Soil Organic Carbon Stabilization: Influence of Tillage on Mineralogical and Chemical Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Particle Size Physical Fractionation and Soil Organic Matter Removal
2.3. Mineralogical and Chemical Analyses of Particle Size Fractions
2.4. Spectroscopic Analyses of Particle Size Fractions
2.5. Statistical Analysis
3. Results and Discussion
3.1. Elemental and Mineralogical Analyses
3.2. Interactions between Inorganic and Organic Components of the Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kölbl, A.; Kögel-Knabner, I. Content and composition of free and occluded particulate organic matter in a differently textured arable Cambisol as revealed by solid-state13C NMR spectroscopy. J. Plant Nutr. Soil Sci. 2004, 167, 45–53. [Google Scholar] [CrossRef]
- Baldock, J.A.; Skjemstad, J.O. Soil organic carbon/soil organic matter. In Soil Analysis: An Interpretation Manual; Peverill, K.I., Sparrow, L.A., Reuter, D.J., Eds.; CSIRO Publishing: Collingwood, Australia, 1999; pp. 159–170. [Google Scholar]
- Kaiser, K.; Guggenberger, G. Mineral surfaces and soil organic matter. Eur. J. Soil Sci. 2003, 54, 219–236. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Pronk, G.J.; Heister, K.; Kögel-Knabner, I. Is turnover and development of organic matter controlled by mineral composition? Soil. Biol. Biochem. 2013, 67, 235–244. [Google Scholar] [CrossRef]
- Wiseman, C.L.S.; Püttmann, W. Interactions and mineral phases in the preservation of soil organic matter. Geoderma 2006, 134, 109–118. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Baldock, J.; Skjemstad, J. Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org. Geochem. 2000, 31, 697–710. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org. Geochem. 2000, 31, 711–725. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Feng, W.; Plante, A.F.; Six, J. Improving estimates of maximal organic carbon stabilization by fine soil particles. Biogeochemistry 2013, 112, 81–93. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, H.; Li, Y.; Ma, Y.; Tang, H.; Ran, W.; Shen, Q. The role of poorly crystalline iron oxides in the stability of soil aggregate-associated organic carbon in a rice–wheat cropping system. Geoderma 2016, 279, 1–10. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peths, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Kleber, M.; Mikutta, R.; Torn, M.; Jahn, R. Poorly crystalline mineral phases protect organic matter in acid subsoil horizons. Eur. J. Soil Sci. 2005, 56, 717–725. [Google Scholar] [CrossRef]
