Application of Nematode Community Analyses-Based Models towards Identifying Sustainable Soil Health Management Outcomes: A Review of the Concepts
Abstract
:1. Introduction
1.1. What Are the Characteristics of Sustainable Soil Health?
1.2. Why Nematodes Are Important to Soil Health?
1.3. Agriculture’s Footprint on Soil Health
- ●
- Approximately 46% of the maize and soybean acreage was high-yielding, 26% stable low yielding, and 28% unstable (variable) yielding.
- ●
- Low-yielding areas contributed ~44% and variable-yielding areas during years of poor yield 31% of total N loss to the environment.
- ●
- Total loss to farmers from overfertilization in low- and variable-yielding areas was ~$485 million. The loss in fertilizer value corresponded to greenhouse gas (GHG) of 6.8 MMT CO2 equivalents.
2. Conceptual Understanding of the Cycle of Soil Health Degradation
3. Barriers to Developing Sustainable Soil Health and How to Overcome the Gaps Using Nematodes
4. How the SFW Model Uses Nematodes to Identify Agroecosystem Suitability of Soil Conditions
4.1. Description of the Ferris et al. SFW Model
4.2. Examples of the SFW Model as a Decision-Making Tool to Identify Soil Health Conditions
4.3. Potential Use of the SFW Model as an Integration Platform for Soil Health Indicators
5. The FUE Model Analysis to Identify Integrated Efficiency Outcomes
5.1. The Concept and the Calculations
5.2. Visualization of the Outcomes
5.3. Examples of How the FUE Model Visualization Can Detect Hidden Patterns
6. Future Perspectives for Increased Implementation of the Models
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. Soil health and climate change: An overview. In Soil Health and Climate Change; Singh, B.P., Cowie, A.L., Chan, K.Y., Eds.; Springer: Berlin/Heidelberg, Germany; Dordrech, The Netherlands; London, UK; New York, NY, USA, 2011; pp. 3–24. [Google Scholar]
- Assefa, F.; Elias, E.; Soromessa, T.; Ayele, G. Effect of changes in land-use management practices on soil physiochemical properties in Kabe Watershed, Ethiopia. Air Soil Water Res. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Fagodiya, R.K.; Pathak, H.; Kumar, A.; Bhatia, A.; Jain, N. Global temperature change potential of N use in agriculture: A 50 year assessment. Sci. Rep. 2016, 7, 44928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. Climate Chang. 2013 Physical Science Basis; Contributing Working Group I to Fifth Assessment Representation. Intergovernmental Panel Climate Change 33; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Jankowski, K.; Neill, C.; Davidson, E.A.; Macedo, M.N.; Costa, C.; Galford, G.L.; Santos, L.M.; Lefebvre, P.; Nunes, D.; Cerri, C.E.; et al. Deep soils modify environmental consequences of increased nitrogen fertilizer use in intensifying Amazon agriculture. Sci. Rep. 2018, 8, 13478. [Google Scholar] [CrossRef] [Green Version]
- Millar, N.; Robertson, G.P.; Grace, P.R.; Gehl, R.J.; Hoben, J.P. Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (Maize) production: An emissions reduction protocol for US Midwest agriculture. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D.; Giampietro, M. Food, Land, Population and the US Economy. 1994. Available online: http://www.dieoff.com/page40.htm (accessed on 19 May 2021).
- Robertson, G.P.; Bruulsema, T.D.; Gehl, R.J.; Kanter, D.; Mauzerall, D.L.; Rotz, C.A.; Williams, C.O. Nitrogen-climate interactions in US agriculture. Biogeochemistry 2012, 114, 41–70. [Google Scholar] [CrossRef] [Green Version]
- Anon. Soil Health. NRCS; 2016. Available online: http://www.nrcs.usda.gov/wps/portal/nr.cs/main/soils/health/ (accessed on 19 May 2021).
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Moreno, S. Biodiversity and soil health: The role of the soil food web in soil fertility and suppressiveness to soil-borne diseases. Acta Hortic. 2018, 1196, 95–104. [Google Scholar] [CrossRef]
- The Soil Food Web and Laboratories in the Continental USA. Available online: https://www.soilfoodweb.com/ (accessed on 19 May 2021).
