A Test of the Inadvertent Uptake Hypothesis Using Plant Species Adapted to Serpentine Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Experimental Design
2.3. Tissue Collection and Chemical Analysis
2.4. Statistical Analysis
3. Results
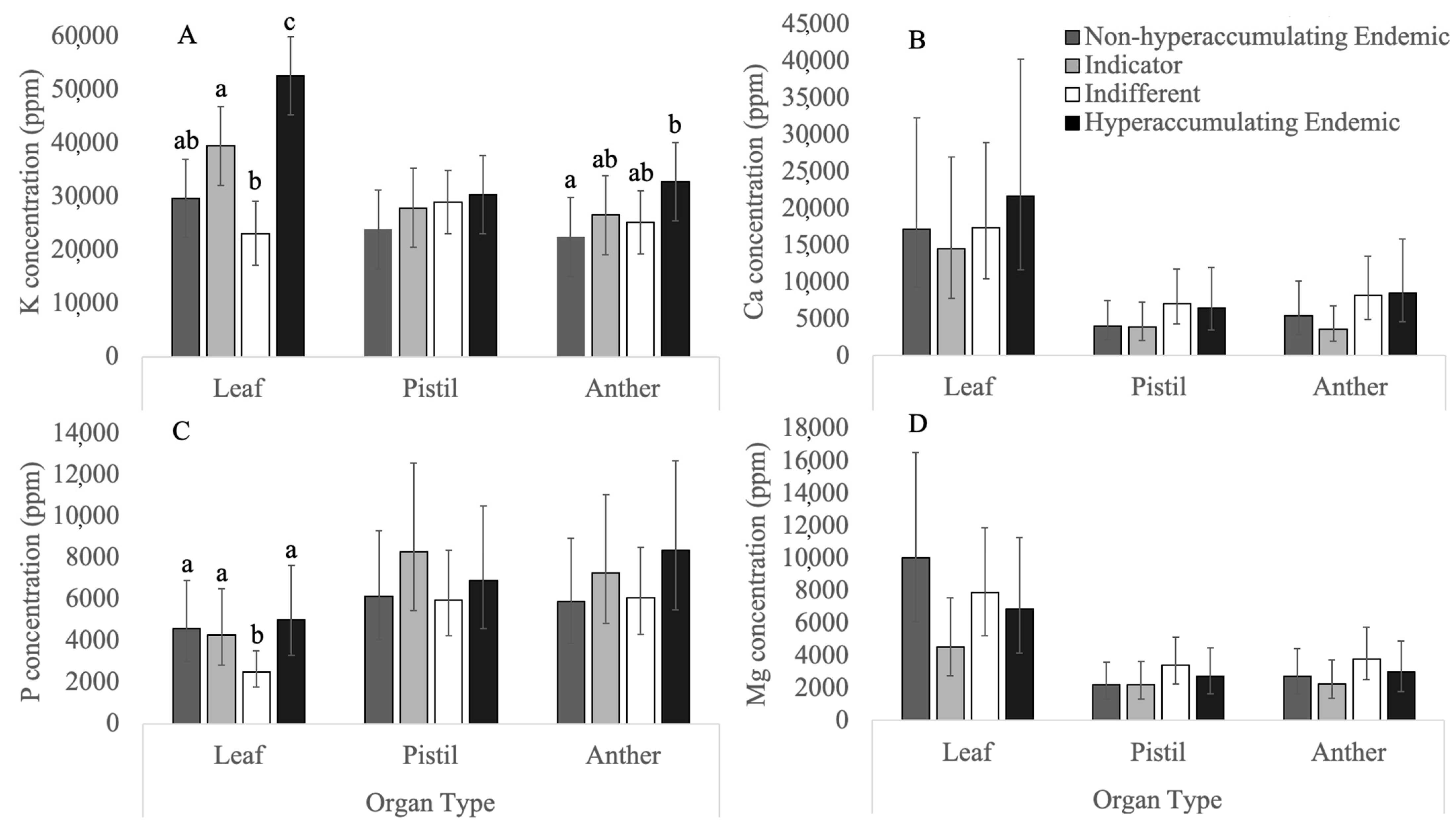
3.1. Macronutrients
