Most Plant Nutrient Elements Are Retained by Biochar in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples
2.2. Preparation of Biochars
2.3. Incubation Experiment
2.4. Chemical Analysis
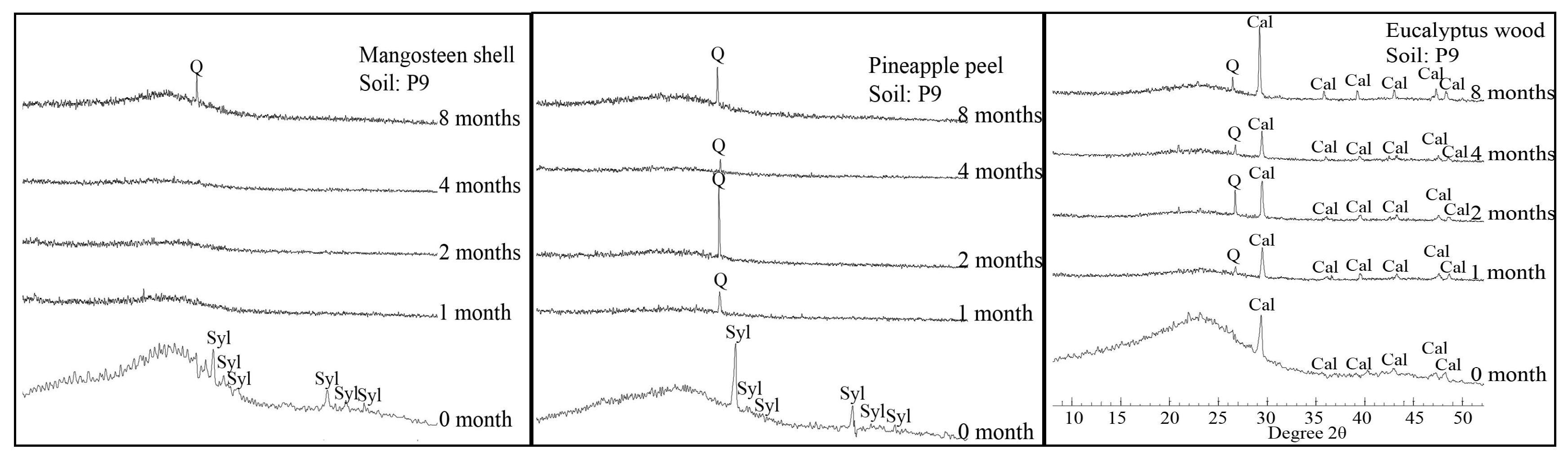
2.5. XRD
2.6. SEM, EDS, and X-ray Mapping
3. Results
3.1. Properties of Biochars
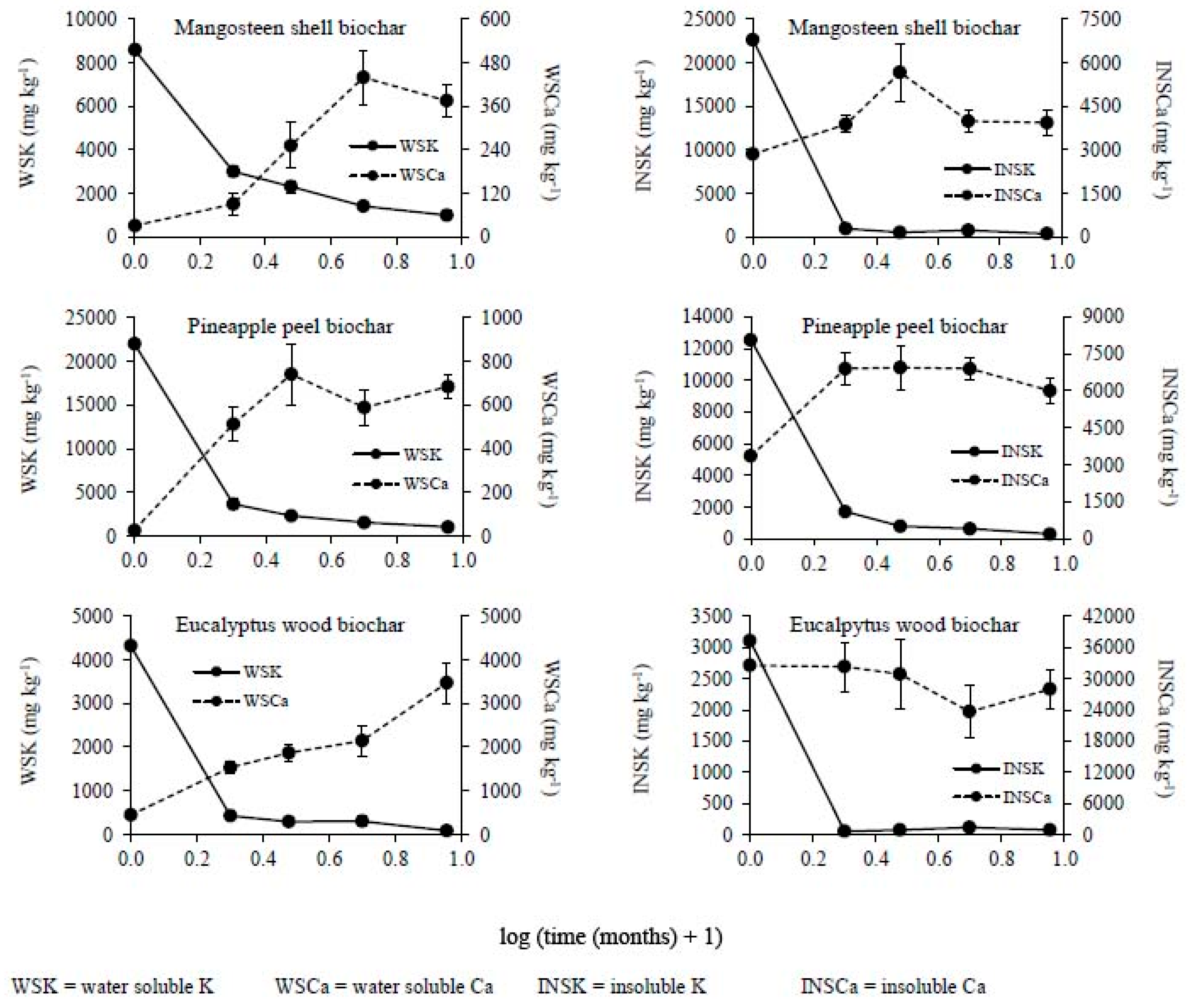
