Biochar Additions Alter the Abundance of P-Cycling-Related Bacteria in the Rhizosphere Soil of Portulaca oleracea L. under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil, Biochar, and Plant Seeds
2.2. Plant Growth Experiments
2.3. Soil Enzyme Activities
2.4. Soil Nutrient Contents
2.5. Isolation of Root-Associated Bacteria
2.6. Identification of Bacterial Isolates
2.7. Statistical Analysis
3. Results
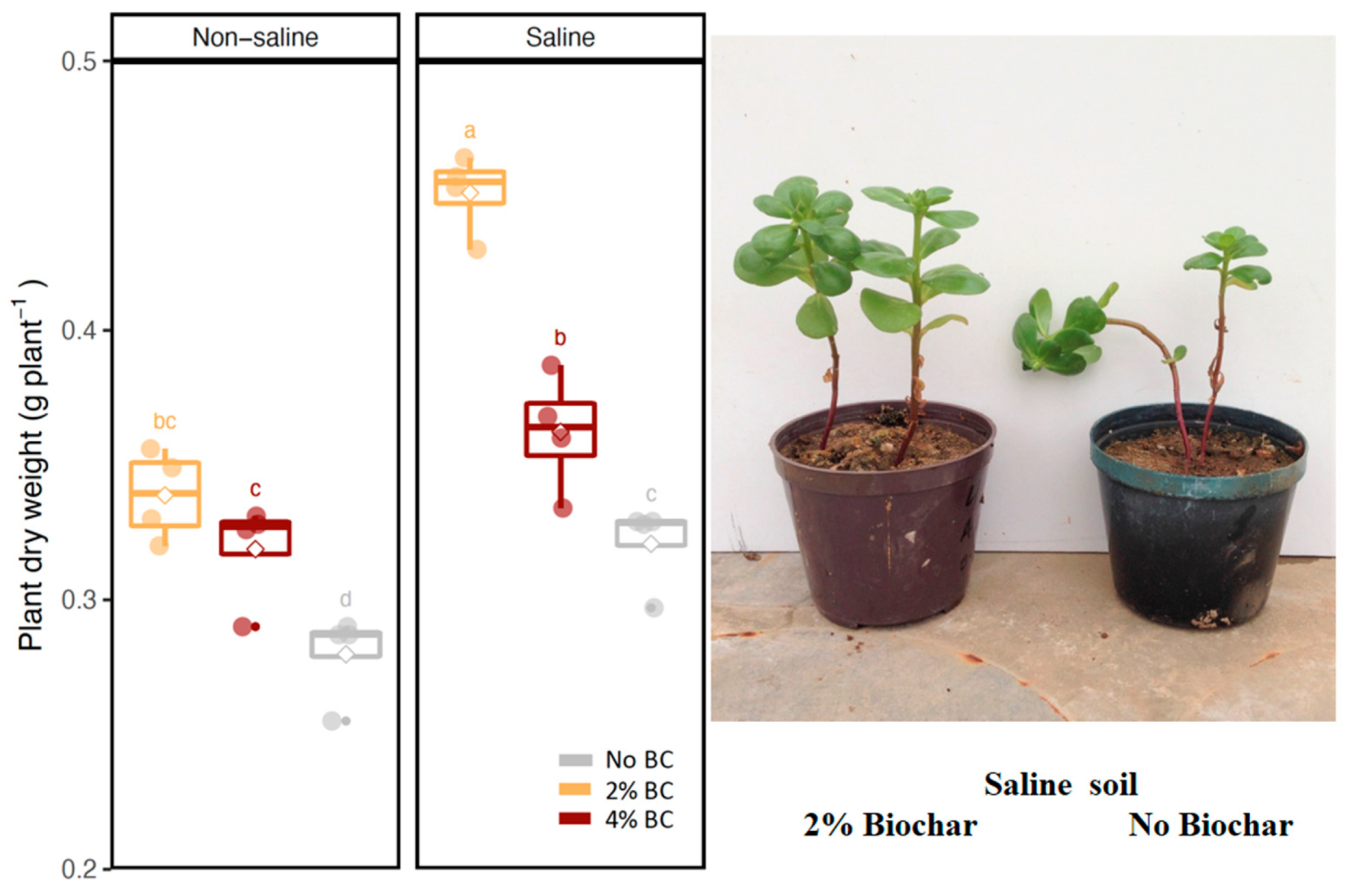
3.1. Plant Growth
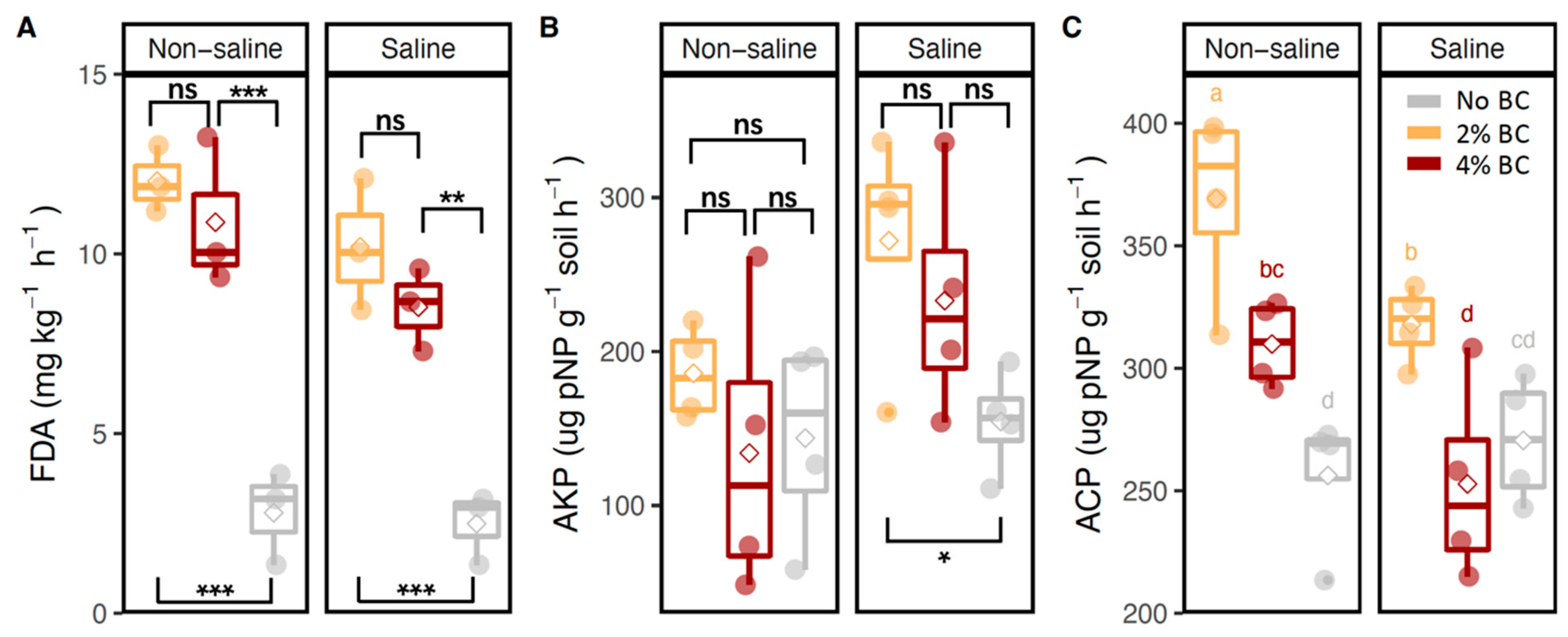
3.2. Soil Enzyme Activities and Soil Nutrient Contents
3.3. Abundance of Phosphate-Solubilising Bacteria
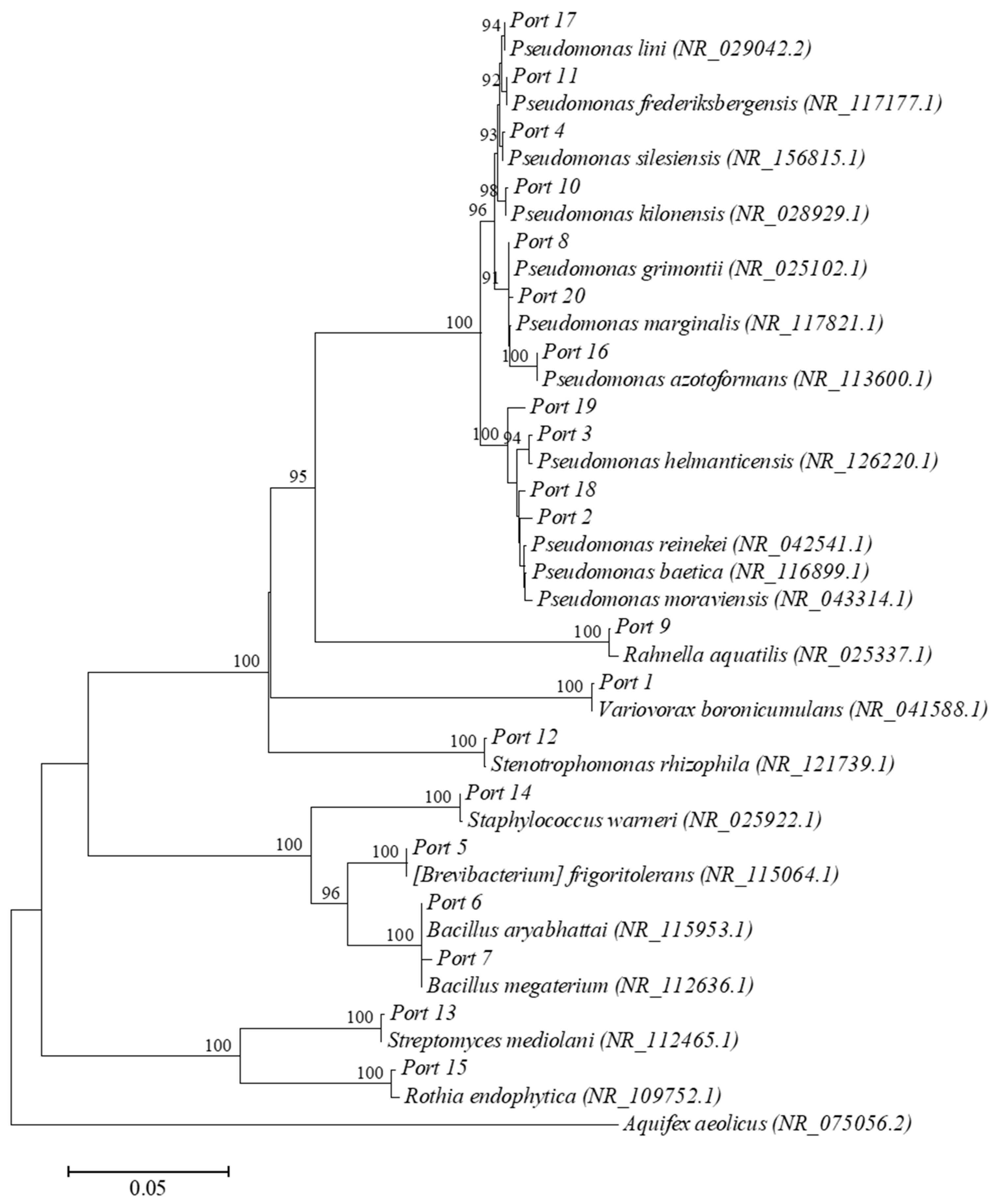
3.4. Isolation and Identification of Rhizosphere Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Akram, N.A.; Shafiq, F.; Ashraf, M.; Iqbal, M.; Ahmad, P. Advances in salt tolerance of some major fiber crops through classical and advanced biotechnological tools: A Review. J. Plant Growth Regul. 2021, 40, 891–905. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Mwando, E.; Angessa, T.T.; Han, Y.; Li, C. Salinity tolerance in barley during germination—Homologs and potential genes. J Zhejiang Univ. Sci. B. 2020, 21, 93–121. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, 206. [Google Scholar] [CrossRef]
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Renella, G.; Wirth, S.; Islam, R. Secondary salinity effects on soil microbial biomass. Biol. Fertil. Soils 2010, 46, 445–449. [Google Scholar] [CrossRef]
- Morrissey, E.M.; Franklin, R.B. Evolutionary history influences the salinity preferences of bacterial taxa in wetland soils. Front. Microbiol. 2015, 6, 1013. [Google Scholar] [CrossRef] [Green Version]