- Steffens, M.; Rogge, D.M.; Mueller, C.W.; Hoschen, C.; Lugmeier, J.; Kolbl, A.; Kogel-Knabner, I. Identification of distinct functional microstructural domains controlling C storage in soil. Environ. Sci. Technol. 2017, 51, 12182–12189. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Zhuang, L.; Zhou, S.-G.; Yuan, Y.; Yuan, T.; Li, F.-B. Humic substance-mediated reduction of iron (III) oxides and degradation of 2,4-D by an alkaliphilic bacterium, Corynebacterium humireducens MFC-5. Microb. Biotechnol. 2013, 6, 141–149. [Google Scholar] [CrossRef]
- Gu, B.; Chen, J. Enhanced microbial reduction of Cr(VI) and U(VI) by different natural organic matter fractions. Geochim. Cosmochim. Acta 2003, 67, 3575–3582. [Google Scholar] [CrossRef]
- Duiker, S.W.; Rhoton, F.E.; Torrent, J.; Smeck, N.E.; Lal, R. Iron(hydr)oxide crystallinity effects on soil aggregation. Soil Sci. Soc. Am. J. 2003, 67, 606–611. [Google Scholar] [CrossRef]
- Hubert, F.; Caner, L.; Meunier, A.; Ferrage, E. Unraveling complex b2 μm clay mineralogy from soils using X-ray diffraction profile modeling on particle-size subfractions: Implications for soil pedogenesis and reactivity. Am. Mineral. 2012, 97, 384–398. [Google Scholar] [CrossRef]
- Wagai, R.; Kajiura, M.; Uchida, M.; Asano, M. Distinctive roles of two aggregate binding agents in allophanic Andisols: Young carbon and poorly-crystalline metal phases with old carbon. Soil Syst. 2018, 2, 29. [Google Scholar] [CrossRef]
- De Mastro, F.; Cocozza, C.; Brunetti, G.; Traversa, A. Chemical and spectroscopic investigation of different soil fractions as affected by soil management. Appl. Sci. 2020, 10, 2571. [Google Scholar] [CrossRef]
- Xie, X.-L.; Pan, X.-Z.; Sun, B. Visible and near-infrared diffuse reflectance spectroscopy for prediction of soil properties near a copper smelter. Pedosphere 2012, 22, 351–366. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Z.; Zhang, B.; Du, S.; Liu, G. The effects of agricultural management on selected soil properties of the arable soils in Tibet, China. Catena 2012, 93, 1–8. [Google Scholar] [CrossRef]