3.1.1. Potassium
3.1.2. Phosphorus
3.1.3. Calcium
3.1.4. Magnesium
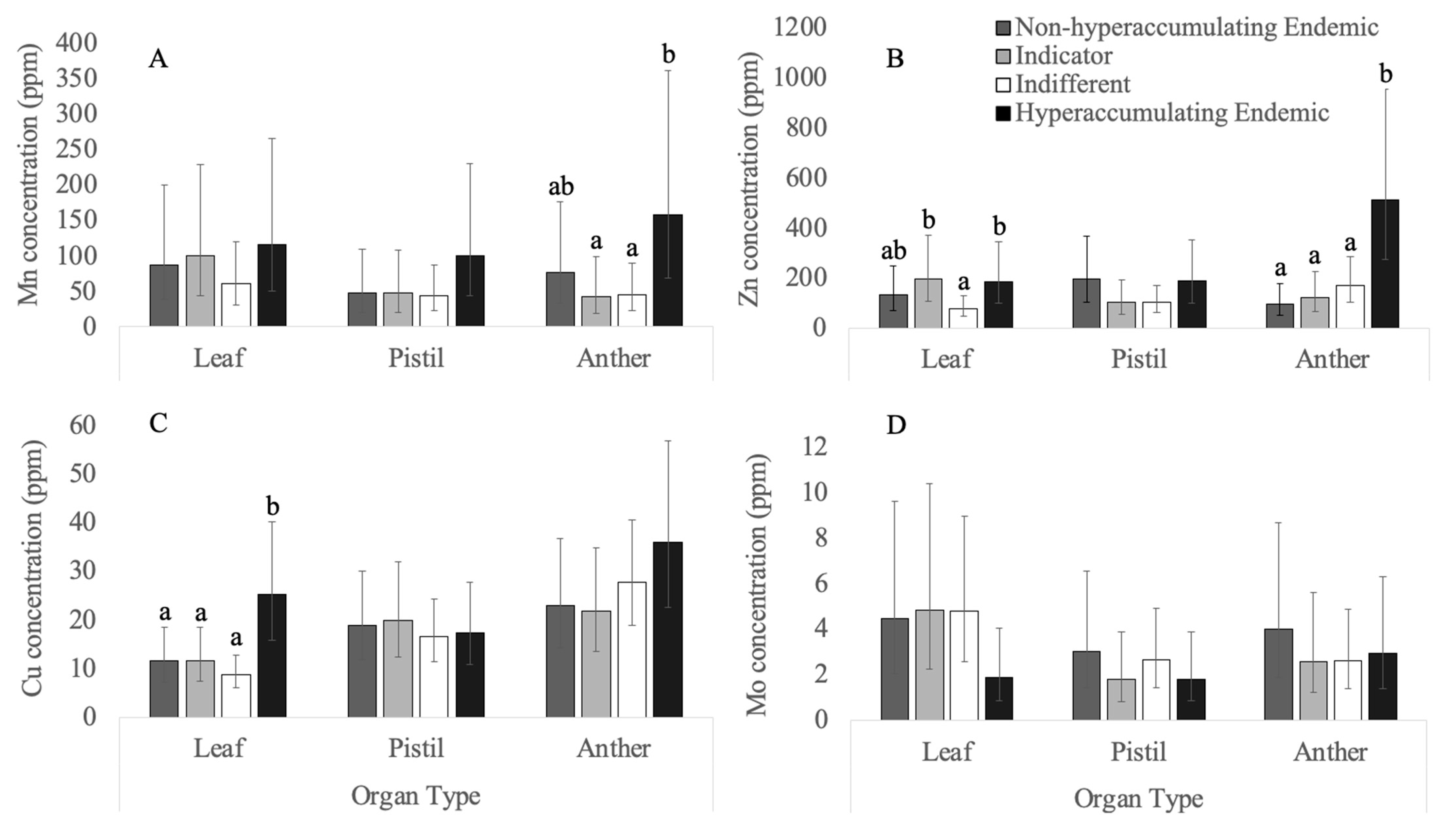
3.2. Micronutrients
3.2.1. Copper
3.2.2. Manganese
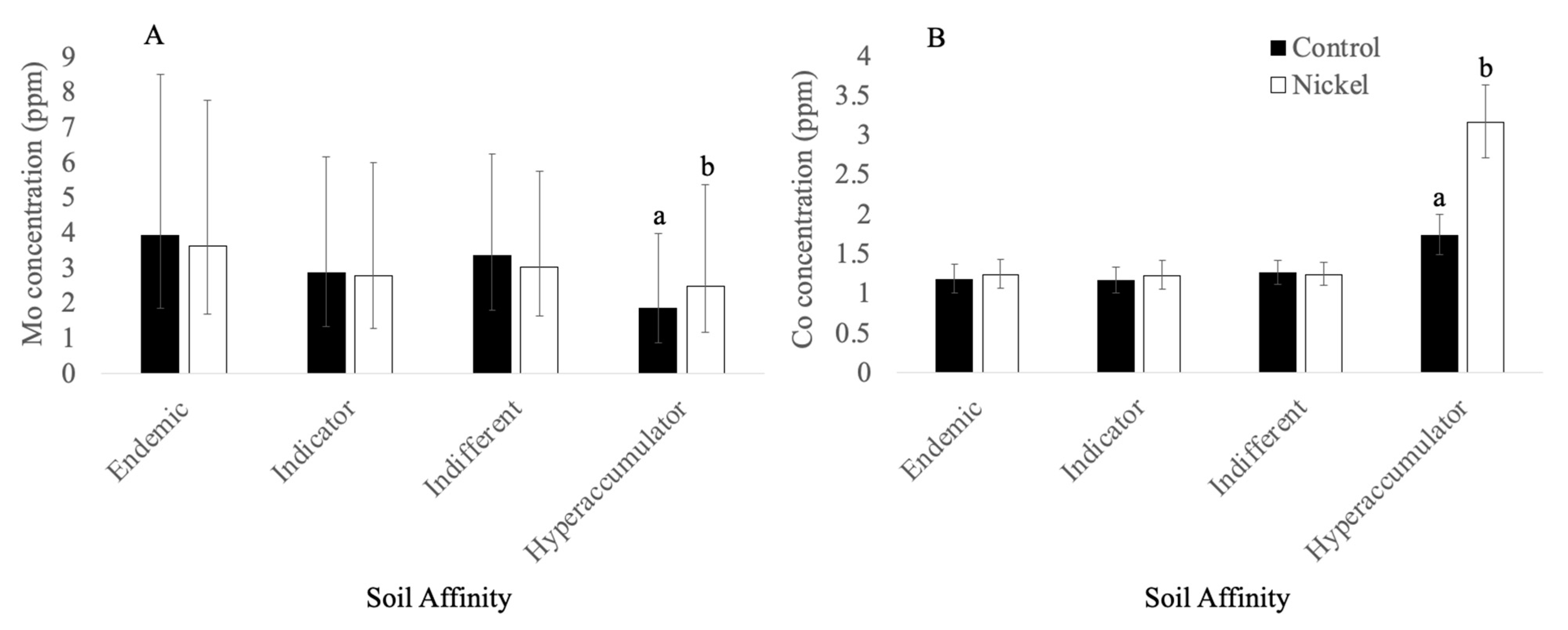
3.2.3. Molybdenum
3.2.4. Zinc
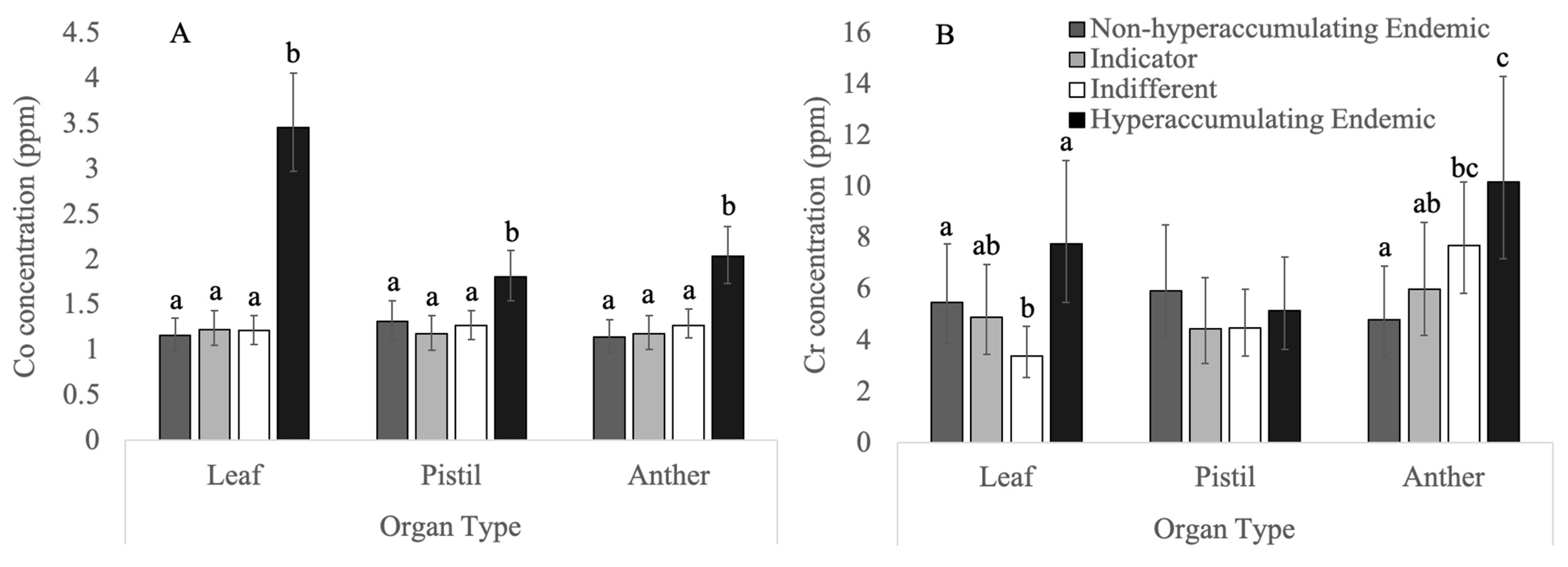
3.3. Non-Essential Heavy Metals
3.3.1. Chromium
3.3.2. Cobalt
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary ecology of plant adaptation to serpentine soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Harrison, S.; Rajakaruna, N. Serpentine: The Evolution and Ecology of a Model System; University of California Press: Berkeley, CA, USA, 2011; Available online: https://muse.jhu.edu/chapter/954521 (accessed on 1 February 2021).
- Rajakaruna, N.; Boyd, R. Serpentine Soils. Oxf. Bibliogr. Ecol. 2014. Available online: https://scholarworks.sjsu.edu/biol_pub/53 (accessed on 1 February 2021).