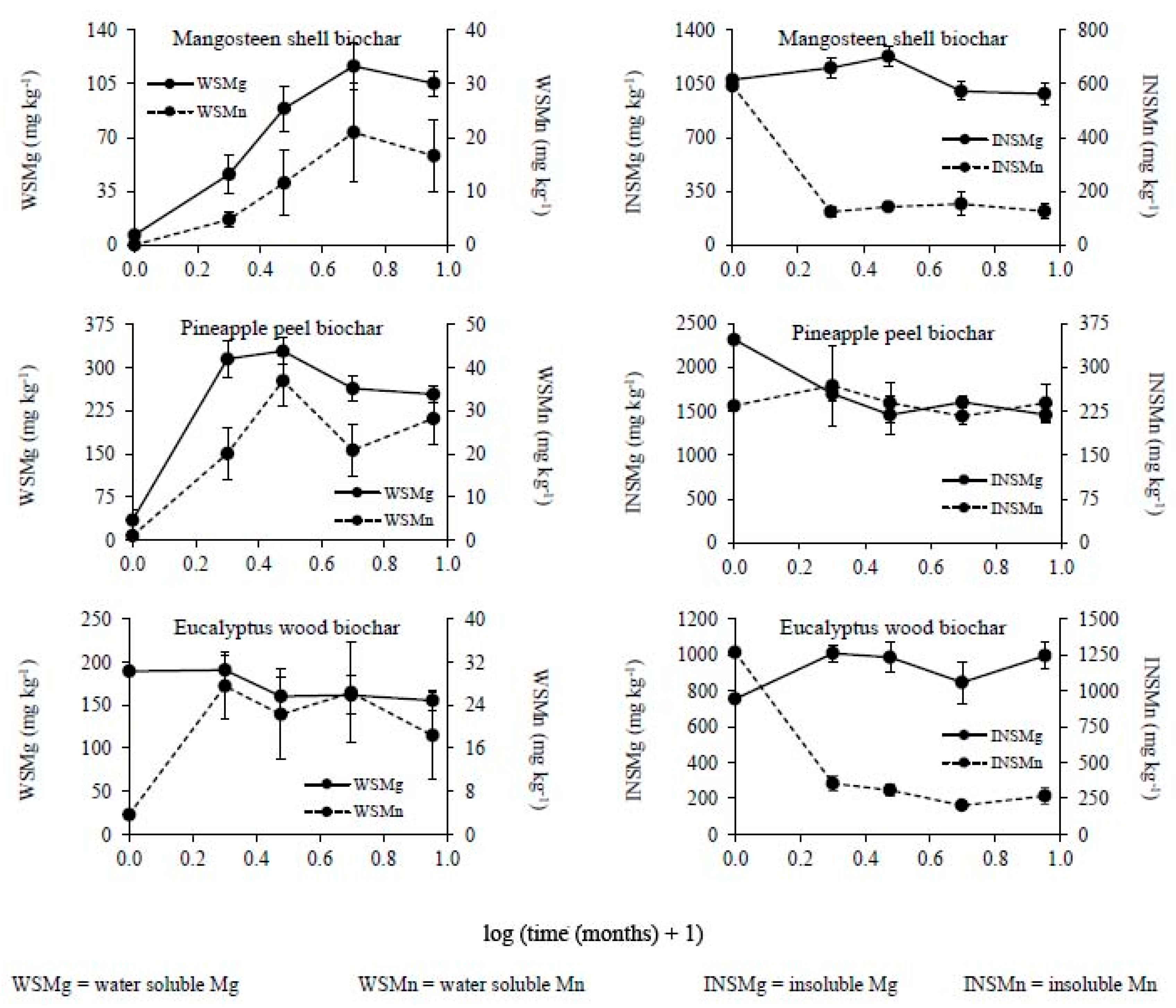
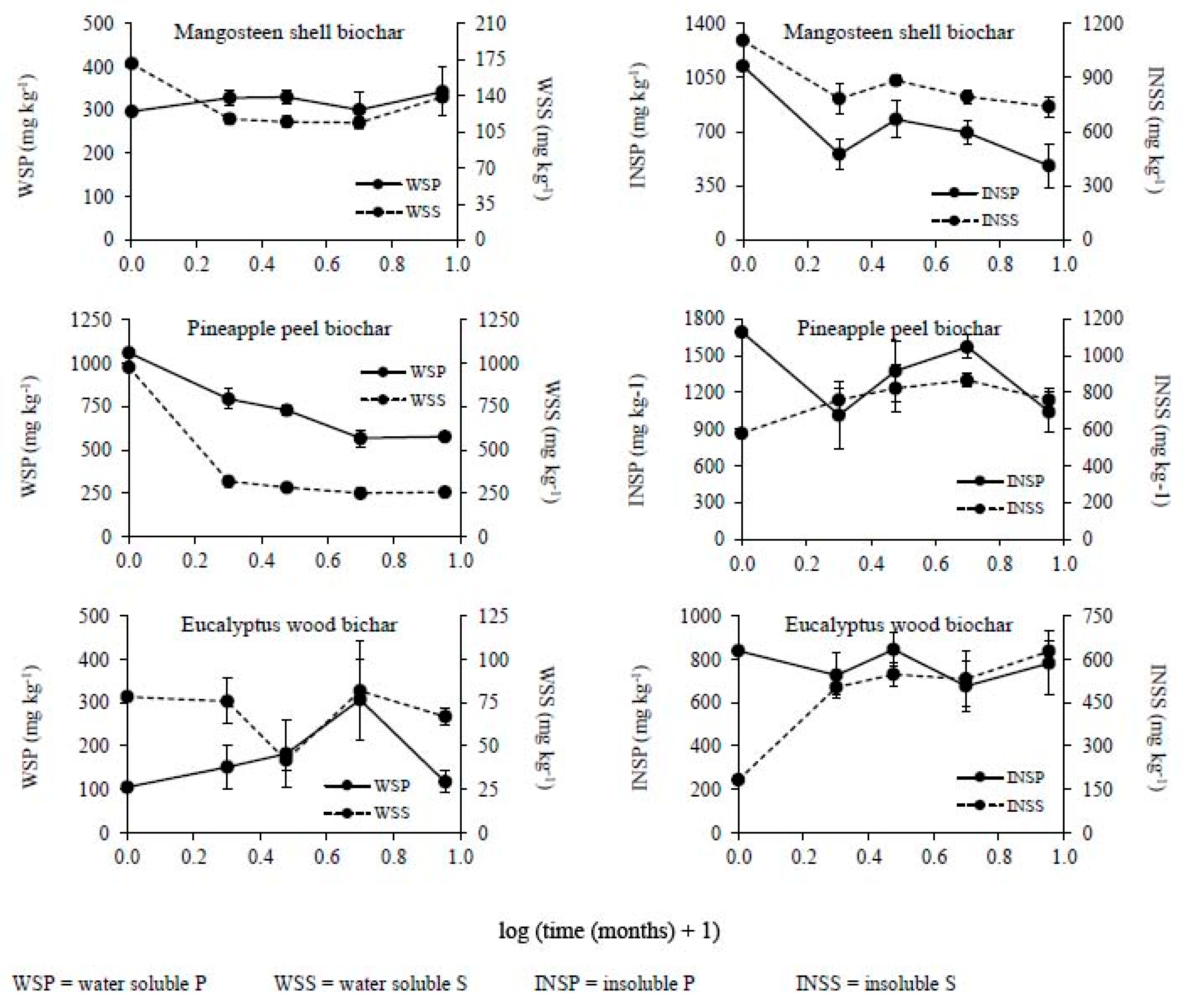
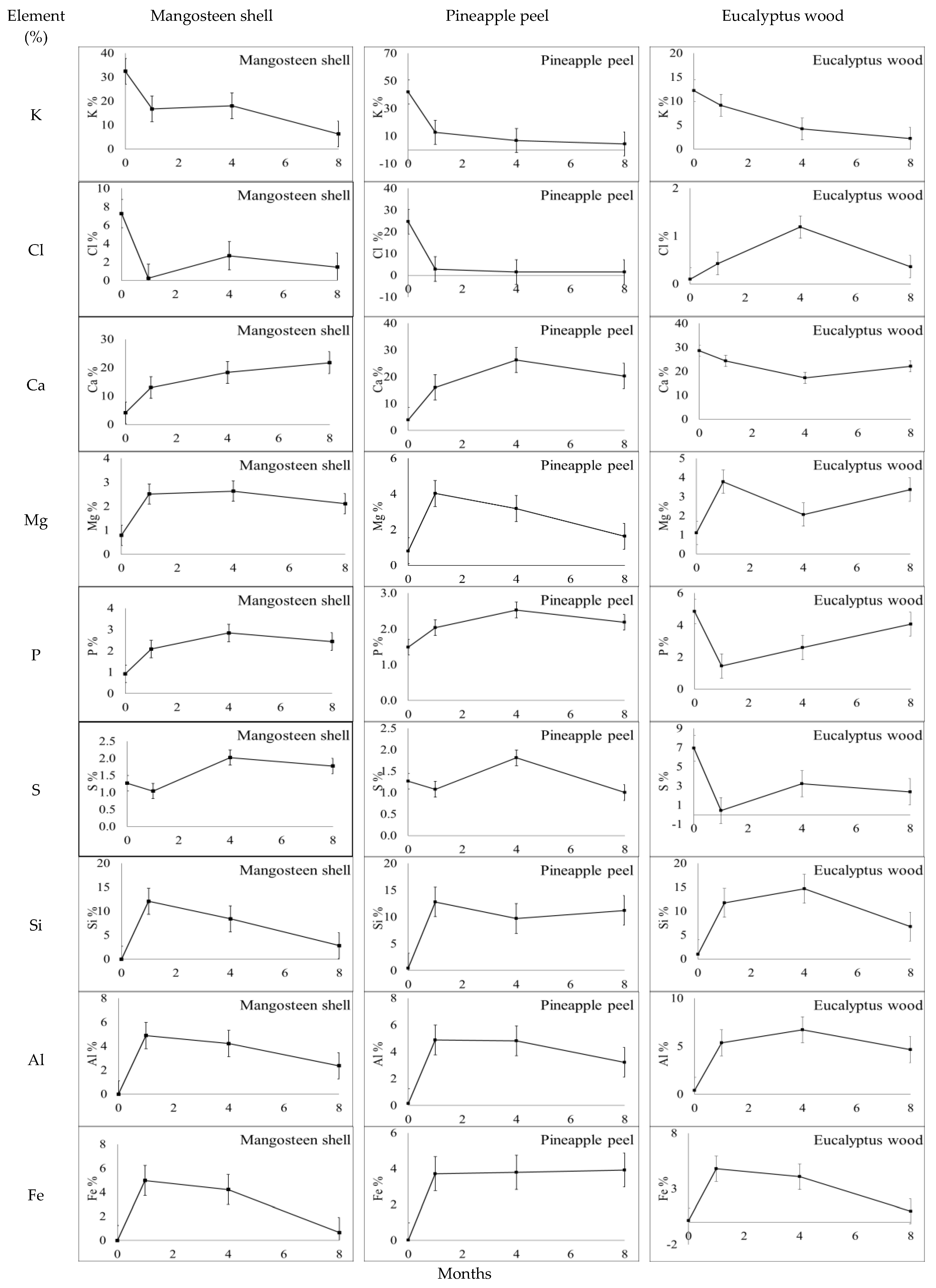
3.2. Changes in the Chemical Composition of Biochar during Incubation
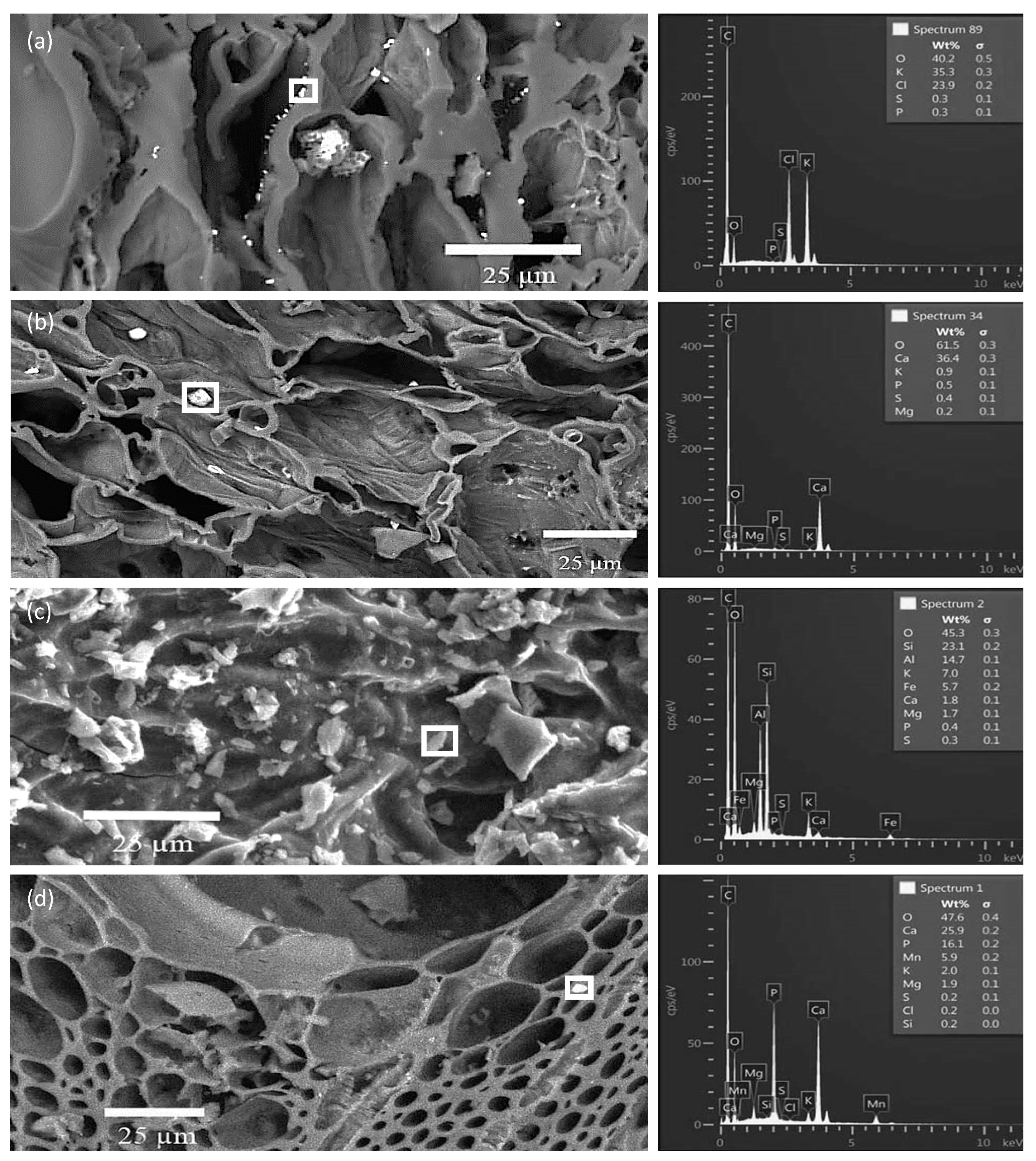
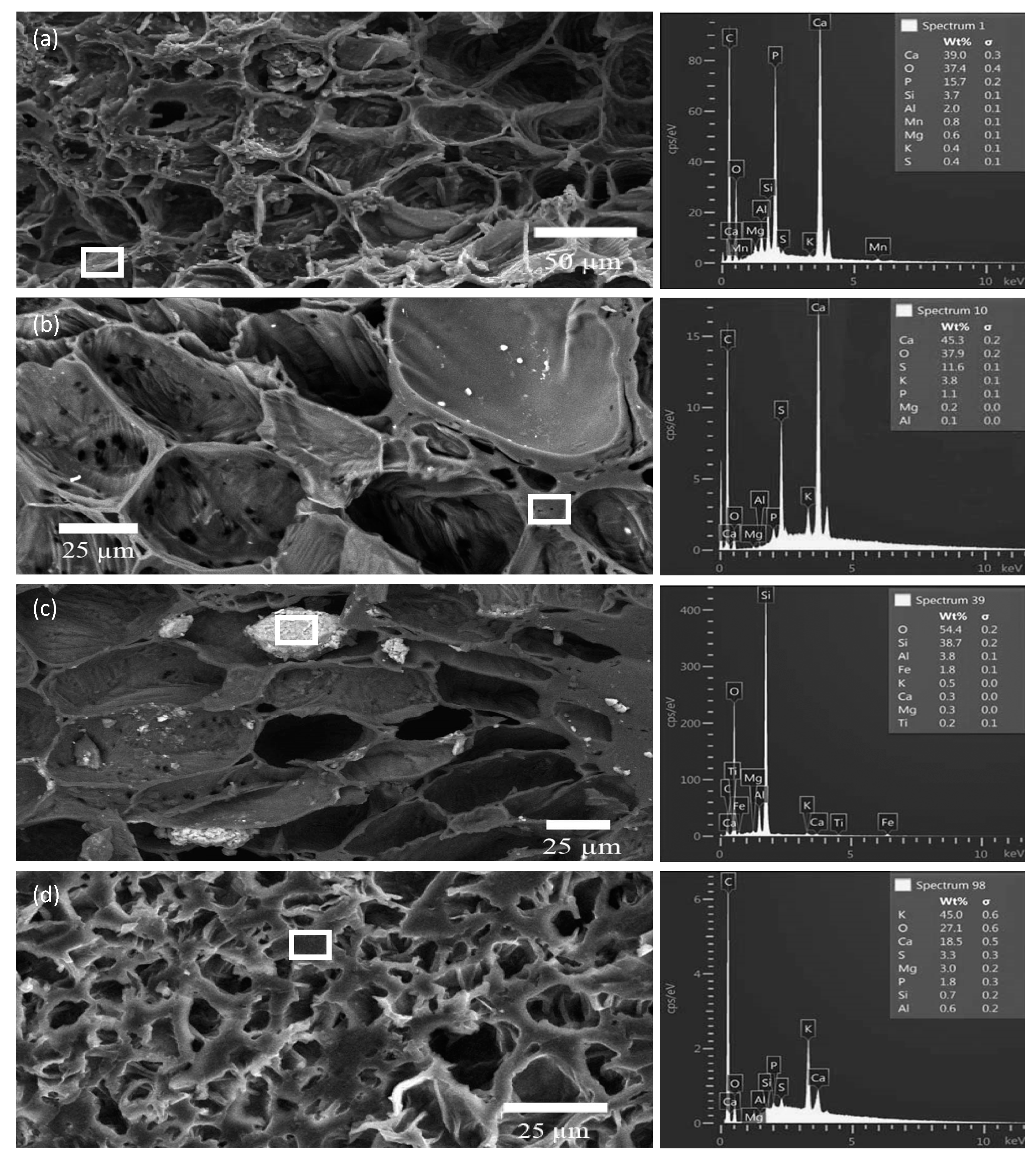
3.3. SEM/EDS Analyses of Biochar Particles Recovered from Soil
3.4. Point and Particle Analyses of Minerals in Biochar