- Ferguson, R.B. Groundwater quality and nitrogen use efficiency in Nebraska’s central platte river valley. J. Environ. Qual. 2015, 44, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Lark, T.J.; Salmon, J.M.; Gibbs, H.K. Cropland expansion outpaces agricultural and biofuel policies in the United States. Environ. Res. Lett. 2015, 10, e044003. [Google Scholar] [CrossRef] [Green Version]
- Mladenoff, D.J.; Sahajpal, R.; Johnson, C.P.; Rothstein, R.E. Recent land use change to agriculture in the US lake states: Impacts on cellulosic biomass potential and natural lands. PLoS ONE 2016, 11, e0148566. [Google Scholar] [CrossRef]
- Beehler, J.; Fry, J.; Negassa, W.; Kravchenko, A.K. Impact of cover crop on soil carbon accrual in topographically diverse terrain. J. Soil. Water. Conserv. 2017, 72, 272–279. [Google Scholar] [CrossRef] [Green Version]
- Brainard, D.C.; Noyes, D.C. Strip-tillage and compost influence carrot quality, yield and net returns. HortScience 2012, 47, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Melakeberhan, H.; Mennan, S.; Grewal, P.S. Relationship between soybean cyst nematode Heterodera glycines and soil nematode community under long-term tillage and crop rotation. Nematropica 2018, 48, 101–115. [Google Scholar]
- Collins, H.P.; Alva, A.; Bydston, R.A.; Cochran, R.L.; Hamm, P.B.; McGuire, A.; Riga, E. Soil microbial, fungal, and nematode responses to soil fumigation and cover crops under potato production. Biol. Fertil. Soils 2006, 42, 247–257. [Google Scholar] [CrossRef]
- García-Orenes, F.; Morugán-Coronado, A.; Zornoza, R.; Scow, K. Changes in soil microbial community structure influenced by agricultural management practices in a Mediterranean agro-ecosystem. PLoS ONE 2013, 8, e80522. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Bert, W.; Maenhout, P.; Sleutel, S.; De Neve, S. Quantifying the contribution of entire free-living nematode communities to carbon mineralization under contrasting C and N availability. PLoS ONE 2015, 10, e0136244. [Google Scholar] [CrossRef] [Green Version]
- Habteweld, A.W.; Brainard, D.C.; Kravchenko, A.N.; Grewal, P.S.; Melakeberhan, H. Effects of plant and animal waste-based compost amendments on soil food web, soil properties, and yield and quality of fresh market and processing carrot cultivars. Nematology 2018, 20, 147–168. [Google Scholar] [CrossRef]
- Helms, I.V.; Ijelu, J.A.; Willis, B.D.; Landis, D.A.; Haddad, N.M. Ant biodiversity and ecosystem services in bioenergy landscapes. Agr. Ecosyst. Environ. 2020, 290. [Google Scholar] [CrossRef]
- Toosi, E.R.; Kravchenko, A.N.; Guber, A.K.; Rivers, M.L. Pore characteristics regulate priming and fate of carbon from plant residue. Soil Biol. Biochem. 2017, 113, 219–230. [Google Scholar] [CrossRef]
- The 4R Principles of Nutrient Management—Do You Really Know Them? Meister Media Worldwide: Willoughby, OH, USA, 2021; Available online: https://www.croplife.com/special-reports/crop-nutrition/4r-principles-nutrient-management-really-know/ (accessed on 10 March 2021).