- Pantenburg, F.J.; Beier, T.; Hennrich, F.; Mommsen, H. The fundamental parameter method applied to X-ray fluorescence analysis with synchrotron radiation. Nucl. Instrum. Methods Phys. Res. Sect. B 1992, 68, 125–132. [Google Scholar] [CrossRef]
- Hunt, G.R.; Salisbury, J.W. Visible and near infrared spectra of minerals and rocks. I. Silicate minerals. Mod. Geol. 1970, 1, 283–300. [Google Scholar]
- Post, J.L.; Noble, P.N. The near-infrared combination band frequencies of dioctahedral smectites, micas, and illites. Clay Miner. 1993, 41, 639–644. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Irons, J.R.; Epema, G.F. Soil reflectance. In Remote Sensing for the Earth Sciences: Manual of Remote Sensing; Rencz, A.N., Ed.; Wiley: New York, NY, USA, 1999; pp. 111–188. [Google Scholar]
- Shepherd, K.D.; Walsh, M.G. Development of reflectance spectral libraries for characterization of soil properties. Soil Sci. Soc. Am. J. 2002, 66, 988–998. [Google Scholar] [CrossRef]
- Cozzolino, D.; Morón, A. The potential of near-infrared reflectance spectroscopy to analyse soil chemical and physical characteristics. J. Agric. Sci. 2003, 140, 65–71. [Google Scholar] [CrossRef]
- Sorensen, L.K.; Dalsgaard, S. Determination of clay and other soil properties by near infrared spectroscopy. Soil Sci. Soc. Am. J. 2005, 69, 159–167. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- De Mastro, F.; Brunetti, G.; Traversa, A.; Cocozza, C. Effect of crop rotation, fertilization and tillage on main soil properties and its water extractable organic matter. Soil Res. 2019, 57, 365–373. [Google Scholar] [CrossRef]
- Bornemann, L.; Welp, G.; Amelung, W. Particulate organic matter at the field scale: Rapid acquisition using MID-Infrared spectroscopy. Soil Sci. Am. J. 2010, 74, 1147–1156. [Google Scholar] [CrossRef]
