Association of SARS-CoV-2 Seropositivity with Persistent Immune Activation in HIV/Tuberculosis Co-Infected Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Settings and Participants
2.2. Sample Collection and Data Collection
2.3. Assessment of Matricellular Proteins, Other Inflammatory Markers, Cytokines, and Anti-SARS-CoV-2 Antibodies
2.4. Calculation of Inflammatory Score (INS)
2.5. Statistical Analysis
3. Results
3.1. Characterization of the Patients
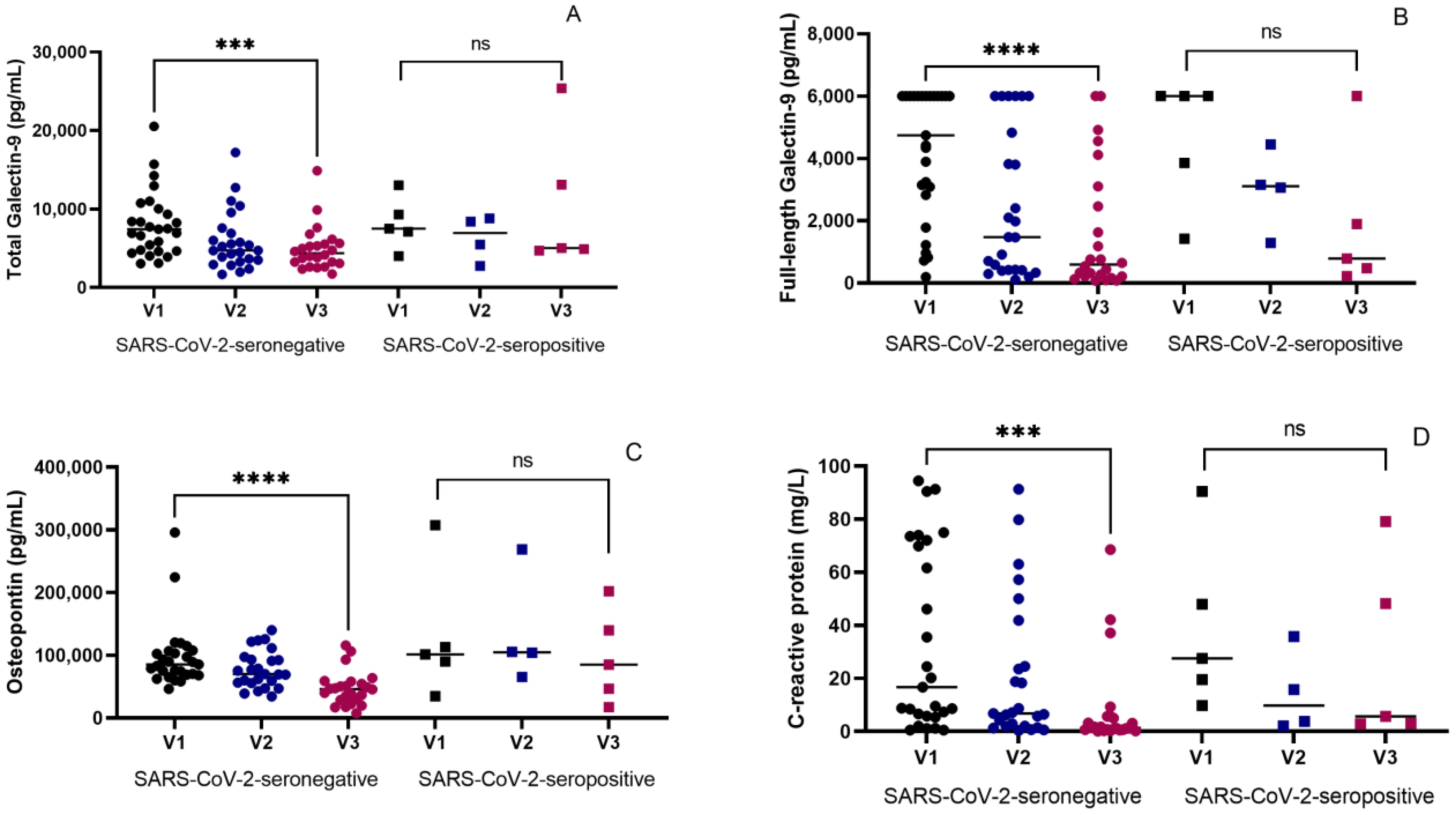
3.2. Therapeutic Effects on Inflammatory Markers in Seronegative and Seropositive Groups
3.3. Inverse Association of SARS-CoV-2 Seropositivity with Changes in the Levels of the Inflammatory Markers at the End of ATT
3.4. Association of SARS-CoV-2 Seropositivity and INS with Changes in the Levels of Various Cytokines at the End of ATT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuerculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Mee, P.; Wagner, R.G.; Gómez-Olivé, F.X.; Kabudula, C.; Kahn, K.; Madhavan, S.; Collinson, M.; Byass, P.; Tollman, S.M. Changing use of traditional healthcare amongst those dying of HIV related disease and TB in rural South Africa from 2003–2011: A retrospective cohort study. BMC Complement. Altern. Med. 2014, 14, 504. [Google Scholar] [CrossRef]
- Suthar, A.B.; Lawn, S.D.; del Amo, J.; Getahun, H.; Dye, C.; Sculier, D.; Sterling, T.R.; Chaisson, R.E.; Williams, B.G.; Harries, A.D.; et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001270. [Google Scholar] [CrossRef]
- Osei, E.; Amu, H.; Kye-Duodu, G.; Kwabla, M.P.; Danso, E.; Binka, F.N.; Kim, S.Y. Impact of COVID-19 pandemic on Tuberculosis and HIV services in Ghana: An interrupted time series analysis. PLoS ONE 2023, 18, e0291808. [Google Scholar] [CrossRef]
- Pai, M.; Kasaeva, T.; Swaminathan, S. COVID-19’s Devastating Effect on Tuberculosis Care—A Path to Recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef]
- Central TB Division. India TB Report 2022; Ministry of Health and Family Welfare: New Delhi, India, 2022. [Google Scholar]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, P.; Yan, B.; Zheng, F.; Yang, Y.; Xi, X.; Xia, L.; Shen, Y. Impact of Tuberculosis on Disease Severity and Viral Shedding Duration in COVID-19 Patients. Viruses 2024, 16, 260. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, S.L.; Wahnich, A.; Lazar, R. COVID-19 Outcomes Among People With HIV and COVID-19 in New York City. J. Infect. Dis. 2023, 228, 1571–1582. [Google Scholar] [CrossRef]
- Prommongkol, B.; Putcharoen, O.; Patamatamkul, S. Prevalence and incidence rates of tuberculosis in people with HIV during the coronavirus 2019 pandemic: A single center retrospective analysis. HIV Res. Clin. Pract. 2024, 25, 2348935. [Google Scholar]