- Basso, B.G.; Zhang, S.J.; Robertson, G.P. Yield stability analysis reveals sources of large-scale nitrogen loss from the US Midwest. Nat. Sci. Rep. 2019, 10, 5774. [Google Scholar] [CrossRef]
- Kang, G.S.; Beri, V.; Sidhu, B.S.; Rupela, O.P. A new index to assess soil quality and sustainability of wheat-based cropping systems. Biol. Fertil. Soils. 2005, 41, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Melakeberhan, H. Fertiliser use efficiency of soybean cultivars infected with Meloidogyne incognita and Pratylenchus penetrans. Nematology 2006, 8, 129–137. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Avendaño, M.F. Spatio-temporal consideration of soil conditions and site-specific management of nematodes. Precis. Agric. 2008, 9, 341–354. [Google Scholar] [CrossRef]
- Melakeberhan, H. Cross-disciplinary efficiency assessment of agronomic and soil amendment practices designed to suppress biotic yield-limiting factors. J. Nematol. 2010, 42, 73–74. [Google Scholar] [PubMed]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.H.; Endale, M.B.; Coleman, D.C.; Whitman, W.B. Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Schutter, M.E.; Sandeno, J.M.; Dick, R.P. Seasonal, soil type, and alternative management influences on microbial communities of vegetable cropping systems. Biol. Fertil. Soils. 2001, 34, 397–410. [Google Scholar]
- Glavatska, O.; Muller, K.; Boutenschoen, O.; Schmalwasser, A.; Kandeler, E.; Scheu, S.; Totsche, K.U.; Ruess, L. Disentangling the root- and detritus-based food chain in the micro-food webs of an arable soil by plant removal. PLoS ONE 2017, 13, e0180264. [Google Scholar] [CrossRef] [Green Version]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Evol. Ecol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Van der Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; De Goede, R.G.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Yeates, G.W. Modification and qualification of the nematode maturity index. Pedobiologia 1995, 38, 97–101. [Google Scholar]
- Yeates, G.W.; Bongers, T.; de Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.C.; Scow, K.M. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralization function. Appl. Soil Ecol. 2004, 24, 19–35. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Chen, S. Influence of long-term corn-soybean crop sequences on soil ecology as indicated by the nematode community. Appl. Soil Ecol. 2016, 100, 172–185. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Maung, Z.T.Z.; Noyes, C.; Baas, D.; Werling, B.P.; Brainard, D.C.; Melakeberhan, H. Effects of cover crops on Pratylenchus penetrans and the nematode community in carrot production. J. Nematol. 2017, 49, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs-Hostyanszki, A.; Elek, Z.; Balazs, K.; Centeri, C.; Falusi, E.; Jeanneret, P.; Penksza, K.; Podmaniczky, L.; Szalkovszki, O.; Baldi, A. Earthworms, spiders and bees as indicators of habitat quality and management in low-input farming region—A whole farm approach. Ecol. Indic. 2013, 33, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Fixen, P.; Brentrup, F.; Bruulsema, T.W.; Garcia, F.; Norton, R.; Zingore, S. Nutrient/fertilizer use efficiency: Measurements, current situation and trends. In Managing Water and Fertilizer for Sustainable Agricultural Intensification, 1st ed.; Drechsel, P., Heffer, P., Magen, H., Mikkelsen, R., Wichelns, D., Eds.; International Fertilizer Industry Association, International Water Management Institute, International Plant Nutrition Institute, and International Potash Institute: Paris, France, 2011; pp. 8–38. Available online: http://www.ipni.net/ipniweb/portal.nsf/0/B9C003FF28F9C9EF85257DE1007607CC/$FILE/2015_ifa_ipni_iwmi_ipi.pdf (accessed on 19 May 2021).
- Melakeberhan, H. Effect of starter nitrogen on soybeans under Heterodera glycines infestation. Plant Soil. 2007, 301, 111–121. [Google Scholar] [CrossRef]
- Bulluck, L.R., III; Barker, K.R.; Ristaino, J.B. Influences of organic and synthetic soil fertility amendments on nematode trophic groups and community dynamics under tomatoes. Appl. Soil Ecol. 2002, 21, 233–250. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Snapp, S.S.; Robertson, G.P. Field-scale experiments reveal persistent yield gaps in low-input and organic cropping systems. Proc. Natl. Acad. Sci. USA 2017, 114, 926–931. [Google Scholar] [CrossRef] [Green Version]
- Carrera, L.M.; Buyer, J.S.; Vinyard, B.; Abdul-Baki, A.A.; Sikora, L.J.; Teasdale, J.R. Effects of cover crops, compost and manure amendments on soil microbial community structure in tomato production systems. Appl. Soil Ecol. 2007, 37, 247–255. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Wang, W.; Kravchenko, A.; Thelen, K. Effects of agronomic practices on the timeline of Heterodera glycines establishment in a new location. Nematology 2015, 17, 705–713. [Google Scholar] [CrossRef]
- Miguez, F.E.; Bollero, G.A. Review of corn yield response under winter cover cropping systems using metadata analytic methods. Crop Sci. 2005, 45, 2318–2329. [Google Scholar] [CrossRef] [Green Version]
- Renco, M.; Gomoryova, E.; Cerevkova, A. The effect of soil type and ecosystems on the soil nematode and microbial communities. Helminthologia 2020, 57, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.W.; Brenneman, T.; Bonito, G.; Smith, M.E. Soil pH and mineral nutrients strongly influence truffles and other ectomycorrhizal fungi associated with commercial pecans (Carya illinoinensis). Plant Soil. 2017, 418, 493–505. [Google Scholar] [CrossRef]
- Bonito, G.; Reynolds, H.; Hodkinson, B.; Nelson, J.; Tuskan, G.; Robeson, M.; Schadt, C.; Vilgalys, R. Plant host and soil origin influence fungal and bacterial assemblages in the rhizosphere of woody plants. Mol. Ecol. 2014, 23, 3356–3370. [Google Scholar] [CrossRef] [PubMed]
- World Fertilizer Trends and Outlook to 2022; FAO: Rome, Italy, 2018; Available online: http://www.fao.org/3/ca6746en/ca6746en.pdf (accessed on 19 May 2021).