- Sparks, D.L. (Ed.) The Chemistry of Saline and Sodic Soils. In Environmental Soil Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 285–300. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.V.; Öztürk, M.; Fujita, M. Potential use of halophytes to remediate saline soils. BioMed Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.R.; Franco, J.G.; King, S.R.; Volder, A. Intercropping Halophytes to Mitigate Salinity Stress in Watermelon. Sustainability 2018, 10, 681. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef] [Green Version]
- Dalavi, J.; Deshmukh, P.; Jadhav, V.; Yadav, S. Two new species of Portulaca (Portulacaceae) from India. Phytotaxa 2018, 376, 68–76. [Google Scholar] [CrossRef]
- Zuccarini, P. Ion uptake by halophytic plants to mitigate saline stress in Solarium lycopersicon L., and different effect of soil and water salinity. Soil Water Res. 2008, 3, 62–73. [Google Scholar] [CrossRef]
- Zaier, H.; Ghnaya, T.; Lakhdar, A.; Baioui, R.; Ghabriche, R.; Mnasri, M.; Sghair, S.; Lutts, S.; Abdelly, C. Comparative study of Pb-phytoextraction potential in Sesuvium portulacastrum and Brassica juncea: Tolerance and accumulation. J. Hazard. Mater. 2010, 183, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Li, Y.F.; Zheng, C.Y.; Zhang, Y.F.; Sun, Z.J. Organic amendment application influence soil organism abundance in saline alkali soil. Eur. J. Soil Biol. 2013, 54, 32–40. [Google Scholar] [CrossRef]