- Margenot, A.J.; Calderón, F.J.; Magrini, K.A.; Evans, R.J. Application of DRIFTs, 13 C NMR, and PY-MBMS to characterize the effects of soil science oxidation assays on soil organic matter composition in a Mollic Xerofluvent. Appl. Spectr. 2017, 71, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.G. Quantitative interpretation of mineralogical composition from X-ray and chemical data or Pierre Shale. U.S. Geol. Surv. Profess. Paper 1964, 391-c, 1–31. [Google Scholar]
- Shaw, D.B.; Stevenson, R.G.; Weaver, C.E.; Bradley, W.F. Interpretation of X-ray diffraction data. In Procedure in Sedimentary Petrology; Carver, R.E., Ed.; Wiley and Sons: New York, NY, USA, 1971; pp. 554–557. [Google Scholar]
- Laviano, R. Analisi mineralogica quantitativa di argille mediante diffrattometria di raggi X. In Atti Workshop “Procedure di Analisi di Materiali Argillosi”; ENEA, Ed.; ENEA: Rome, Italy, 1987; pp. 215–234. [Google Scholar]
- Franzini, M.; Leoni, L.; Saitta, M. A simple method to evaluate the matrix effects in X-ray fluorescence. X-ray Spectrom. 1972, 1, 151–154. [Google Scholar] [CrossRef]
- Franzini, M.; Leoni, L.; Saitta, M. Determination of the X-Ray Mass Absorption Coefficient by measurement of the AgKα Compton Scattered Radiation. X-Ray Spectrom. 1976, 5, 84–87. [Google Scholar] [CrossRef]
- Franzini, M.; Leoni, L.; Saitta, M. Enhancement Effects in X-ray fluorescence analysis of rocks. X-ray Spectrom. 1976, 5, 208–211. [Google Scholar] [CrossRef]
- Leoni, L.; Saitta, M. X-ray fluorescence analysis of 29 trace elements in rock and mineral standards. Rend. Soc. It. Mineral. Petrogr. 1976, 32, 497–510. [Google Scholar]
- Schwertmann, U. Differenzierung der Eisenoxide des Bodensdurch Extraktion mitsaurer Ammonium oxalat-Losung. Z. Pflanz. Bodenk. 1964, 105, 194–202. [Google Scholar] [CrossRef]
- Summa, V.; Tateo, F.; Medici, L.; Giannossi, M.L. The role of mineralogy, geochemistry and grain size in badland development in Pisticci (Basilicata, Southern Italy). Earth Surf. Process Landform 2007, 32, 980–997. [Google Scholar] [CrossRef]
- Akmad, N. Pedogenesis and soil taxonomy. In The Soil Orders, Volume 2; Wilding, L.P., Smeck, N.E., Hall, G.F., Eds.; Elsevier: New York, NY, USA, 1983; pp. 91–123. [Google Scholar]
- Brunetti, G.; Mezzapesa, G.N.; Traversa, A.; Bonifacio, E.; Farrag, K.; Senesi, N.; D’Orazio, V. Characterization of clay- and silt-sized fractions and corresponding humic acids along a Terra Rossa soil profile. CLEAN 2016, 44, 1–10. [Google Scholar] [CrossRef]