- Adesemoye, A.O.; Kloepper, J.W. Plant-microbe interactions in enhanced fertilizer-use efficiency-Mini-review. Appl. Microbio. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chagas, W.F.T.; Emrich, E.B.; Guelfi, D.R.; Caputo, A.L.C.; Faquin, V. Productive characteristics, nutrition and agronomic efficiency of polymer-coated MAP in lettuce crops. Cienc. Agron. 2015, 46, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Nissen, T.M.; Wander, M.M. Management and soil-quality effects on fertilizer-use efficiency and leaching. Soil Sci. Soc. Am. J. 2003, 67, 1524–1532. [Google Scholar] [CrossRef] [Green Version]
- Olk, D.C.; Cassman, K.G.; Simbaha, G.; Sta Cruz, P.C.; Abdulrachman, S.; Nagarajan, R.; Tan, P.S.; Satawathananon, S. Interpreting fertilizer-use efficiency in relation to soil nutrient-supplying capacity, factor productivity, and agronomic efficiency. Nutr. Cycl. Agroecosyst 1999, 53, 35–41. [Google Scholar] [CrossRef]
- Biesiada, A.; Koota, E. The Effect of nitrogen fertilization on yield and quality of Radicchio. J. Elementol. 2008, 13, 175–180. [Google Scholar]
- Blanc, C.; Sy, M.; Djigal, D.; Brauman, A.; Normand, P.; Villenave, C. Nutrition on bacteria by bacterial-feeding nematodes and consequences on the structure of soil microbial community. Eur. J. Soil Biol. 2006, 42, S70S78. [Google Scholar] [CrossRef]
- Gibson, J.; Shokralla, S.; Porter, T.M.; King, I.; van Konynenburg, S.; Janzen, D.H.; Hallwachs, W.; Hajibabaei, M. Simultaneous assessment of the macrobiome and microbiome in a bulk sample of tropical arthropods through DNA metasystematics. Proc. Natl. Acad. Sci. USA 2014, 111, 8007–8012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riga, E.; Mojtahedi, H.; Ingham, R.E.; McGuire, A.M. Green manure amendments and management of root knot nematodes on potato in the Pacific Northwest of USA. Nematol. Monogr. Perspect. 2003, 2, 151–158. [Google Scholar]
- Schorpp, Q.; Schrader, S. Earthworm functional groups respond to the perennial energy cropping system of the cup plant (Silphium perforliatum). Biomass Bioenergy 2016, 87, 61–68. [Google Scholar] [CrossRef]
- Toosi, E.R.; Kravchenko, A.N.; Quigley, M.M.; Mao, J.; Rivers, M.L. Effects of management and pore characteristics on organic matter composition of macroaggregates, evidence from X-ray µ-tomography, FTIR and 13C-NMR. Eur. J. Soil Sci. 2017, 68, 200–211. [Google Scholar] [CrossRef]
- van Leeuwen, J.P.; Djukic, I.; Bloem, J.; Lehtinen, T.; Hemerick, L.; de Ruiter, P.C.; Lair, G.J. Effects of land use on soil microbial biomass, activity and community structure at different soil depth in the Danube floodplain. Eur. J. Soil Biol. 2017, 79, 14–20. [Google Scholar] [CrossRef]
