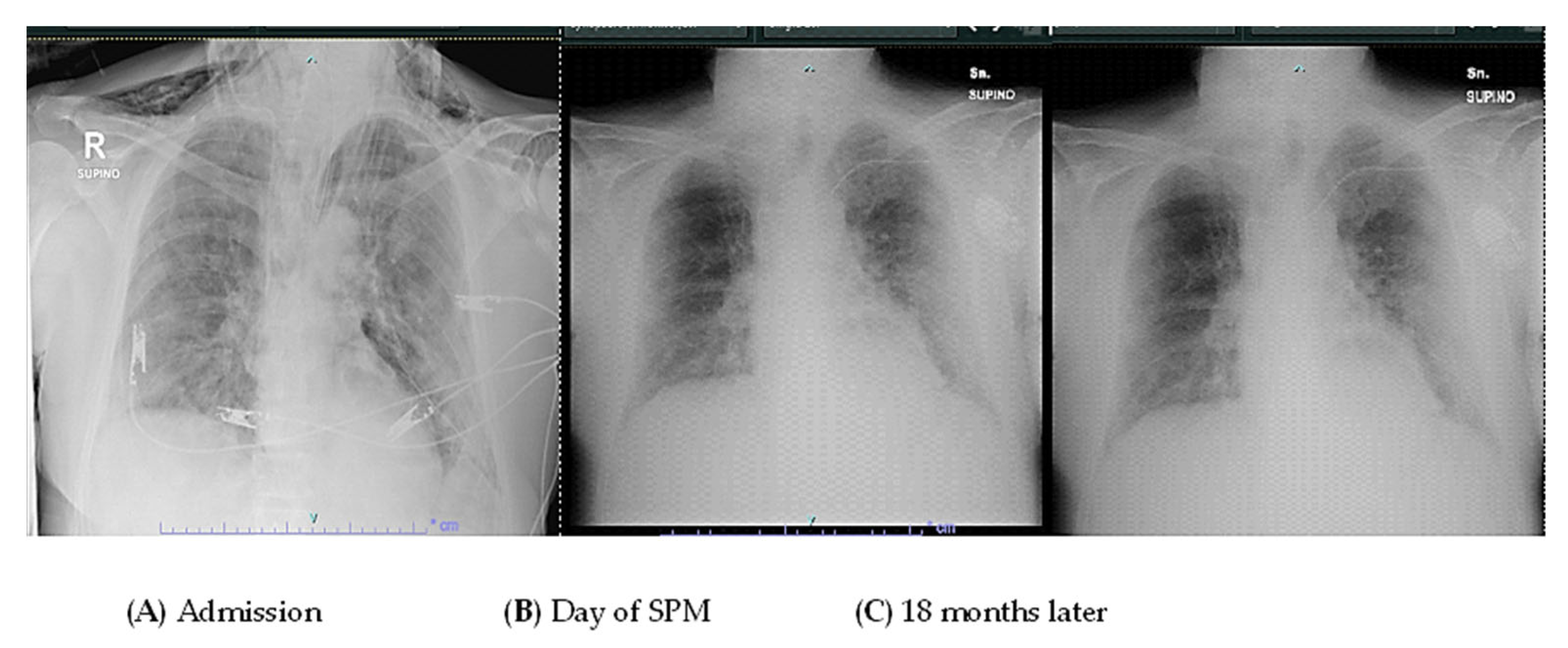
2. Case Presentation
3. Discussion
4. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elhakim, T.S.; Abdul, H.S.; Pelaez Romero, C.; Rodriguez-Fuentes, Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: A rare case and literature review. BMJ Case Rep. 2020, 13, e239489. [Google Scholar] [CrossRef] [PubMed]
- Ganessane, E.; Devendiran, A.; Ramesh, S.; Uthayakumar, A.; Chandrasekar, V.; Sadasivam, A.S.; Nathan, B.; Ayyan, M. Pneumomediastinum in COVID-19 disease: Clinical review with emphasis on emergency management. J. Am. Coll. Emerg. Physicians Open 2023, 4, e12935. [Google Scholar] [CrossRef] [PubMed]
- Muley, M.; Finamore, P.; Pedone, C.; Margiotta, D.P.E.; Gilardi, E.; Sambuco, F.; De Vincentis, A.; Vespasiani-Gentilucci, U.; Travaglino, F.; Antonelli-Incalzi, R. Incidence and Outcome of Pneumomediastinum in Non-ICU Hospitalized COVID-19 Patients. Crit. Care Med. 2023, 51, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, S.; Finkelstein, M.; Pagano, A.; Manna, S.; Toussie, D.; Chung, M.; Bernheim, A.; Concepcion, J.; Gupta, S.; Eber, C.; et al. Barotrauma in COVID 19: Incidence, pathophysiology, and effect on prognosis. Clin. Imaging 2022, 90, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Manenti, A.; Roncati, L.; Melegari, G. Deepening Pathology of SARS-CoV-2 Pneumonia Explains Lung Ventilation Complications. Ann. Thorac. Surg. 2022, 113, 1389. [Google Scholar] [CrossRef] [PubMed]
- Belletti, A.; Pallanch, O.; Bonizzoni, M.A.; Guidi, L.; De Cobelli, F.; Landoni, G.; Zangrillo, A.; De Bonis, M.; Palumbo, D. Clinical use of Macklin-like radiological sign (Macklin effect): A systematic review. Respir. Med. 2023, 210, 107178. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, G.; Belmonte, G.; Scarano, E.; Rotondo, P.; Palumbo, D.; Belletti, A.; Corradi, F.; Bertini, P.; Landoni, G.; Guarracino, F. Macklin effect on baseline chest CT scan accurately predicts barotrauma in COVID-19 patients. Respir. Med. 2022, 197, 106853. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, C.; Xie, Y.; Xu, M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect. Dis. 2020, 20, 510. [Google Scholar] [CrossRef]