- Owliaie, H.R. Micromorphology of Pedogenic Carbonate Features in Soils of Kohgilouye, Southwestern Iran. J. Agric. Sci. Technol. 2012, 14, 225–239. [Google Scholar]
- Dubroeucq, D.; Volkoff, B. From Oxisols to Spodosols and Histosols: Evolution of the soil mantles in the Rio Negro basin (Amazonia). Catena 1998, 32, 245–280. [Google Scholar] [CrossRef]
- Sandler, A. Clay Distribution over the Landscape of Israel: From the Hyper-Arid to the Mediterranean Climate Regimes. Catena 2013, 110, 119–132. [Google Scholar] [CrossRef]
- Comino, F.; Ayora-Cañada, M.J.; Aranda, V.; Díaz, A.; Domínguez-Vidal, A. Near-infrared spectroscopy and X-ray fluorescence data fusion for olive leaf analysis and crop nutritional status determination. Talanta 2018, 188, 676–684. [Google Scholar] [CrossRef]
- Bishop, J.L.; Pieters, C.M.; Edwards, J.O. Infrared spectroscopic analyses on the nature of water in montmorillonite. Clay Clay Miner. 1994, 42, 702–716. [Google Scholar] [CrossRef]
- Heckman, K.; Throckmorton, H.; Horwath, W.R.; Swanston, C.W.; Rasmussen, C. Variation in the molecular structure and radiocarbon abundance of mineral-associated organic matter across a lithosequence of forest soils. Soil Syst. 2018, 2, 36. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gelinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Wagai, R.; Mayer, L.M. Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim. Cosmochim. Acta 2007, 71, 25–35. [Google Scholar] [CrossRef]
- Xue, B.; Huang, L.; Huang, Y.; Kubar, K.A.; Li, X.; Lu, J. Straw management influences the stabilization of organic carbon by Fe (oxyhydr)oxides in soil aggregates. Geoderma 2020, 358, 113987. [Google Scholar] [CrossRef]
- Spielvogel, S.; Prietzel, J.; Kogel-Knabner, I. Soil organic matter stabilization in acidic forest soils is preferential and soil type—Specific. Eur. J. Soil Sci. 2008, 59, 674–692. [Google Scholar] [CrossRef]
- Chenu, C.; Virto, I.; Plante, A.; Elsass, F. Clay-Size organo-mineral complexes in temperate soils. In Carbon Stabilization by Clays in the Environment; Laird, D., Cervini-Silva, J., Eds.; Clay Minerals Society: Chantilly, VA, USA, 2009; pp. 119–135. [Google Scholar]