- Singh, S.; Gupta, S.; Jha, A.; Dhamnetiya, D.; Jha, R.P. An Insight Into Tuberculosis Patients in the Chest Clinic of North India: Epidemiological Profile and Treatment Outcomes in the Wake of COVID-19. Cureus 2023, 15, e47161. [Google Scholar] [CrossRef] [PubMed]
- Degli Antoni, M.; Maifredi, G.; Storti, S.; Tiecco, G.; Di Gregorio, M.; Rossi, B.; Gasparotti, C.; Focà, E.; Castelli, F.; Quiros-Roldan, E. Long-term symptoms after SARS-CoV-2 infection in a cohort of people living with HIV. Infection, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Merani, S.; Chen, W.; Elahi, S. The bitter side of sweet: The role of Galectin-9 in immunopathogenesis of viral infections. Rev. Med. Virol. 2015, 25, 175–186. [Google Scholar] [CrossRef]
- Moar, P.; Linn, K.; Premeaux, T.A.; Bowler, S.; Sardarni, U.K.; Gopalan, B.P.; Shwe, E.E.; San, T.; Han, H.; Clements, D.; et al. Plasma Galectin-9 relates to cognitive performance and inflammation among adolescents with vertically acquired HIV. AIDS 2024, 38, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.T.; Niki, T.; Furushima, D.; Bai, G.; Chagan-Yasutan, H.; Telan, E.F.; Tactacan-Abrenica, R.J.; Maeda, Y.; Solante, R.; Hattori, T. Plasma Levels of a Cleaved Form of Galectin-9 Are the Most Sensitive Biomarkers of Acquired Immune Deficiency Syndrome and Tuberculosis Coinfection. Biomolecules 2020, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Bichare, S.; Pujari, V.; Virkar, R.; Thakar, M.; Ghate, M.; Patil, S.; Vyakarnam, A.; Gangakhedkar, R.; Bai, G.; et al. Elevated Levels of Galectin-9 but Not Osteopontin in HIV and Tuberculosis Infections Indicate Their Roles in Detecting MTB Infection in HIV Infected Individuals. Front. Microbiol. 2020, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Furushima, D.; Niki, T.; Matsuba, T.; Maeda, Y.; Takahashi, A.; Hattori, T.; Ashino, Y. High Levels of the Cleaved Form of Galectin-9 and Osteopontin in the Plasma Are Associated with Inflammatory Markers That Reflect the Severity of COVID-19 Pneumonia. Int. J. Mol. Sci. 2021, 22, 4978. [Google Scholar] [CrossRef]
- Iwasaki-Hozumi, H.; Maeda, Y.; Niki, T.; Chagan-Yasutan, H.; Bai, G.; Matsuba, T.; Furushima, D.; Ashino, Y.; Hattori, T. Plasma N-Cleaved Galectin-9 Is a Surrogate Marker for Determining the Severity of COVID-19 and Monitoring the Therapeutic Effects of Tocilizumab. Int. J. Mol. Sci. 2023, 24, 3591. [Google Scholar] [CrossRef]
- Hasibuan, F.M.; Shiratori, B.; Senoputra, M.A.; Chagan-Yasutan, H.; Koesoemadinata, R.C.; Apriani, L.; Takahashi, Y.; Niki, T.; Alisjahbana, B.; Hattori, T. Evaluation of matricellular proteins in systemic and local immune response to Mycobacterium tuberculosis infection. Microbiol. Immunol. 2015, 59, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The role of osteopontin in inflammatory processes. J. Cell Commun. Signal. 2009, 3, 311–322. [Google Scholar] [CrossRef]