- Eid, E.M.; Alrumman, S.A.; El-Bebany, A.F.; Fawy, K.F.; Taher, M.A.; Hesham, A.E.-L.; El-Shaboury, G.A.; Ahmed, M.T. Evaluation of the potential of sewage sludge as a valuable fertilizer for wheat (Triticum aestivum L.) crops. Environ. Sci. Pollut. Res. Int. 2019, 26, 392–401. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, Y.; Luo, X.; Xing, B. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 2017, 41, 517–532. [Google Scholar] [CrossRef]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Li, Q.; Omari, R.A.; Hou, M.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on the interrelationships among soybean growth, root nodulation, plant P uptake, and soil nutrients in a sandy field. Sustainability 2019, 11, 6542. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Alaylar, B.; Zoghi, Z.; Kistaubayeva, A.; Ma, H.; Wirth, S.; Bellingrath-Kimura, S. Biochar amendments improve licorice (Glycyrrhiza uralensis Fish.) growth and nutrient uptake through altering the root system and soil enzyme activities in loamy sand under salt stress. Plants 2021, 10, 2135. [Google Scholar] [CrossRef]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on soybean-rhizobium symbiotic performance and soil enzymatic activity in field rhizosphere. Agronomy 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.T.N.; Farrell, C.; Kristiansen, P.E.; Rayner, J.P. Biochar makes green roof substrates lighter and improves water supply to plants. Ecol. Eng. 2014, 71, 368–374. [Google Scholar] [CrossRef]
- He, K.; He, G.; Wang, C.; Zhang, H.; Xua, Y.; Wang, S.; Kong, Y.; Zhou, G.; Hu, R. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020, 155, 103674. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Abd_Allah, E.F.; Berg, G. Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Front. Microbiol. 