- Torn, M.S.; Trumbore, S.E.; Chadwick, O.A.; Vitousek, P.M.; Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 1997, 389, 170–173. [Google Scholar] [CrossRef]
- Rasmussen, C.; Southard, R.J.; Horwath, W.R. Mineral control of organic carbon mineralization in a range of temperate conifer forest soils. Glob. Change Biol. 2006, 12, 834–847. [Google Scholar] [CrossRef]
- Kramer, M.; Sanderman, J.; Chadwick, O.A.; Chorover, J.; Vitousek, P.M. Long-term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Glob. Chang. Biol. 2012, 18, 2594–2605. [Google Scholar] [CrossRef]
- Velde, B. Introduction to Clay Minerals: Chemistry, Origins, Uses and Environmental Significances; Chapman and Hall: London, UK, 1992. [Google Scholar]
- Khormali, F.; Abtahi, A. Origin and distribution of clay minerals in calcareous arid and semi-arid soils of Fars Province, southern Iran. Clay Miner. 2003, 38, 511–527. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. Sorptive stabilization of organic matter by microporous goethite: Sorption into small pores vs. surface complexation. Eur. J. Soil Sci. 2007, 58, 45–59. [Google Scholar] [CrossRef]
- Chorover, J.; Amistadi, M.K. Reaction of forest floor organic matter at goethite, birnessite and smectite surfaces. Geochim. Cosmochim. Acta 2001, 65, 95–109. [Google Scholar] [CrossRef]
- Huang, X.; Feng, C.; Zhao, G.; Ding, M.; Kang, W. Carbon sequestration potential promoted by oxalate extractable iron oxides through organic fertilization. Soil Sci. Soc. Am. J. 2018, 81, 1359–1370. [Google Scholar] [CrossRef]
- Saidy, A.R.; Smernik, R.J.; Baldock, J.A.; Kaiser, K.; Sanderman, J. The sorption of organic carbon onto differing clay minerals in the presence and absence of hydrous iron oxide. Geoderma 2013, 209, 15–21. [Google Scholar] [CrossRef]
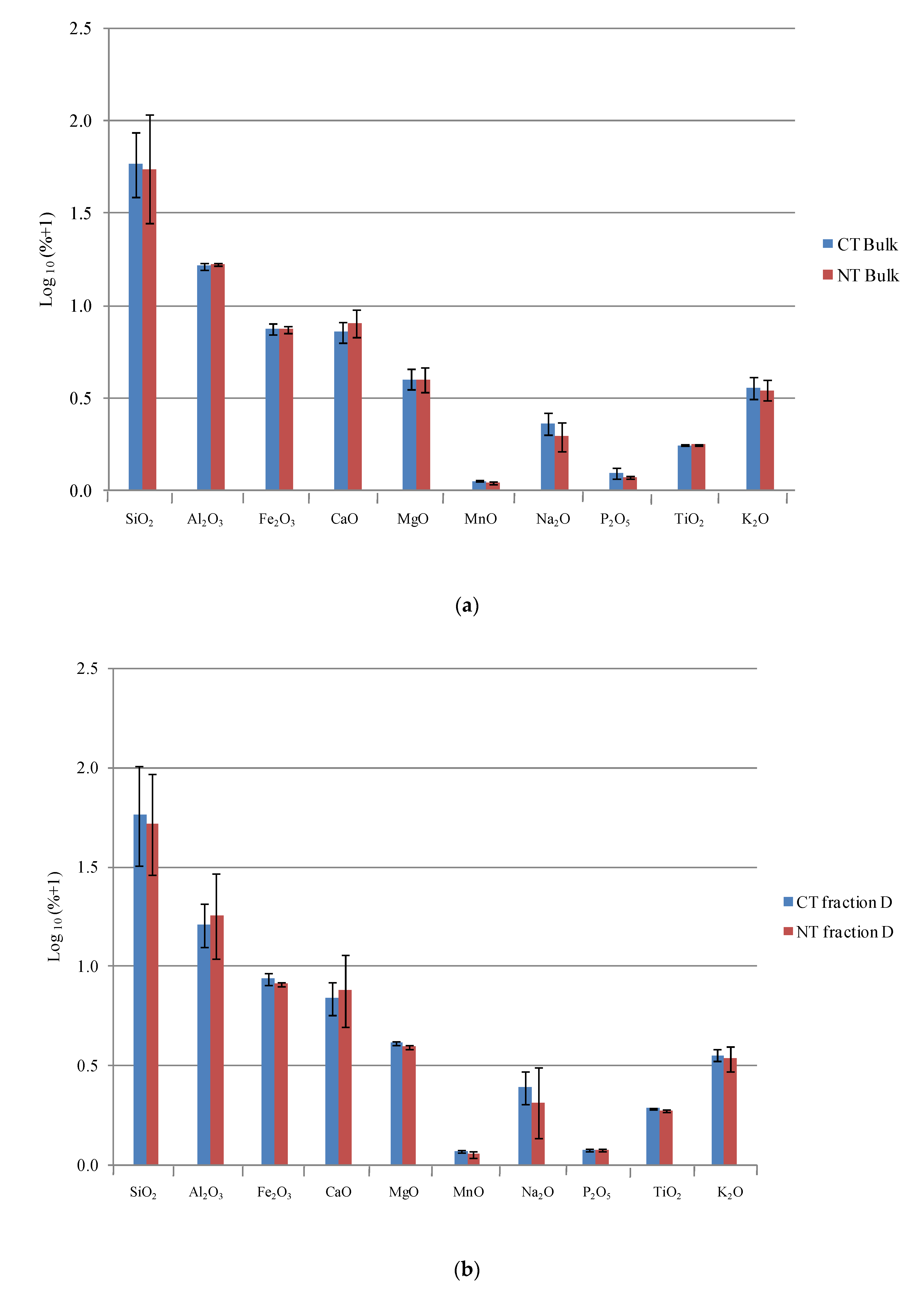
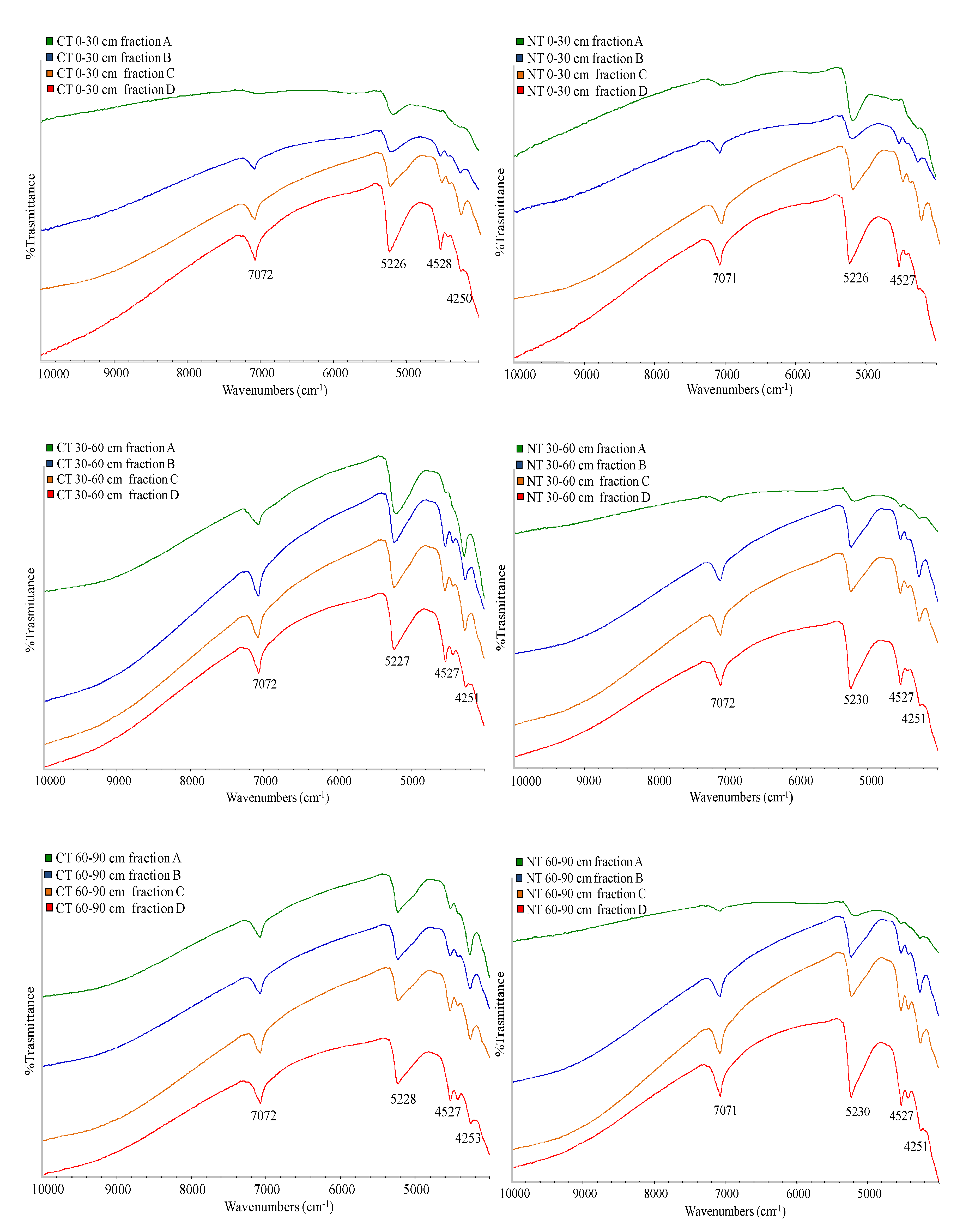
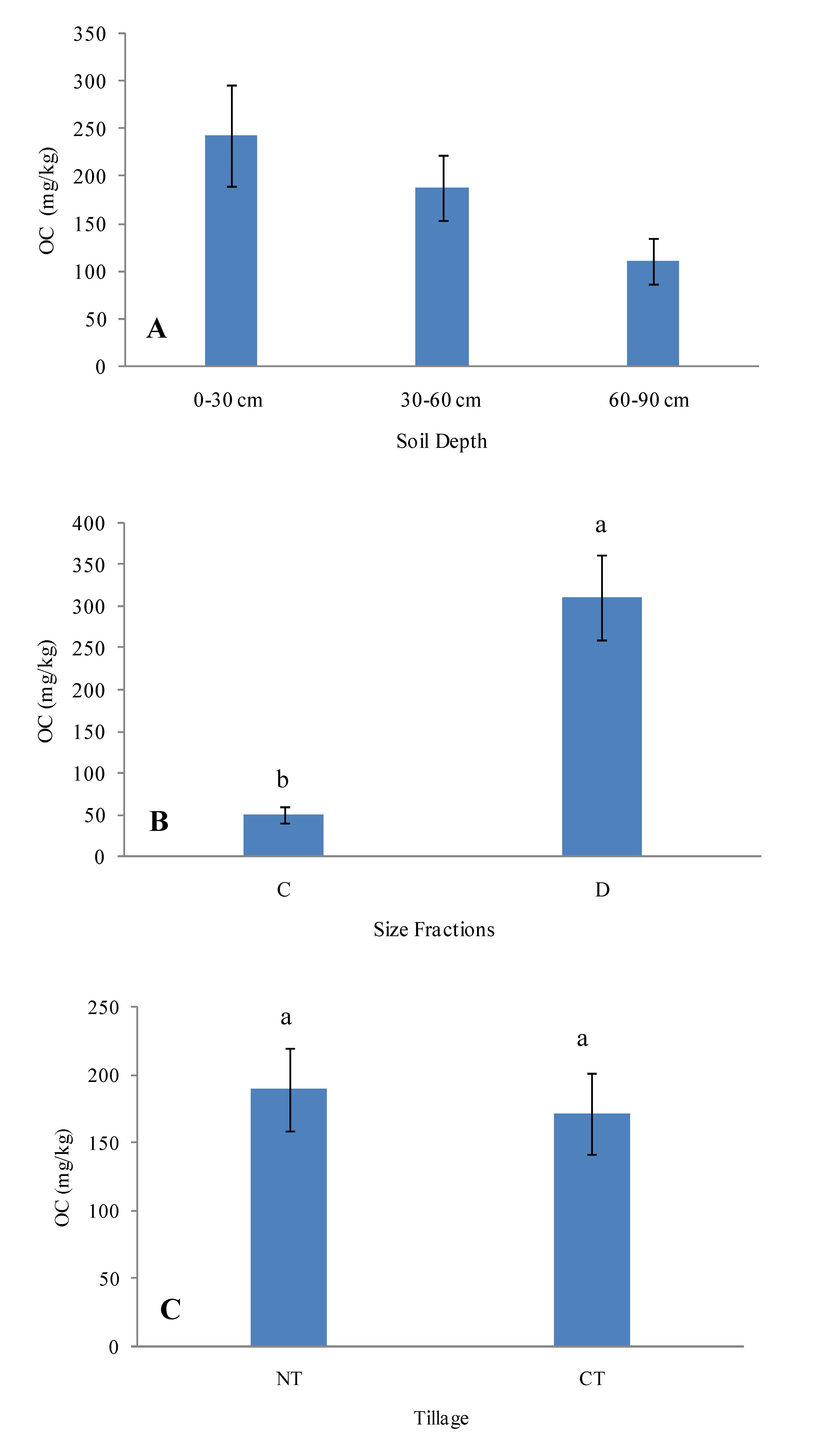
| SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI | Si/Al | Rb | Sr | Y | Zr | Nb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | ppm | ||||||||||||||||
| Size | *** | *** | *** | *** | * | * | *** | *** | *** | n.s. | *** | ** | *** | *** | *** | *** | *** |
| Depth | * | ** | n.s. | n.s. | n.s. | n.s. | * | * | n.s. | n.s. | n.s. | n.s. | n.s. | ** | * | n.s. | ** |