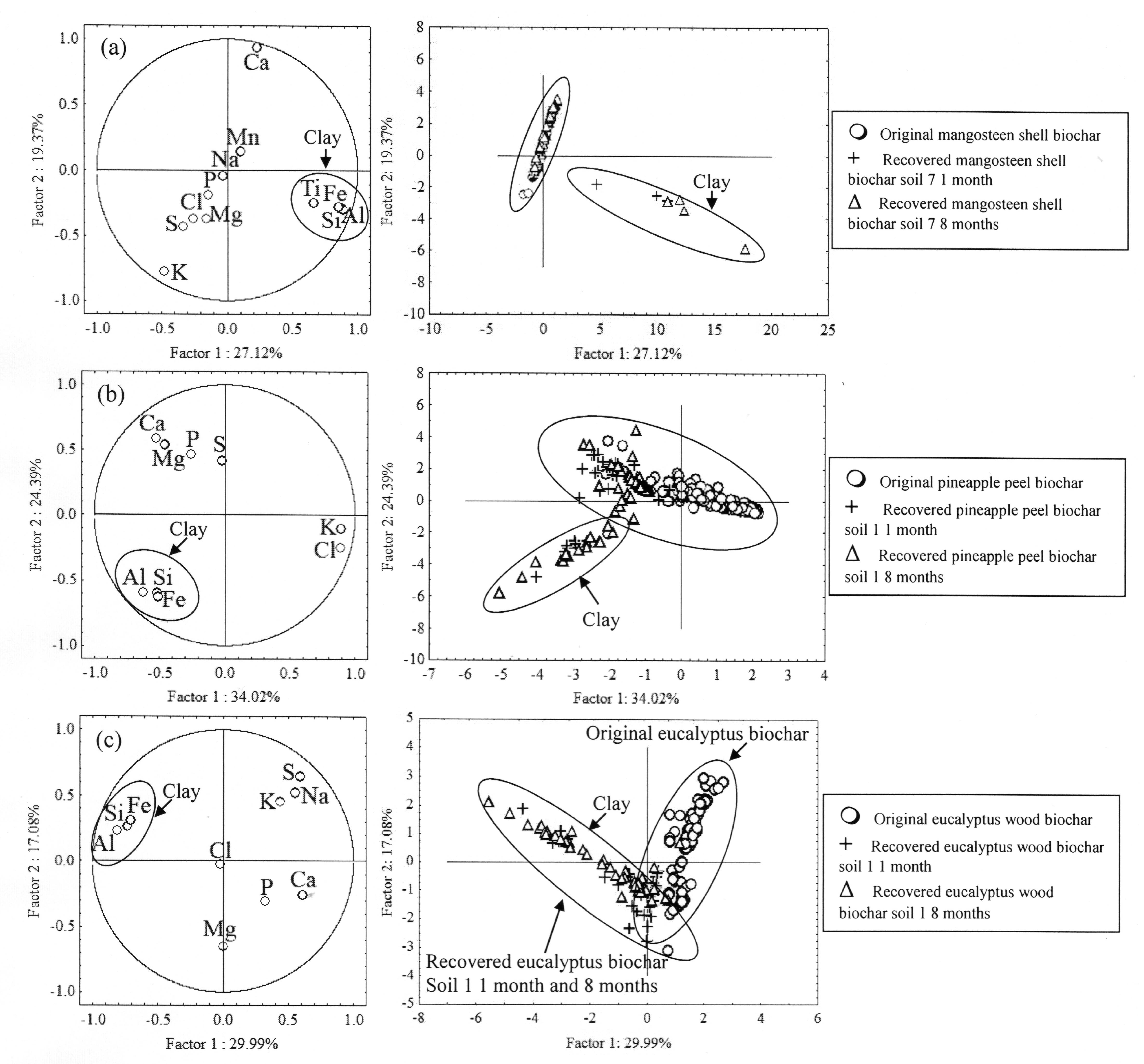
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W. Forms and solubility of plant nutrient elements in tropical plant waste biochars. J. Plant Nutr. Soil Sci. 2015, 178, 732–740. [Google Scholar] [CrossRef]
- Archanjo, B.S.; Mendoza, M.E.; Albu, M.; Mitchell, D.R.G.; Hagemann, N.; Mayrhofer, C.; Mai, T.L.A.; Weng, Z.; Kappler, A.; Behrens, S.; et al. Nanoscale analyses of the surface structure and composition of biochars extracted from field trials or after co-composting using advanced analytical electron microscopy. Geoderma 2017, 294, 70–79. [Google Scholar] [CrossRef]
- Limwikran, T.; Kheoruenromne, I.; Suddhiprakarn, A.; Prakongkep, N.; Gilkes, R.J. Dissolution of K, Ca, and P from biochar grains in tropical soils. Geoderma 2018, 312, 139–150. [Google Scholar] [CrossRef]
- Bolland, M.D.A.; Weatherley, A.J.; Gilkes, R.J. The long-term residual value of rock phosphate and superphosphate fertilizers for various plant species under field conditions. Fertil. Res. 1989, 20, 89–100. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Miller, R.O. Nitric-perchloric acid wet digestion in an open vessel. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC press: Washington, DC, USA, 1998; pp. 57–61. [Google Scholar]
- National Soil Survey Center. Soil Survey Laboratory Methods Manual; United States Department of Agriculture: Washington, DC, USA, 1996.