- Rajakaruna, N.; Boyd, R. Edaphic Factor. Encycl. Ecol. 2008, 46, 1201–1207. [Google Scholar]
- Kazakou, E.; Dimitrakopoulos, P.G.; Baker, A.J.M.; Reeves, R.D.; Troumbis, A.Y. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: From speciesto ecosystemlevel. Biol. Rev. 2008, 83, 495–508. [Google Scholar] [CrossRef]
- DeHart, K.S.; Meindl, G.A.; Bain, D.J.; Ashman, T.L. Elemental composition of serpentine plants depends on habitat affinity and organ type. J. Plant Nutr. Soil Sci. 2014, 177, 851–859. [Google Scholar] [CrossRef]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Boyd, R.S.; Martens, S.N. Nickel hyperaccumulation by Thlaspi montanum var. montanum (Brassicaceae): A constitutive trait. Am. J. Bot. 1998, 85, 259–265. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Manara, A.; Fasani, E.; Furini, A.; DalCorso, G. Evolution of the metal hyperaccumulation and hypertolerance traits. Plant Cell Environ. 2020, 43, 2969–2986. [Google Scholar] [CrossRef] [PubMed]
- Jhee, E.M.; Boyd, R.S.; Eubanks, M.D. Nickel hyperaccumulation as an elemental defense of Streptanthus polygaloides (Brassicaceae): Influence of herbivore feeding mode. New Phytol. 2005, 168, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Vesk, P.A.; Reichman, S.M. Hyperaccumulators and herbivores—A bayesian meta-analysis of feeding choice Trials. J. Chem. Ecol. 2009, 35, 289–296. [Google Scholar] [CrossRef]
- Cappa, J.J.; Pilon-Smits, E.A.H. Evolutionary aspects of elemental hyperaccumulation. Planta 2014, 239, 267–275. [Google Scholar] [CrossRef]
- Eskandari, B.S.; Ghaderian, S.M.; Schat, H. The role of nickel (Ni) and drought in serpentine adaptation: Contrasting effects of Ni on osmoprotectants and oxidative stress markers in the serpentine endemic, Cleome heratensis, and the related non-serpentinophyte, Cleome foliolosa. Plant Soil 2017, 417, 183–195. [Google Scholar] [CrossRef]
- Boyd, R.S.; Martens, S.N. The raison d’être for metal hyperaccumulation by plants. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept: Andover, UK, 1992; pp. 279–289. [Google Scholar]
- Boyd, R.; Jaffré, T. Elemental concentrations of eleven new caledonian plant species from serpentine soils: Elemental correlations and leaf-age effects. Northeast. Nat. 2009, 16, 93–110. [Google Scholar] [CrossRef]
- Liu, X.; Feng, H.; Fu, J.; Chen, Y.; Liu, Y.; Ma, L.Q. Arsenic-induced nutrient uptake in As-hyperaccumulator Pteris vittata and their potential role to enhance plant growth. Chemosphere 2018, 198, 425–431. [Google Scholar] [CrossRef]
- Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012; pp. 171–178. [Google Scholar]
- Rahimi, A. Kupfermangel bei hoheren Pflanzen. Landwirtch. Forssch. Sonderh. 1970, 25, 27–42. [Google Scholar]
- van der Ent, A.; Mak, R.; de Jonge, M.D.; Harris, H.H. Simultaneous hyperaccumulation of nickel and cobalt in the tree Glochidion cf. sericeum (Phyllanthaceae): Elemental distribution and chemical speciation. Sci. Rep. 2018, 8, 9683. [Google Scholar] [CrossRef] [PubMed]
- Nkrumah, P.N.; Tisserand, R.; Chaney, R.L.; Baker, A.J.M.; Morel, J.L.; Goudon, R.; Erskine, P.D.; Echevarria, G.; van der Ent, A. The first tropical ‘metal farm’: Some perspectives from field and pot experiments. J. Geochem. Explor. 2019, 198, 114–122. [Google Scholar] [CrossRef]
- Taylor, S.I.; Macnair, M.R. Within and between population variation for zinc and nickel accumulation in two species of Thlaspi (Brassicaceae). New Phytol. 2006, 169, 505–514. [Google Scholar] [CrossRef]