- Reyes, S.; Roche, B.; Kazzaz, F.; Ocazionez, D.; Lal, A.P.; Estrada, Y.M.R.M.; Cherian, S.V. Pneumothorax and pneumomediastinum in COVID-19: A case series. Am. J. Med. Sci. 2022, 363, 548–551. [Google Scholar] [CrossRef]
- Woo, W.; Kipkorir, V.; Marza, A.M.; Hamouri, S.; Albawaih, O.; Dhali, A.; Kim, W.; Udwadia, Z.F.; Nashwan, A.J.; Shaikh, N.; et al. Prognosis of Spontaneous Pneumothorax/Pneumomediastinum in Coronavirus Disease 2019: The CoBiF Score. J. Clin. Med. 2022, 11, 7132. [Google Scholar] [CrossRef]
- Chowdhary, A.; Nirwan, L.; Abi-Ghanem, A.S.; Arif, U.; Lahori, S.; Kassab, M.B.; Karout, S.; Itani, R.M.; Abdalla, R.; Naffaa, L.; et al. Spontaneous Pneumomediastinum in Patients Diagnosed with COVID-19: A Case Series with Review of Literature. Acad. Radiol. 2021, 28, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Maron, S.Z.; Cedillo, M.A.; Voutsinas, N.; Toussie, D.; Finkelstein, M.; Steinberger, S.; Chung, M.; Bernheim, A.; Eber, C.; et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin. Imaging 2020, 67, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, M.S.; Thiara, S.; Kanji, H.D.; Ronco, J.J. Spontaneous Pneumomediastinum in COVID-19: The Macklin Effect? Am. J. Respir. Crit. Care Med. 2021, 204, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Chun, W.; Lee, H.J.; Min, J.H.; Kim, S.M.; Seo, J.Y.; Ahn, K.S.; Oh, S.R. The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells 2021, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2017, 195, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K.E.; Swenson, E.R. Pathophysiology of Acute Respiratory Distress Syndrome and COVID-19 Lung Injury. Crit. Care Clin. 2021, 37, 749–776. [Google Scholar] [CrossRef]
- Matthay, M.A.; Arabi, Y.M.; Siegel, E.R.; Ware, L.B.; Bos, L.D.J.; Sinha, P.; Beitler, J.R.; Wick, K.D.; Curley, M.A.Q.; Constantin, J.M.; et al. Phenotypes and personalized medicine in the acute respiratory distress syndrome. Intensive Care Med. 2020, 46, 2136–2152. [Google Scholar] [CrossRef]
- Caceres, M.; Ali, S.Z.; Braud, R.; Weiman, D.; Garrett, H.E., Jr. Spontaneous pneumomediastinum: A comparative study and review of the literature. Ann. Thorac. Surg. 2008, 86, 962–966. [Google Scholar] [CrossRef]