- Wang, K.H.; McSorley, R.; Kokalis-Burelle, N. Effects of cover cropping, solarization, and fumigation on nematode communities. Plant Soil. 2006, 286, 229–243. [Google Scholar] [CrossRef]
- Wang, K.H.; Radovich, T.; Pant, A.; Cheng, Z. Integration of cover crops and vermicompost tea for soil and plant health management in a short-term vegetable cropping system. Appl. Soil Ecol. 2014, 82, 26–37. [Google Scholar] [CrossRef]
- Wickings, K.; Grandy, A.S.; Kravchenko, A.N. Going with the flow: Landscape position drives differences in microbial biomass and activity in conventional, low input, and organic agricultural systems in the Midwestern, U.S. Agric. Ecosyst. Environ. 2016, 218, 1–10. [Google Scholar] [CrossRef]
- Xiao, H.; Li, G.; Li, D.-M.; Hu, F.; Li, H.-X. Effect of different bacterial feeding nematode species on soil bacterial numbers, activity and community composition. Pedosphere 2014, 24, 116–124. [Google Scholar] [CrossRef]
- Zhang, X.; Ferris, H.; Mitchell, J.; Liang, W. Ecosystem services of the soil food web after long-term application if agricultural management practices. Soil Biol. Biochem. 2017, 111, 36–43. [Google Scholar] [CrossRef]
- Emery, S.M.; Reid, M.L.; Bell-Dereske, L.; Gross, K.L. Soil mycorrhizal and nematode diversity vary in response to bioenergy crop identity and fertilization. Glob. Chang. Biol. Bioenergy 2017, 9, 1644–1656. [Google Scholar] [CrossRef] [Green Version]
- Kokalis-Burelle, N.; Mahaffee, W.F.; Rodriguez-Kabana, R.; Klopper, J.W.; Brown, K.L. Effects of switchgrass (Panicum virgatum) rotations with peanut (Arachis hypogaea L.) on nematode populations and soil microflora. J. Nematol. 2002, 34, 98–105. [Google Scholar]
- Jian, J.; Du, X.; Stewart, R.D. A database for global soil health assessment. Nature. Sci. Data 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wander, M.M.; Cihacek, L.J.; Coyne, M.; Drijber, R.A.; Grossman, J.M.; Gutknecht, J.L.M.; Horwath, W.R.; Jagandamma, S.; Olk, D.C.; Ruark, M.; et al. Developments in agricultural soil quality and health: Reflections by the research committee on soil organic matter management. Front. Environ. Sci. 2019, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fine, A.K.; van Es, H.M.; Schindelbeck, R.R. Statistics, Scoring Functions, and Regional Analysis of a Comprehensive Soil Health Database. Soil Sci. Soc. Am. J. 2017, 81, 589. [Google Scholar] [CrossRef]
- Kihara, J.; Bolo, P.; Kinyua, M.; Nyawira, S.S.; Sommer, R. Soil health and ecosystem services: Lessons from sub-Saharan Africa. Geoderma 2019, 370, 141342. [Google Scholar]