- Robinson, B.S.; Arthur, C.M.; Evavold, B.; Roback, E.; Kamili, N.A.; Stowell, C.S.; Vallecillo-Zúniga, M.L.; Van Ry, P.M.; Dias-Baruffi, M.; Cummings, R.D.; et al. The Sweet-Side of Leukocytes: Galectins as Master Regulators of Neutrophil Function. Front. Immunol. 2019, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Krautter, F.; Hussain, M.T.; Zhi, Z.; Lezama, D.R.; Manning, J.E.; Brown, E.; Marigliano, N.; Raucci, F.; Recio, C.; Chimen, M.; et al. Galectin-9: A novel promoter of atherosclerosis progression. Atherosclerosis 2022, 363, 57–68. [Google Scholar] [CrossRef]
- Vianello, E.; Kalousová, M.; Dozio, E.; Tacchini, L.; Zima, T.; Romanelli, M.M.C. Osteopontin: The Molecular Bridge between Fat and Cardiac-Renal Disorders. Int. J. Mol. Sci. 2020, 21, 5568. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; D’onghia, D.; Apostolo, D.; Matino, E.; Costanzo, M.; Casciaro, G.F.; Croce, A.; Rizzi, E.; Zecca, E.; Pedrinelli, A.R.; et al. Baseline Plasma Osteopontin Protein Elevation Predicts Adverse Outcomes in Hospitalized COVID-19 Patients. Viruses 2023, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.G.; Eleftheriou, K.; Vlahakos, V.; Magkouta, S.F.; Riba, T.; Dede, K.; Siampani, R.; Kompogiorgas, S.; Polydora, E.; Papalampidou, A.; et al. High Plasma Osteopontin Levels Are Associated with Serious Post-Acute-COVID-19-Related Dyspnea. J. Clin. Med. 2024, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Ghate, M.; Iwasaki-Hozumi, H.; Patil, S.; Shidhaye, P.; Bai, G.; Matsuba, T.; Pharande, P.; Mahajan, B.; Randive, A.; et al. Dynamics of Matricellular Protein Levels in Blood Predict Recovery in Patients with Human Immunodeficiency Virus-Tuberculosis Coinfection. Viruses 2024, 16, 664. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, N.; Dabó, H.; Gomes, I.; Marques, A. C-reactive protein in pulmonary tuberculosis-correlation with extent and severity of the disease. Eur. Respir. J. 2012, 40 (Suppl. 56), 2597. [Google Scholar]
- Gebrecherkos, T.; Challa, F.; Tasew, G.; Gessesse, Z.; Kiros, Y.; Gebreegziabxier, A.; Abdulkader, M.; Desta, A.A.; Atsbaha, A.H.; Tollera, G.; et al. Prognostic Value of C-Reactive Protein in SARS-CoV-2 Infection: A Simplified Biomarker of COVID-19 Severity in Northern Ethiopia. Infect. Drug Resist. 2023, 16, 3019–3028. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J.; et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: A cross-sectional cohort study in China. Lancet Infect. Dis. 2024, 24, 845–855. [Google Scholar] [CrossRef]
- Abstracts of the papers presented in the international conference of Indian Virological Society, VIROCON 2023 on “Advancements in global virus research towards one health” held during 01–03 December, 2023 at ICAR-National Research Centre for Banana, Tiruchirappalli, Tamil Nadu, India. VirusDisease 2024, 35, 69–229.