- Bolaños-Morales, F.V.; Santibáñez-Salgado, J.A.; Guadarrama-Pérez, C.; Herrera-Zamora, J.J.; Armas-Zárate, F.J.; Santillán-Doherty, P.J. Spontaneous pneumomediastinum in COVID-19 patients. Case series. Gac. Méd. Méx. 2021, 157, 110–114. [Google Scholar] [CrossRef]
- Lal, A.; Mishra, A.K.; Akhtar, J.; Nabzdyk, C. Pneumothorax and pneumomediastinum in COVID-19 acute respiratory distress syndrome. Monaldi Arch. Chest Dis. 2021, 91, 1608. [Google Scholar] [CrossRef]
- Belletti, A.; Todaro, G.; Valsecchi, G.; Losiggio, R.; Palumbo, D.; Landoni, G.; Zangrillo, A. Barotrauma in Coronavirus Disease 2019 Patients Undergoing Invasive Mechanical Ventilation: A Systematic Literature Review. Crit. Care Med. 2022, 50, 491–500. [Google Scholar] [CrossRef]
- Hossain, S.; Pastores, S.M. Bursting at the Seams: Barotrauma in Coronavirus Disease 2019 Acute Respiratory Distress Syndrome Patients. Crit. Care Med. 2022, 50, 531–534. [Google Scholar] [CrossRef]
- Marza, A.M.; Cindrea, A.C.; Petrica, A.; Stanciugelu, A.V.; Barsac, C.; Mocanu, A.; Critu, R.; Botea, M.O.; Trebuian, C.I.; Lungeanu, D. Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with COVID-19: Three-Year Debriefing across Five Pandemic Waves. J. Pers. Med. 2023, 13, 1497. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspari, A.; Carrieri, F.; Villani, M.; Bertellini, E. Significant Spontaneous Pneumomediastinum and Extensive Subcutaneous Emphysema in a COVID-19 Patient. Reports 2024, 7, 15. https://doi.org/10.3390/reports7010015
Gaspari A, Carrieri F, Villani M, Bertellini E. Significant Spontaneous Pneumomediastinum and Extensive Subcutaneous Emphysema in a COVID-19 Patient. Reports. 2024; 7(1):15. https://doi.org/10.3390/reports7010015
Chicago/Turabian StyleGaspari, Arianna, Francesca Carrieri, Matteo Villani, and Elisabetta Bertellini. 2024. "Significant Spontaneous Pneumomediastinum and Extensive Subcutaneous Emphysema in a COVID-19 Patient" Reports 7, no. 1: 15. https://doi.org/10.3390/reports7010015
APA StyleGaspari, A., Carrieri, F., Villani, M., & Bertellini, E. (2024). Significant Spontaneous Pneumomediastinum and Extensive Subcutaneous Emphysema in a COVID-19 Patient. Reports, 7(1), 15. https://doi.org/10.3390/reports7010015