2016, 7, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egamberdieva, D.; Reckling, M.; Wirth, S. Biochar-based inoculum of Bradyrhizobium sp. improves plant growth and yield of lupin (Lupinus albus L.) under drought stress. Eur. J. Soil Biol. 2017, 78, 38–42. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Q.; Tian, Z.; Cui, Y.; Liang, Y.; Wang, H. Apply biochar to ameliorate soda saline-alkali land, improve soil function and increase corn nutrient availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, J.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Kul, R.; Arjumend, T.; Ekinci, M.; Yildirim, E.; Turan, M.; Argin, S. Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Soil Sci. Plant Nutr. 2021, 67, 693–706. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Li, L.; Ma, H.; Wirth, S.; Bellingrath-Kimura, S.D. Soil amendment with different maize biochars improves chickpea growth under different moisture levels by improving symbiotic performance with Mesorhizobium ciceri and soil biochemical properties to varying degrees. Front. Microbiol. 2019, 10, 2423. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amend-ment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Ma, H.; Shurigin, V.; Jabborova, D.; dela Cruz, J.A.; dela Cruz, T.E.; Wirth, S.; Bellingrath-Kimura, S.D.; Egamberdieva, D. The integrated effect of microbial inoculants and biochar types on soil biological properties, and plant growth of lettuce (Lactuca sativa L.). Plants 2022, 11, 423. [Google Scholar] [CrossRef]