- Rayment, G.E. Soil Chemical Methods: Australia; CSIRO Publishing: Collingwood, VIC, Australia, 2011. [Google Scholar]
- Wuhrer, R.; Moran, K.; Moran, L. Characterisation of materials through X-ray mapping. Mater. Forum. 2006, 30, 63–70. [Google Scholar]
- Singh, B.; Raven, M.D. X-ray diffraction analysis of biochar. In Biochar A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CSIRO Publishing: Collingwood, VIC, Australia, 2017; pp. 245–252. [Google Scholar]
- Camps-Arbestain, M.; Shen, Q.; Wang, T.; van Zwieten, L.; Novak, J. Available nutrients in biochar. In Biochar A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CSIRO Publishing: Collingwood, VIC, Australia, 2017; pp. 109–125. [Google Scholar]
- Kilmer, V.J.; Younts, S.E.; Brady, N.C. The Role of Potassium in Agriculture; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1968. [Google Scholar]
- Benbi, D.; Gilkes, R. The movement into soil of P from superphosphate grains and its availability to plants. Fertil. Res. 1987, 12, 21–36. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef]
- Garvie, L.A.J. Mineralogy of paloverde (Parkinsonia microphylla) tree ash from the Sonoran Desert: A combined field and laboratory study. Am. Mineral. 2016, 101, 1584–1595. [Google Scholar] [CrossRef]
- Hocking, P.J. The composition of phloem exudate and xylem sap from tree tobacco (Nicotiana glauca Grah.). Ann. Bot. 1980, 45, 633–643. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Canti, M.G. Aspects of the chemical and microscopic characteristics of plant ashes found in archaeological soils. CATENA 2003, 54, 339–361. [Google Scholar] [CrossRef]
- Ahmed, J.; Ojha, K.; Vaidya, S.; Ganguli, J.; Ganguli, A. Formation of calcium oxalate nanoparticles in leaves: Significant role of water content and age of leaves. Curr. Sci. 2012, 103, 293–298. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, M.A. Rock-Forming Minerals Vol. 5 Non-Silicates; Longman: London, UK, 1962. [Google Scholar]
- Hughes, J.M.; Cameron, M.; Crowley, K.D. Ordering of divalent cations in the apatite structure: Crystal structure refinements of natural Mn- and Sr-bearing apatite. Am. Mineral. 1991, 76, 1857–1862. [Google Scholar]
- Kumar, V.; Gilkes, R.; Bolland, M. Residual phosphate fertilizer compounds in soils—II. Their influence on soil tests for available phosphate. Fertil. Res. 1991, 30, 31–38. [Google Scholar] [CrossRef]
- Yusiharni, E.; Gilkes, R. Minerals in the ash of Australian native plants. Geoderma 2012, 189–190, 369–380. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 35–53. [Google Scholar] [CrossRef]
- Novak, J.M.; Johnson, M.G.; Spokas, K.A. Concentration and release of phosphorus and potassium from Lignocellulosic- and manure-based biochars for fertilizer reuse. Front. Sustain. Food Syst. 2018, 54, 1–9. [Google Scholar] [CrossRef]
- Gupta, A. Extraction and Characterization of Phosphorus from Organic Algal Biochar. Master’s Thesis, University of Kansas, Lawrence, KS, USA, 2014. [Google Scholar]
- Prendergast-Miller, M.T.; Duvalla, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Khalil, R.A.; Seljeskog, M.; Hustad, J.E. Sulfur abatement in pyrolysis of straw pellets. Energy Fuel 2008, 22, 2789–2795. [Google Scholar] [CrossRef]
- Bolland, M.; Gilkes, R.J. The poor performance of rock phosphate fertilizers in Western Australia: Part I. The crop and pasture responses. Agric. Sci. 1990, 3, 43–48. [Google Scholar]
- Gilkes, R.; Bolland, M. Australian field and glasshouse research with rock phosphates: History, explanations and current developments. Terra (Mexico) 1994, 12, 79–105. [Google Scholar]
- Bolland, M.; Gilkes, R. Long-term residual value of North Carolina and Queensland rock phosphates compared with triple superphosphate. Fertil. Res. 1995, 41, 151–158. [Google Scholar] [CrossRef]