- Deng, T.H.B.; Tang, Y.T.; Sterckeman, T.; Echevarria, G.; Morel, J.L.; Qiu, R.L. Effects of the interactions between nickel and other trace metals on their accumulation in the hyperaccumulator Noccaea caerulescens. Environ. Exp. Bot. 2019, 158, 73–79. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Bleeker, P.; Bookum, W.M.T.; Vooijs, R.; Schat, H. Intraspecific variation of metal preference patterns for hyperaccumulation in Thlaspi caerulescens: Evidence from binary metal exposures. Plant Soil 2007, 303, 289–299. [Google Scholar] [CrossRef]
- Keeling, S.M.; Stewart, R.B.; Anderson, C.W.N.; Robinson, B. Nickel and Cobalt Phytoextraction by the HyperaccumulatorBerkheya coddii: Implications for Polymetallic Phytomining and Phytoremediation. Int. J. Phytoremediation 2003, 5, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.-H.-B.; van der Ent, A.; Tang, Y.-T.; Sterckeman, T.; Echevarria, G.; Morel, J.-L.; Qiu, R.-L. Nickel hyperaccumulation mechanisms: A review on the current state of knowledge. Plant Soil 2018, 423, 1–11. [Google Scholar] [CrossRef]
- Minnie, M. Phytoextraction of soil cobalt using hyperaccumulator plants. Int. J. Phytoremediation 2001, 2, 319–329. [Google Scholar]
- Homer, F.A.; Morrison, R.S.; Brooks, R.R.; Clemens, J.; Reeves, R.D. Comparative studies of nickel, cobalt, and copper uptake by some nickel hyperaccumulators of the genus Alyssum. Plant Soil 1991, 138, 195–205. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Chaney, R.L.; Angle, J.S.; Erbe, E.F.; Maugel, T.K. Nickel localization and response to increasing Ni soil levels in leaves of the Ni hyperaccumulator Alyssum murale. Plant Soil 2004, 265, 225–242. [Google Scholar] [CrossRef]
- Safford, H.D.; Viers, J.H.; Harrison, S.P. Serpentine endemism in California flora: A database of serpentine affinity. Madroño 2005, 52, 222–257. [Google Scholar] [CrossRef]
- Safford, H.; Miller, J.E.D. An updated database of serpentine endemism in the California flora. Madroño 2020, 67, 85–104. [Google Scholar] [CrossRef]
- Gall, J.E.; Rajakaruna, N. The physiology, functional genomics, and applied ecology of heavy metal-tolerant Brassicaceae. In Brassicaceae: Characterization, Functional Genomics and Health Benefits; Lang, M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 121–148. [Google Scholar]
- Baldwin, B.G.; Goldman, D.H.; Keil, D.J.; Patterson, R.; Rosatti, T.J.; Wilken, D.H. (Eds.) The Jepson Manual: Vascular Plants of California; University of California Press: Berkeley, CA, USA, 2012. [Google Scholar]
- Meindl, G.A.; Bain, D.J.; Ashman, T.-L. Nickel accumulation in leaves, floral organs and rewards varies by serpentine soil affinity. AoB PLANTS 2014, 6, plu036. [Google Scholar] [CrossRef]
- L’Huillier, L.L.; Edighoffer, S. Extractability of nickel and its concentration in cultivated plants in Ni rich ultramafic soils of New Caledonia. Plant Soil. 1996, 186, 255–264. [Google Scholar] [CrossRef]
- Chardot, V.; Massoura, S.T.; Echevarria, G.; Reeves, R.D.; Morel, J.-L. Phytoextraction potential of the nickel hyperaccumulators leptoplax emarginata and bornmuellera tymphaea. Int. J. Phytoremediation 2005, 7, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Echevarria, G.; Massoura, S.T.; Sterckeman, T.; Becquer, T.; Schwartz, C.; Morel, J.L. Assessment and control of the bioavailability of nickel in soils. Environ. Toxicol. Chem. 2006, 25, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Littell, R.C.; Stroup, W.W.; Freund, R.J. SAS for Linear Models; SAS Institute: Cary, NC, USA, 2002. [Google Scholar]