- Shiratori, B.; Zhao, J.; Okumura, M.; Chagan-Yasutan, H.; Yanai, H.; Mizuno, K.; Yoshiyama, T.; Idei, T.; Ashino, Y.; Nakajima, C.; et al. Immunological Roles of Elevated Plasma Levels of Matricellular Proteins in Japanese Patients with Pulmonary Tuberculosis. Int. J. Mol. Sci. 2016, 18, 19. [Google Scholar] [CrossRef]
- Bai, G.; Motoda, H.; Ozuru, R.; Chagan-Yasutan, H.; Hattori, T.; Matsuba, T. Synthesis of a Cleaved Form of Osteopontin by THP-1 Cells and Its Alteration by Phorbol 12-Myristate 13-Acetate and BCG Infection. Int. J. Mol. Sci. 2018, 19, 418. [Google Scholar] [CrossRef]
- Hattori, T.; Iwasaki-Hozumi, H.; Bai, G.; Chagan-Yasutan, H.; Shete, A.; Telan, E.F.; Takahashi, A.; Ashino, Y.; Matsuba, T. Both Full-Length and Protease-Cleaved Products of Osteopontin Are Elevated in Infectious Diseases. Biomedicines 2021, 9, 1006. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Bhatnagar, T.; Thangaraj, J.W.V.; Saravanakumar, V.; Kumar, M.S.; Selvaraju, S.; Rade, K.; Girish Kumar, C.P.; Sabarinathan, R.; Asthana, S.; et al. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June-July 2021: A population-based cross-sectional study. PLoS Med. 2021, 18, e1003877. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; André, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef]
- Alemu, A.; Bitew, Z.W.; Seid, G.; Diriba, G.; Gashu, E.; Berhe, N.; Mariam, S.H.; Gumi, B. Tuberculosis in individuals who recovered from COVID-19: A systematic review of case reports. PLoS ONE 2022, 17, e0277807. [Google Scholar] [CrossRef] [PubMed]
- Besevic, J.; Lacey, B.; Callen, H.; Omiyale, W.; Conroy, M.; Feng, Q.; Crook, D.W.; Doherty, N.; Ebner, D.; Eyre, D.W.; et al. Persistence of SARS-CoV-2 antibodies over 18 months following infection: UK Biobank COVID-19 Serology Study. J. Epidemiol. Community Health 2023, 78, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Ghate, M.; Shidhaye, P.; Gurav, S.; Gadhe, K.; Kale, V.; Jain, P.; Thakar, M. Seroprevalence of Anti-SARS-CoV-2 IgG Antibodies among HIV Infected Individuals Attending ART Centre at Pune: A Cross-Sectional Study. J. Int. Assoc. Provid. AIDS Care 2022, 21, 23259582221077943. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.A.; Lynch, K.L.; Yun, C.; Glidden, D.V.; Peluso, M.J.; Henrich, T.J.; Gandhi, M.; Brown, L.B. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: A matched case-control observational study. Lancet HIV 2021, 8, e334–e341. [Google Scholar] [CrossRef]
- Kwedi Nolna, S.; Niba, M.; Djadda, C.; Netongo, P.M. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in HIV-positive and HIV-negative patients in clinical settings in Douala, Cameroon. Front. Epidemiol. 2023, 3, 1212220. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Raizada, A.; Dewan, P.; Nirmal, K.; Saini, V.; Khan, A.M.; Mogha, N.S.; Jain, S.; Gomber, S.; Singh, N.P. Antibody Response to SARS-CoV-2 in HIV Patients Co-Infected with COVID-19. Int. J. Virol. AIDS 2021, 8, 079. [Google Scholar]