- Stewart, Z.P.; Pierzynski, G.M.; Middendorf, B.J.; Prasad, P.V.V. Approaches to improve soil fertility in sub-Saharan Africa. J. Exp. Bot. 2020, 71, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Hu, F.; Li, H. Spatial ecology of soil nematodes: Perspectives from global to micro scales. Soil Biol. Biochem. 2019, 137, 107565. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Maung, Z.T.Z.; Lee, C.-L.; Poindexter, S.; Stewart, J. Soil type-driven variable effects on cover- and rotation-crops, nematodes and soil food web in sugar beet fields reveal a roadmap for developing healthy soils. Eur. J. Soil Biol. 2018, 85, 53–63. [Google Scholar] [CrossRef]
- Thuo, A.K.; Karuku, G.N.; Kimenju, J.W.; Kariuku, G.M.; Wendot, P.K.; Melakeberhan, H. Factors influencing the relationship between nematode communities and edaphic factors on selected soil groups in Kenya: Vertisols, Cambisols and Arenosols. Trop. Subtrop. Agroecosyst. 2020, 23, 49. [Google Scholar]
- Domene, X.; Mattana, S.; Sanchez-Moreno, S. Biochar addition rate determines contrasting shifts in soil nematode trophic groups in outdoor mesocosms: An appraisal of underlying mechanisms. Appl. Soil Ecol. 2021, 158, 103788. [Google Scholar] [CrossRef]
- Habteweld, A.; Brainard, D.; Kravchenko, A.; Parwinder, P.S.; Melakeberhan, H. Characterizing nematode communities in carrot fields and their bioindicator role for soil health. Nematropica 2020, 50, 201–210. [Google Scholar]
- Habteweld, A.; Brainard, D.; Kravchenko, A.; Parwinder, P.S.; Melakeberhan, H. Effects of integrated application of plant-based compost and urea on soil food web, soil properties, and yield and quality of a processing carrot cultivar. J. Nematol. 2020, 52. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Maung, Z.; Lartey, I.; Yildiz, S.; Gronseth, J.; Qi, J.; Karuku, G.N.; Kimenju, J.W.; Kwoseh, C.; Adjei-Gyapong, T. Nematode community-based soil food web analysis of Ferralsol, Lithosol and Nitosol soil groups in Ghana, Kenya and Malawi reveals distinct soil health degradations. Diversity 2021, 13, 101. [Google Scholar] [CrossRef]
- Moore-Kucera, J.; Azarenko, A.N.; Brutcher, L.; Chozinski, A.; Myrold, D.D.; Ingham, R. In search of key soil functions to assess soil community management for sustainable sweet cherry orchards. HortScience 2008, 43, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Kravchenko, A.N.; Guber, A.K.; Rasavi, B.S.; Koestel, J.; Quigley, M.Y.; Robertson, G.P.; Kuzyakov, Y. Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Comm. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Publications—Soil Food Web School; Soil Foodweb School LLC: Corvallis, OR, USA, 2021; Available online: https://www.soilfoodweb.com/publications/ (accessed on 10 March 2021).
- State of Knowledge of Soil Biodiversity—Status, Challenges and Potentialities (Fao.org); FAO: Rome, Italy, 2020; Available online: http://www.fao.org/3/cb1928en/CB1928EN.pdf (accessed on 10 March 2021).