- Bolan, N.S.; Syers, J.K.; Sumner, M.E. Dissolution of various sources of gypsum in aqueous solutions and in soil. J. Sci. Food Agric. 1991, 57, 527–541. [Google Scholar] [CrossRef]
| Soil Series [4] | Coordinates | Classification [6] | Parent Material |
|---|---|---|---|
| Oxisols | |||
| Pak Chong1 (Pc1) | 47P 0,759,730 E 1,624,306 N | Rhodic Kandiustox | Residuum and colluvium from limestone |
| Pak Chong2 (Pc2 | 47P 0,758,389 E 1,616,034 N | Rhodic Kandiustox | Residuum and colluvium from limestone |
| Chok Chai (Ci) | 48P 0,210,727 E 1,633,381 N | Rhodic Kandiustox | Residuum and colluvium from basalt |
| Ultisols | |||
| Roi Et1 (Re1) | 47P 0,795,612 E 1,680,167 N | Aeric Kandiaquults | Wash deposit from sandstone |
| Roi Et2 (Re2) | 47P 0,788,656 E 1,652,504 N | Aeric Kandiaquults | Wash deposit from sandstone |
| Khorat (Kt) | 47P 0,792,694 E 1,678,613 N | Typic Kandiustults | Wash deposit from sandstone |
| Yasothon (Yt) | 47P 0,793,502 E 1,675,845 N | Typic Paleustults | Wash deposit from sandstone |
| Sung Noen1 (Sn1) | 47P 0,791,130 E 1,666,752 N | Typic Paleustults | Wash deposit over siltstone/shale |
| Sung Noen2 (Sn2) | 47P 0,790,298 E 1,664,657 N | Typic Paleustults | Wash deposit over siltstone/shale |
| Soil Series [4] | pH | EC | Clay | OC | CEC | K | Na | Ca | Mg | Avail. P |
|---|---|---|---|---|---|---|---|---|---|---|
| µS cm−1 | (%) | cmol kg−1 | (cmol kg−1) | mg kg−1 | ||||||
| Oxisols | ||||||||||
| Pc1 | 6.4 | 53 | 32 | 0.66 | 12 | 2.1 | 0.0 | 5.2 | 2.1 | 76 |
| Pc2 | 4.8 | 51 | 36 | 0.54 | 12 | 0.3 | 0.0 | 4.1 | 1.4 | 65 |
| Ci | 5.0 | 61 | 32 | 0.47 | 10 | 0.3 | 0.1 | 2.3 | 0.5 | 47 |
| Ultisols | ||||||||||
| Re1 | 6.3 | 199 | 20 | 0.42 | 12 | 0.5 | 0.1 | 5.7 | 2.1 | 113 |
| Re2 | 5.4 | 73 | 24 | 0.41 | 11 | 0.5 | 0.1 | 5.3 | 0.7 | 18 |
| Kt | 5.2 | 81 | 12 | 0.27 | 6 | 0.2 | 0.1 | 2.3 | 1.1 | 26 |
| Yt | 5.1 | 35 | 8 | 0.13 | 3 | 0.2 | 0.0 | 0.9 | 0.2 | 15 |
| Sn1 | 4.7 | 86 | 28 | 0.40 | 12 | 0.9 | 0.1 | 4.3 | 1.6 | 41 |
| Sn2 | 5.8 | 94 | 20 | 0.40 | 10 | 0.5 | 0.2 | 3.3 | 1.3 | 34 |
| Raw Materials | pH | EC | Ca | K | Mg | Mn | Na | P | S |
|---|---|---|---|---|---|---|---|---|---|
| µS cm−1 | (mg kg−1) | ||||||||
| Mangosteen shell | 8.2 | 6070 | 4796 | 28,758 | 1084 | 97 | 388 | 10,346 | 1036 |
| Pineapple peel | 7.9 | 13,273 | 6466 | 32,528 | 2231 | 235 | 333 | 4211 | 1555 |
| Eucalyptus wood | 10.2 | 2677 | 14,362 | 5552 | 944 | 1273 | 1001 | 3463 | 261 |
| Raw Materials | Ca | K | Mg | Mn | Na | P | S | K/Cl |
|---|---|---|---|---|---|---|---|---|
| Mangosteen shell | 0.05 | 0.26 | 0.01 | 0.00 | 0.23 | 0.03 | 0.17 | 0.69 |
| Pineapple peel | 0.07 | 0.54 | 0.02 | 0.00 | 0.24 | 0.17 | 0.63 | 0.87 |
| Eucalyptus wood | 0.04 | 0.42 | 0.20 | 0.00 | 0.30 | 0.02 | 0.30 | 1.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limwikran, T.; Kheoruenromne, I.; Suddhiprakarn, A.; Prakongkep, N.; Gilkes, R.J. Most Plant Nutrient Elements Are Retained by Biochar in Soil. Soil Syst. 2019, 3, 75. https://doi.org/10.3390/soilsystems3040075
Limwikran T, Kheoruenromne I, Suddhiprakarn A, Prakongkep N, Gilkes RJ. Most Plant Nutrient Elements Are Retained by Biochar in Soil. Soil Systems. 2019; 3(4):75. https://doi.org/10.3390/soilsystems3040075
Chicago/Turabian StyleLimwikran, Tanawan, Irb Kheoruenromne, Anchalee Suddhiprakarn, Nattaporn Prakongkep, and Robert J. Gilkes. 2019. "Most Plant Nutrient Elements Are Retained by Biochar in Soil" Soil Systems 3, no. 4: 75. https://doi.org/10.3390/soilsystems3040075
APA StyleLimwikran, T., Kheoruenromne, I., Suddhiprakarn, A., Prakongkep, N., & Gilkes, R. J. (2019). Most Plant Nutrient Elements Are Retained by Biochar in Soil. Soil Systems, 3(4), 75. https://doi.org/10.3390/soilsystems3040075