- Samandari, T.; Ongalo, J.B.; McCarthy, K.D.; Biegon, R.K.; Madiega, P.A.; Mithika, A.; Orinda, J.; Mboya, G.M.; Mwaura, P.; Anzala, O.; et al. Prevalence and functional profile of SARS-CoV-2 T cells in asymptomatic Kenyan adults. J. Clin. Investig. 2023, 133, e170011. [Google Scholar] [CrossRef]
- Nkosi, T.; Chasara, C.; Papadopoulos, A.O.; Nguni, T.L.; Karim, F.; Moosa, M.-Y.S.; Gazy, I.; Jambo, K.; COMMIT-KZN-Team; Hanekom, W.; et al. Unsuppressed HIV infection impairs T cell responses to SARS-CoV-2 infection and abrogates T cell cross-recognition. eLife 2022, 11, e78374. [Google Scholar] [CrossRef] [PubMed]
- Al-Kayali, R.S.; Kashkash, M.F.; Alhajji, A.H.A.; Khouri, A. Activation of tuberculosis in recovered COVID-19 patients: A case report. Ann. Med. Surg. 2023, 85, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Deshpande, S.; Sawant, J.; Warthe, N.; Thakar, M.; Madkaikar, M.; Pradhan, V.; Rao, P.; Rohatgi, S.; Mukherjee, A.; et al. Higher proinflammatory responses possibly contributing to suppressed cytotoxicity in patients with COVID-19 associated mucormycosis. Immunobiology 2023, 228, 152384. [Google Scholar] [CrossRef] [PubMed]
- Maro, A.; Rosenthal, E.M.; Abdallah, M.; Tesoriero, J.; Dehovitz, J. Are persons living with diagnosed HIV capable of mounting a strong inflammatory response to the new coronavirus? Int. J. STD AIDS 2023, 34, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Cottam, A.; Manneh, I.L.; Gindeh, A.; Sillah, A.K.; Cham, O.; Mendy, J.; Barry, A.; Coker, E.G.; Daffeh, G.K.; Badjie, S.; et al. The impact of prior SARS-CoV-2 infection on host inflammatory cytokine profiles in patients with TB or other respiratory diseases. Front. Immunol. 2023, 14, 1292486. [Google Scholar] [CrossRef] [PubMed]
- Najafi-Fard, S.; Aiello, A.; Navarra, A.; Cuzzi, G.; Vanini, V.; Migliori, G.B.; Gualano, G.; Cerva, C.; Grifoni, A.; Sette, A.; et al. Characterization of the immune impairment of patients with tuberculosis and COVID-19 coinfection. Int. J. Infect. Dis. 2023, 130 (Suppl. 1), S34–S42. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Mahmood, K.; Ahmad, L.; Gul, H.; Hayat, A.; Rehman, M.U. Clinical manifestations of active tuberculosis patients coinfected with severe acute respiratory syndrome coronavirus-2. J. Clin. Tuberc. Other Mycobact. Dis. 2023, 31, 100359. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-M.; Zhao, J.-Y.; Zhang, Q.-Y.; Liu, S.-Q.; Zhu, X.-H.; An, Q.-Q.; Xu, T.-T.; Li, S.-J.; Liu, J.-Y.; Tao, N.-N.; et al. COVID-19 and Tuberculosis Coinfection: An Overview of Case Reports/Case Series and Meta-Analysis. Front. Med. 2021, 8, 657006. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki-Hozumi, H.; Chagan-Yasutan, H.; Ashino, Y.; Hattori, T. Blood Levels of Galectin-9, an Immuno-Regulating Molecule, Reflect the Severity for the Acute and Chronic Infectious Diseases. Biomolecules 2021, 11, 430. [Google Scholar] [CrossRef]