- Lišková, M.; Čerevková, A.; Hanel, L. Nematode communities of forest ecosystems in association with various soil orders. Russ. J. Nematol. 2008, 16, 129–142. [Google Scholar]
- Lima da Silva, J.V.C.; Hirschfeld, M.N.C.; Cares, J.E.; Esteves, A.M. Land use, soil properties and climate variables influence the nematode communities in the Caatinga dry forest. Appl. Soil Ecol. 2019, 103474. [Google Scholar] [CrossRef]
- Lazarova, S.; Coyne, D.; Rodríguez, M.G.; Peteira, B.; Ciancio, A. Functional diversity of soil nematodes in relation to the impact of agriculture—A review. Diversity 2021, 13, 64. [Google Scholar] [CrossRef]
- Baveye, P.C.; Otten, W.; Kravchenko, A.; Balseiro-Romero, M.; Beckers, É.; Chalhoub, M.; Darnault, C.; Eickhorst, T.; Garnier, P.; Hapca, S.; et al. Emergent properties of microbial activity in heterogeneous soil microenvironments: Different research approaches are slowly converging, yet major challenges remain. Frontiers in Microbiology, for special topic Elucidating Microbial Processes in Soils and Sediments. Micro. Measu. Model. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lallias, D.; Hiddink, J.G.; Fonseca, V.G.; Gaspar, J.M.; Sung, W.; Neill, S.P.; Barnes, N.; Ferrero, T.; Hall, N.; Lambshead, P.J.D.; et al. Environmental metabarcoding reveals heterogeneous drivers of microbial eukaryote diversity in contrasting estuarine ecosystems. Int. Soc. Micro. Ecol. J. 2015, 9, 1208–1221. [Google Scholar] [CrossRef]
- Peraza-Padilla, W.; Archidona-Yuste, A.; Ferris, H.; Zamora-Araya, T.; Cantalapiedra-Navarrete, C.; Palmores-Rius, J.E.; Subbotin, S.A.; Castillo, P. Molecular characterization of pseudomonodelphic dagger nematodes of the genus Xiphinema Cobb, 1913 (Nematoda: Longidoridae) in Costa Rica, with notes on Xiphinema setariae Tarjan, 1964. Eur. J. Plant Pathol. 2017, 148, 739–747. [Google Scholar] [CrossRef]
- Powers, T.O.; Bernard, E.C.; Harris, T.; Higgins, R.; Olson, M.; Olson, S.; Lodema, M.; Matczyszyn, J.; Mullin, P.; Sutton, L.; et al. Species discovery and diversity in Lobocriconema (Criconematidae: Nematoda) and related plant-parasitic nematodes from North American ecoregions. Zootaxa 2016, 4085, 301–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Omita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonito, G.; Hameed, K.; Krishnan, J.; Ventura, R.; Vilgalys, R. Isolating a functionally relevant guild of fungi from the root microbiome of Populus. Fungal Ecol. 2016, 22, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Uehling, J.; Gryganskyi, A.; Hameed, K.; Tschaplinski, T.; Misztal, P.K.; Wu, S.; Desirò, A.; Vande Pol, N.; Du, Z.; Zienkiewicz, A.; et al. Comparative Genome sequencing reveals origins of a unique bacterial endosymbiosis in the earliest lineages of terrestrial Fungi. Environ Micro. 