| Size | |||||||||||||||||
| Bulk | 50.3 bc (0.79) | 0.77 d (0.00) | 15.6 c (0.05) | 6.4 c (0.00) | 0.13 ab (0.00) | 2.9 b (0.00) | 8.3 a (0.07) | 0.8 b (0.00) | 2.4 c (0.00) | 0.1 a (0.00) | 12.3 a (0.12) | 3.2 a (0.01) | 97 c (2.3) | 206 a (28.43) | 22 d (0.13) | 144 b (12.35) | 15.0 d (0.05) |
| A | 25.5 a (0.40) | 0.16 a (0.00) | 3.4 a (0.01) | 2.4 a (0.00) | 0.09 a (0.00) | 1.8 a (0.00) | 35.9 c (0.33) | 0.3 a (0.00) | 0.5 a (0.00) | 0.3 a (0.00) | 29.5 b (0.29) | 7.6 b (0.03) | 23 a (0.55) | 546 b (75.34) | 6 a (0.04) | 36 a (3.08) | 3 a (0.01) |
| B | 52.9 bc (0.83) | 0.41 b (0.00) | 11.2 b (0.04) | 4.4 b (0.01) | 0.12 ab (0.00) | 2.6 ab (0.00) | 14.0 b (0.13) | 1.1 c (0.00) | 1.8 b (0.00) | 0.1 a (0.00) | 11.1 a (0.11) | 4.7 a (0.02) | 59 b (1.42) | 220 a (30.36) | 10 b (0.06) | 58 a (4.97) | 7 b (0.02) |
| C | 56.1 bc (0.88) | 0.54 c (0.00) | 12.7 b (0.05) | 4.8 b (0.01) | 0.11 ab (0.00) | 2.7 ab (0.00) | 10.4 ab (0.09) | 1.3 c (0.00) | 1.8 b (0.00) | 0.1 a (0.00) | 9.5 a (0.09) | 4.4 a (0.02) | 64 b (1.54) | 207 a (28.6) | 16 c (0.10) | 128 b (10.98) | 10 c (0.03) |
| D | 48.2 b (0.76) | 0.84 d (0.00) | 17.0 c (0.06) | 6.8 c (0.01) | 0.14 b (0.00) | 3.0 b (0.00) | 7.8 a (0.07) | 0.7 b (0.00) | 2.4 c (0.00) | 0.1 a (0.00) | 12.9 a (0.12) | 2.8 a (0.01) | 106 c (2.5) | 220 a (30.36) | 25 d (0.15) | 155 b (13.30) | 17 e (0.06) |
| Depth | |||||||||||||||||
| 0–30 | 48.9 b (0.60) | 0.57 b (0.00) | 12.2 a (0.03) | 5.1 a (0.00) | 0.12 a (0.00) | 2.5 a (0.00) | 13.4 a (0.09) | 0.9 b (0.00) | 1.8 a (0.00) | 0.2 a (0.00) | 14.2 a (0.11) | 4.5 a (0.03) | 72 a (1.33) | 250 a (26.75) | 17 b (0.08) | 118 b (7.84) | 11 b (0.03) |
| 30–60 | 46.7 ab (0.56) | 0.55 ab (0.00) | 11.9 a (0.03) | 5.0 a (0.00) | 0.12 a (0.00) | 2.5 a (0.00) | 15.3 ab (0.11) | 0.8 ab (0.00) | 1.8 a (0.00) | 0.2 a (0.00) | 15.0 a (0.12) | 4.4 a (0.03) | 69 a (1.28) | 276 a (29.53) | 16 ab (0.07) | 104 ab (6.91) | 10 a (0.03) |
| 60–90 | 44.2 a (0.54) | 0.51 a (0.00) | 11.8 a (0.03) | 4.8 a (0.00) | 0.12 a (0.00) | 2.8a (0.00) | 17.1 b (0.12) | 0.7 a (0.00) | 1.7 a (0.00) | 0.1 a (0.00) | 16.0 a (0.12) | 4.8 a (0.03) | 68 a (1.26) | 320 a (34.24) | 15 a (0.07) | 90 a (5.98) | 10 a (0.03) |