- Berentschot, J.C.; Drexhage, H.A.; Mersha, D.G.A.; Wijkhuijs, A.J.M.; GeurtsvanKessel, C.H.; Koopmans, M.P.G.; Voermans, J.J.C.; Hendriks, R.W.; Nagtzaam, N.M.A.; de Bie, M.; et al. Immunological profiling in long COVID: Overall low grade inflammation and T-lymphocyte senescence and increased monocyte activation correlating with increasing fatigue severity. Front. Immunol. 2023, 14, 1254899. [Google Scholar] [CrossRef]
- Shete, A.; Bhat, M.; Sawant, J.; Deshpande, S. Both N- and C-terminal domains of galectin-9 are capable of inducing HIV reactivation despite mediating differential immunomodulatory functionalities. Front. Immunol. 2022, 13, 994830. [Google Scholar] [CrossRef]
- Shete, A.; Wagh, V.; Sawant, J.; Shidhaye, P.; Sane, S.; Rao, A.; Kulkarni, S.; Ghate, M. Antiretroviral Treatment-Induced Galectin-9 Might Impact HIV Viremia in Addition to Contributing to Inflammaging. Int. J. Mol. Sci. 2023, 24, 12273. [Google Scholar] [CrossRef] [PubMed]
- Korobova, Z.R.; Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Dedkov, V.G.; Gladkikh, A.S.; Sharova, A.A.; Adish, Z.; Chernykh, E.I.; Kaschenko, V.A.; et al. Cytokine Profiling in Different SARS-CoV-2 Genetic Variants. Int. J. Mol. Sci. 2022, 23, 14146. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.D.; Kumar, G.; Mukherjee, A.; Nyayanit, D.A.; Shete, A.M.; Sahay, R.R.; Kumar, A.; Majumdar, T.; Patil, S.; Pandit, P.; et al. Delta variant SARS-CoV-2 infections in pediatric cases during the second wave in India. J. Microbiol. Immunol. Infect. 2022, 55 Pt 1, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Kurle, S.; Dhayarkar, S.; Patil, A.; Kulkarni, S.; Ghate, M.; Sangale, S.; Medhe, U.; Rajan, S.; Verma, V.; et al. High IL-5 levels possibly contributing to HIV viremia in virologic non-responders at one year after initiation of anti-retroviral therapy. Microb. Pathog. 2020, 143, 104117. [Google Scholar] [CrossRef]
- Shete, A.; Suryawanshi, P.; Godbole, S.; Pawar, J.; Paranjape, R.; Thakar, M. HIV-infected CD4+ T Cells Use T-bet-dependent Pathway for Production of IL-10 Upon Antigen Recognition. Scand. J. Immunol. 2016, 83, 288–296. [Google Scholar] [CrossRef]
- Chen, Z.; Andreev, D.; Oeser, K.; Krljanac, B.; Hueber, A.; Kleyer, A.; Voehringer, D.; Schett, G.; Bozec, A. Th2 and eosinophil responses suppress inflammatory arthritis. Nat. Commun. 2016, 7, 11596. [Google Scholar] [CrossRef]

| Parameters, Median (IQR) | SARS-CoV-2-Seronegative Patients (n = 27) | SARS-CoV-2-Seropositive Patients (n = 5) | p Value |
|---|---|---|---|
| Age (Years) | 45 (38–50) | 48 (45–50) | NS |
| Gender—M:F | 18:09 | 05:00 | NS |
| Type—Pulmonary: extrapulmonary | 18:09 | 2:3 | NS |
| Body weight (Kg) | 49 (39–56) | 43.9 (40–66.5) | NS |
| Duration of therapy (months) | 6 (6–11.75) | 10 (6–12.5) | NS |
| CD4 count (cells/mL) | 266 (141–387) | 359 (261–509) | NS |