2017, 19, 2964–2983. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.; Noel, Z.A.; Benucci, G.M.N.; Chilvers, M.I.; Trail, F.; Bonito, G. Crop management impacts the soybean (Glycine max) microbiome. Front. Micro. 2020, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Habteweld, A.W. Assessing the Impact of Compost Amendment for Managing Nematodes and the Health of Mineral Soil under carrot Production. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2015. [Google Scholar]





| SG | Country | SI ¥ | EI |
|---|---|---|---|
| FL | Ghana | 77.8 ± 6.6 ab | 18.5 ± 4.1 ed |
| Kenya | 68.2 ± 4.9 bc | 30.6 ± 2.8 abc | |
| Malawi | 31.9 ± 4.9 d | 36.0 ± 2.8 ab | |
| LL | Ghana | 88.3 ± 5.8 a | 28.3 ± 3.4 abc |
| Kenya | 62.4 ± 4.9 bc | 26.0 ± 2.8 cd | |
| Malawi | 52.1 ± 5.0 c | 37.0 ± 2.9 a | |
| NL | Ghana | 77.7 ± 4.9 ab | 27.7 ± 2.8 bcd |
| Kenya | 62.8 ± 4.9 bc | 10.8 ± 2.8 e | |
| Malawi | 42.1 ± 5.4 d | 31.3 ± 3.3 abc |
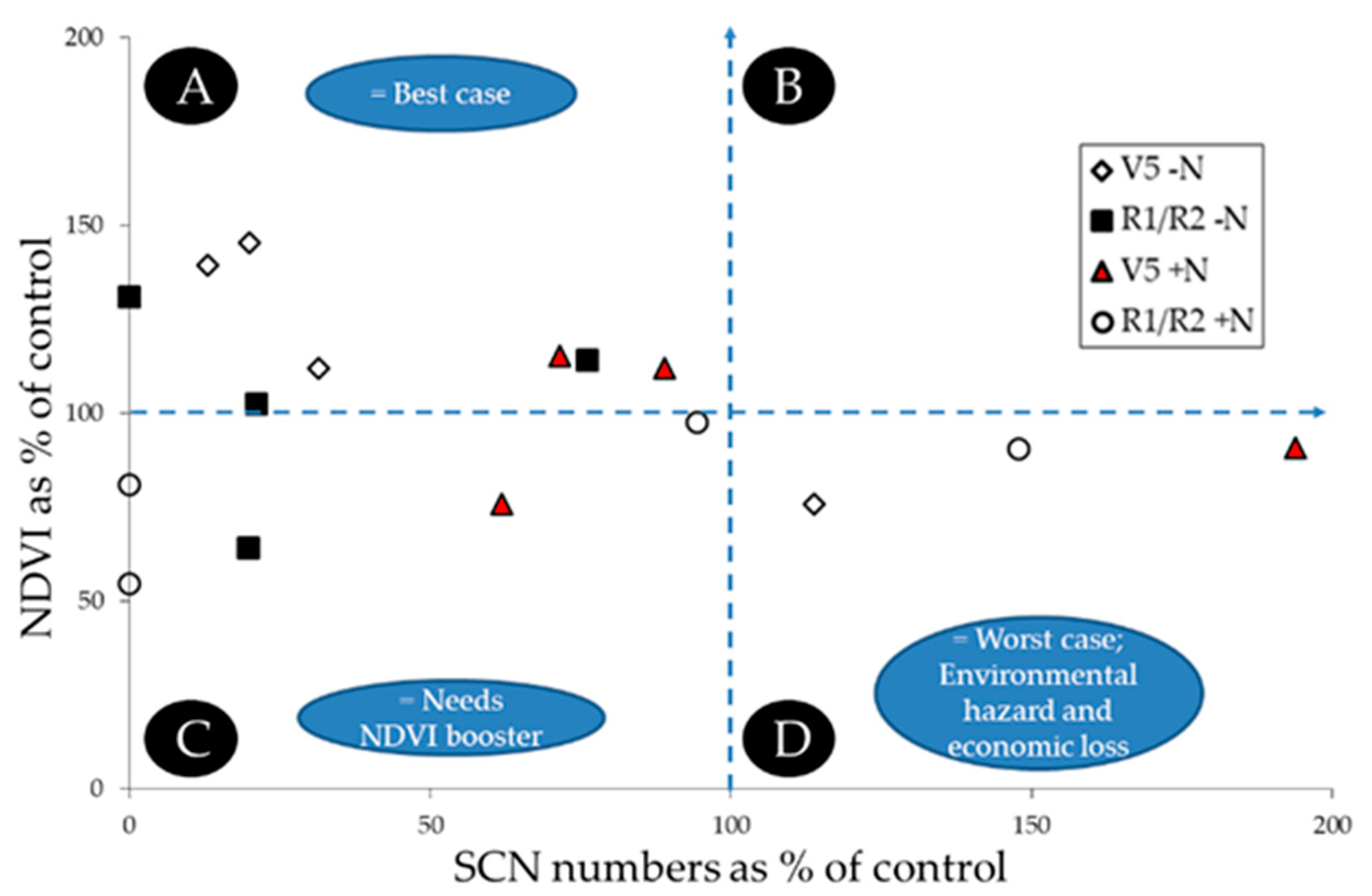
| Factor | SCN ¥ | NDVI | SCN | NDVI | |
|---|---|---|---|---|---|
| Measured Values | AS % of Control | ||||
| Nutrient (Nu) | Control | 2420 a | 0.373 a | na | na |
| No N | 991 a | 0.411 a | 36.9 a | 110.3 a | |
| Plus N | 2269 a | 0.333 a | 82.3 a | 89.8 a | |
| Time (T) | V 5 | 3062 a | 0.348 a | 74.3 a | 108.3 a |
| R1-R2 | 725 b | 0.396 a | 44.9 a | 91.9 a | |
| Nu*T | 0.5398 | 0.6988 | 0.6205 | 0.9296 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melakeberhan, H.; Bonito, G.; Kravchenko, A.N. Application of Nematode Community Analyses-Based Models towards Identifying Sustainable Soil Health Management Outcomes: A Review of the Concepts. Soil Syst. 2021, 5, 32. https://doi.org/10.3390/soilsystems5020032
Melakeberhan H, Bonito G, Kravchenko AN. Application of Nematode Community Analyses-Based Models towards Identifying Sustainable Soil Health Management Outcomes: A Review of the Concepts. Soil Systems. 2021; 5(2):32. https://doi.org/10.3390/soilsystems5020032
Chicago/Turabian StyleMelakeberhan, Haddish, Gregory Bonito, and Alexandra N. Kravchenko. 2021. "Application of Nematode Community Analyses-Based Models towards Identifying Sustainable Soil Health Management Outcomes: A Review of the Concepts" Soil Systems 5, no. 2: 32. https://doi.org/10.3390/soilsystems5020032
APA StyleMelakeberhan, H., Bonito, G., & Kravchenko, A. N. (2021). Application of Nematode Community Analyses-Based Models towards Identifying Sustainable Soil Health Management Outcomes: A Review of the Concepts. Soil Systems, 5(2), 32. https://doi.org/10.3390/soilsystems5020032