- Meindl, G.A.; Bain, D.J.; Ashman, T.-L. Variation in nickel accumulation in leaves, reproductive organs and floral rewards in two hyperaccumulating Brassicaceae species. Plant Soil 2014, 383, 349–356. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, S.; Al Mahmud, J.; Hossen, S.; Masud, A.A.C.; Moumita, M.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- White, P.J. Improving potassium acquisition and utilisation by crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 305–316. [Google Scholar] [CrossRef]
- Chen, S.; Polle, A. Salinity tolerance of Populus. Plant Biol. 2010, 12, 317–333. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Xu, J.; Liang, J.; Lu, X.; Yang, J.; Zhu, Q. Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes. Field Crop. Res. 2003, 83, 271–281. [Google Scholar] [CrossRef]
- Van Der Ent, A.; Mulligan, D. Multi-element concentrations in plant parts and fluids of malaysian nickel hyperaccumulator plants and some economic and ecological considerations. J. Chem. Ecol. 2015, 41, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Reuther, W. Copper and soil fertility. In Soil, the Yearbook of Agriculture; U.S. Gov. Printing Office: Washington, DC, USA, 1957; pp. 128–135. [Google Scholar]
- Stevenson, F.J. Cycles of Soil–Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Van Der Ent, A.; Reeves, R.D. Foliar metal accumulation in plants from copper-rich ultramafic outcrops: Case studies from Malaysia and Brazil. Plant Soil 2015, 389, 401–418. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.P.; Sharma, P.N.; Chatterjee, C.; Agarwala, S.C. Manganese deficiency in maize affects pollen viability. Plant Soil 1991, 138, 139–142. [Google Scholar] [CrossRef]
- Sharma, P.N. Pollen fertility in manganese deficient wheat. Trop. Agric. 1992, 69, 21–24. [Google Scholar]
- Mukhopadhyay, M.J.; Sharma, A. Manganese in cell metabolism of higher plants. Bot. Rev. 1991, 57, 117–149. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C.; Sharma, C.P. Impairment in reproductive development is a major factor limiting yield of black gram under zinc deficiency. Biol. Plant. 2009, 53, 723–727. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Mittal, N.; Srivastava, A.K. Cadmium- and Chromium-Induced Aberrations in the Reproductive Biology of Hordeum vulgare. Cytologia 2014, 79, 207–214. [Google Scholar] [CrossRef][Green Version]
- Broadley, M.R.; Willey, N.; Wilkins, J.C.; Baker, A.J.M.; Mead, A.; White, P.J. Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytol. 2001, 152, 9–27. [Google Scholar] [CrossRef]
- Palacio, S.; Escudero, A.; Montserrat-Martí, G.; Maestro, M.; Milla, R.; Albert, M.J. Plants Living on Gypsum: Beyond the Specialist Model. Ann. Bot. 2007, 99, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mata, D.; De La Fuente, V.; Rufo, L.; Rodríguez, N.; Amils, R. Localization of Nickel in Tissues of Streptanthus polygaloides Gray (Cruciferae) and Endemic Nickel Hyperaccumulators from California. Biol. Trace Element Res. 2013, 157, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Sianta, S.A.; Kay, K.M. Adaptation and divergence in edaphic specialists and generalists: Serpentine soil endemics in the California flora occur in barer serpentine habitats with lower soil calcium levels than serpentine tolerators. Am. J. Bot. 2019, 106, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Share, H.; Sharifi, R.; Boyd, R.S.; Rajakaruna, N. Inducing Ni sensitivity in the Ni hyperaccumulator plant Alyssum inflatum Nyárády (Brassicaceae) by transforming with CAX1, a vacuolar membrane calcium transporter. Ecol. Res. 2018, 33, 737–747. [Google Scholar] [CrossRef]