| T-Gal9 (pg/mL) | 7439 (4657–10,038) | 7507 (5561–11,172) | NS |
| FL-Gal9 (pg/mL) | 4742 (2830–6000) | 6000 (2638–6000) | NS |
| OPN (ng/mL) | 85.5 (69.0–106.9) | 101.5 (62.7–210.3) | NS |
| CRP (mg/L) | 16.72 (5.77–72.05) | 27.57 (14.69–69.23) | NS |
| Correlation Analysis | Correlation Coefficient (r) | p Value |
|---|---|---|
| SARS-CoV-2 seropositivity versus Inflammatory Score | −0.386 | 0.039 |
| SARS-CoV-2 seropositivity versus percent change in total Galectin-9 levels | 0.404 | 0.030 |
| Baseline Value | % Decrease Rate | |||||||
|---|---|---|---|---|---|---|---|---|
| Correlation with INS | Correlation with SARS-CoV-2 Seropositivity | Correlation with INS | Correlation with SARS-CoV-2 Seropositivity | |||||
| Cytokines | r Value | p Value | r Value | p Value | r Value | p Value | r Value | p Value |
| IL-5 | −0.273 | 0.076 | −0.379 | 0.015 | 0.041 | 0.421 | 0.319 | 0.056 |
| IL-4 | −0.128 | 0.258 | −0.082 | 0.330 | 0.062 | 0.384 | 0.364 | 0.037 |
| GM-CSF | −0.265 | 0.082 | −0.192 | 0.142 | 0.005 | 0.491 | −0.007 | 0.487 |
| IL-10 | 0.080 | 0.343 | 0.005 | 0.490 | −0.398 | 0.030 | 0.032 | 0.442 |
| IFN-γ | −0.030 | 0.439 | −0.238 | 0.095 | 0.054 | 0.400 | 0.097 | 0.322 |
| IL-21 | −0.308 | 0.055 | −0.177 | 0.171 | 0.083 | 0.346 | 0.406 | 0.022 |
| TNF-α | 0.203 | 0.146 | 0.079 | 0.333 | −0.022 | 0.459 | −0.125 | 0.276 |
| IL-12 (p70) | −0.175 | 0.182 | −0.112 | 0.270 | −0.189 | 0.177 | 0.404 | 0.020 |
| IL-2 | 0.142 | 0.231 | −0.219 | 0.111 | 0.169 | 0.210 | 0.325 | 0.057 |
| IL-17A | −0.254 | 0.092 | −0.037 | 0.420 | 0.049 | 0.408 | 0.250 | 0.114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shete, A.; Ghate, M.; Iwasaki-Hozumi, H.; Patil, S.; Shidhaye, P.; Matsuba, T.; Bai, G.; Pharande, P.; Hattori, T. Association of SARS-CoV-2 Seropositivity with Persistent Immune Activation in HIV/Tuberculosis Co-Infected Patients. Reports 2024, 7, 61. https://doi.org/10.3390/reports7030061
Shete A, Ghate M, Iwasaki-Hozumi H, Patil S, Shidhaye P, Matsuba T, Bai G, Pharande P, Hattori T. Association of SARS-CoV-2 Seropositivity with Persistent Immune Activation in HIV/Tuberculosis Co-Infected Patients. Reports. 2024; 7(3):61. https://doi.org/10.3390/reports7030061
Chicago/Turabian StyleShete, Ashwini, Manisha Ghate, Hiroko Iwasaki-Hozumi, Sandip Patil, Pallavi Shidhaye, Takashi Matsuba, Gaowa Bai, Pratiksha Pharande, and Toshio Hattori. 2024. "Association of SARS-CoV-2 Seropositivity with Persistent Immune Activation in HIV/Tuberculosis Co-Infected Patients" Reports 7, no. 3: 61. https://doi.org/10.3390/reports7030061
APA StyleShete, A., Ghate, M., Iwasaki-Hozumi, H., Patil, S., Shidhaye, P., Matsuba, T., Bai, G., Pharande, P., & Hattori, T. (2024). Association of SARS-CoV-2 Seropositivity with Persistent Immune Activation in HIV/Tuberculosis Co-Infected Patients. Reports, 7(3), 61. https://doi.org/10.3390/reports7030061