| Sm | Ill + Ms | Kln | Ch | Qz | Cal | Kfs + Pl | ΣC.M. | |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| Size | * | ** | ** | *** | ** | *** | n.s. | *** |
| Depth | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. |
| Size | ||||||||
| Bulk | 11 c | 19 b | 5 c | 19 cd | 21 a | 13 a | 12 a | 54 c |
| A | 2 a | 2 a | 0 a | 7 a | 18 a | 64 b | 8 a | 10 a |
| B | 6 ab | 16 b | 1 ab | 12 ab | 29 ab | 20 a | 16 a | 35 b |
| C | 5 ab | 19 b | 1 ab | 16 bc | 34 b | 15 a | 10 a | 40 b |
| D | 11 c | 19 b | 4 bc | 23 d | 20 a | 12 a | 12 a | 56 c |
| Depth | ||||||||
| 0–30 | 5 a | 15 a | 2 a | 17 a | 27 a | 22 a | 12 a | 39 a |
| 30–60 | 9 a | 14 a | 2 a | 15 a | 25 a | 25 ab | 11 a | 39 a |
| 60–90 | 7 a | 17 a | 2 a | 14 a | 22 a | 28 b | 10 a | 39 a |
| Al | Fe | Mn | |
|---|---|---|---|
| ppm | |||
| Depth | n.s. | n.s. | n.s. |
| Tillage | n.s. | n.s. | n.s. |
| Size | *** | *** | ** |
| Depth | |||
| 0–30 | 704 a | 545 a | 212 a |
| 30–60 | 450 a | 474 a | 161 a |
| 60–90 | 679 a | 466 a | 121 a |
| Tillage | |||
| NT | 539 a | 454 a | 141 a |
| CT | 716 a | 535 a | 189 a |
| Size | |||
| C | 238 a | 211 a | 97 a |
| D | 1017 b | 779 b | 232 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mastro, F.; Traversa, A.; Cocozza, C.; Pallara, M.; Brunetti, G. Soil Organic Carbon Stabilization: Influence of Tillage on Mineralogical and Chemical Parameters. Soil Syst. 2020, 4, 58. https://doi.org/10.3390/soilsystems4030058
De Mastro F, Traversa A, Cocozza C, Pallara M, Brunetti G. Soil Organic Carbon Stabilization: Influence of Tillage on Mineralogical and Chemical Parameters. Soil Systems. 2020; 4(3):58. https://doi.org/10.3390/soilsystems4030058
Chicago/Turabian StyleDe Mastro, Francesco, Andreina Traversa, Claudio Cocozza, Mauro Pallara, and Gennaro Brunetti. 2020. "Soil Organic Carbon Stabilization: Influence of Tillage on Mineralogical and Chemical Parameters" Soil Systems 4, no. 3: 58. https://doi.org/10.3390/soilsystems4030058
APA StyleDe Mastro, F., Traversa, A., Cocozza, C., Pallara, M., & Brunetti, G. (2020). Soil Organic Carbon Stabilization: Influence of Tillage on Mineralogical and Chemical Parameters. Soil Systems, 4(3), 58. https://doi.org/10.3390/soilsystems4030058