- Ghasemi, R.; Chavoshi, Z.Z.; Boyd, R.S.; Rajakaruna, N. Calcium: Magnesium ratio affects environmental stress sensitivity in the serpentine-endemic Alyssum inflatum (Brassicaceae). Aust. J. Bot. 2015, 63, 39–46. [Google Scholar] [CrossRef]
| Species | Plant Category | Affinity Score | Life History | Range | Seed Collection Location |
|---|---|---|---|---|---|
| Streptanthus polygaloides | Hyperaccumulating Endemic | 6 | Annual | CA | N 39° 46′50.4″ |
| W 121°28′41.6″ | |||||
| Noccaea fendleri ssp. glauca | Hyperaccumulating Endemic | 4.4 | Perennial | Western NA | N 41°16′42.4″ |
| W 122°41′48.7″ | |||||
| S. breweri var. breweri | Non-hyperaccumulating Endemic | 5.7 | Annual | CA | N 38°51′52.4″ |
| W 122°24′16.4″ | |||||
| S. morrisonii | Non-hyperaccumulating Endemic | 6.1 | Perennial | CA | N 38°48′45.3″ |
| W 122°22′54.9″ | |||||
| S. glandulosus ssp. glandulosus | Indicator | 1.9 | Annual | CA | N 38°51′43.9″ |
| W 122°23′57.3″ | |||||
| S. tortuosus | Indicator | 1.7 | Perennial | Western NA | N 39°59′18.4″ |
| W 121°17′19.8″ | |||||
| Erysimum capitatum var. capitatum | Indifferent | <1 | Perennial | NA | N 41°16′32.5″ |
| W 122°41′54.4″ | |||||
| Hirschfeldia incana | Indifferent | <1 | Annual | NA, AE | N 38°51′30.0″ |
| W 122°24′35.2″ | |||||
| Boechera breweri | Indifferent | <1 | Perennial | CA | N 39°57′12.3″ |
| W 121°19′4.5″ |
| Macronutrients | Micronutrients | Heavy Metals | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | K | P | Ca | Mg | Zn | Cu | Mo | Fe | Mn | Co | Cr |
| Affinity (A) | 0.05 | 0.32 | 0.34 | 0.37 | 0.16 | 0.36 | 0.62 | 0.25 | 0.29 | <0.001 | 0.16 |
| Ni Treatment (NT) | 0.5 | 0.12 | 0.95 | 0.86 | <0.001 | <0.05 | 0.55 | 0.59 | 0.5 | <0.0001 | 0.07 |
| Organ Type (OT) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.001 | <0.0001 | <0.0001 | <0.0001 | 0.7 | <0.01 | <0.001 |
| A × OT | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.18 | <0.0001 | <0.0001 | <0.001 |
| A × NT | 0.56 | 0.7 | 0.92 | 0.68 | 0.48 | 0.63 | <0.001 | 0.4 | 0.7 | <0.0001 | 0.59 |
| A × OT × NT | 0.71 | 0.77 | 0.98 | 0.66 | 0.5 | 0.61 | 0.24 | 0.39 | 0.79 | 0.74 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meindl, G.A.; Poggioli, M.I.; Bain, D.J.; Colón, M.A.; Ashman, T.-L. A Test of the Inadvertent Uptake Hypothesis Using Plant Species Adapted to Serpentine Soil. Soil Syst. 2021, 5, 34. https://doi.org/10.3390/soilsystems5020034
Meindl GA, Poggioli MI, Bain DJ, Colón MA, Ashman T-L. A Test of the Inadvertent Uptake Hypothesis Using Plant Species Adapted to Serpentine Soil. Soil Systems. 2021; 5(2):34. https://doi.org/10.3390/soilsystems5020034
Chicago/Turabian StyleMeindl, George A., Mark I. Poggioli, Daniel J. Bain, Michael A. Colón, and Tia-Lynn Ashman. 2021. "A Test of the Inadvertent Uptake Hypothesis Using Plant Species Adapted to Serpentine Soil" Soil Systems 5, no. 2: 34. https://doi.org/10.3390/soilsystems5020034
APA StyleMeindl, G. A., Poggioli, M. I., Bain, D. J., Colón, M. A., & Ashman, T.-L. (2021). A Test of the Inadvertent Uptake Hypothesis Using Plant Species Adapted to Serpentine Soil. Soil Systems, 5(2), 34. https://doi.org/10.3390/soilsystems5020034