- El-sayed, M.E.A.; Hazman, M.; Abd El-Rady, A.G.; Almas, L.; McFarland, M.; Shams El Din, A.; Burian, S. Biochar reduces the adverse effect of saline water on soil properties and wheat production profitability. Agriculture 2021, 11, 1112. [Google Scholar] [CrossRef]
- Joniec, J. Enzymatic activity as an indicator of regeneration processes in degraded soil reclaimed with various types of waste. Int. J. Environ. Sci. Technol. 2018, 15, 2241–2252. [Google Scholar] [CrossRef] [Green Version]
- Egamberdiyeva, D.; Hoflich, G. Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekistan. J. Arid Environ. 2004, 56, 293–301. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, W.; Gulaqa, A.; Cui, Y.; Zhou, Y.; Weng, W.; Wang, X.; Liu, Q.; Jin, F. Effects of peanut shell biochar on soil nutrients, soil enzyme activity, and rice yield in heavily saline-sodic paddy field. J. Soil Sci. Plant Nutr. 2021, 21, 655–664. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Alaylar, B.; Kistaubayeva, A.; Wirth, S.; Bellingrath-Kimura, S.D. Biochar for improving soil biological properties and mitigating salt stress in plants on salt-affected soils. Commun. Plant Soil Sci. 2022, 53, 140–152. [Google Scholar] [CrossRef]
- Manasa, M.R.K.; Katukuri, N.R.; Nair, S.S.D.; Haojie, Y.; Yang, Z.; Guo, R.B. Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J. Environ. Manag. 2020, 269, 10737. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Kwapinski, W.; Griffiths, B.S.; Schmalenberger, A. The role of sulfur- and phosphorus-mobilizing bacteria in biochar-induced growth promotion of Lolium perenne. FEMS Microbiol. Ecol. 2014, 90, 78–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, G.; Banerjee, P.; Sharma, R.K.; Maity, J.P.; Etesami, H.; Shaw, A.K.; Huang, Y.-H.; Huang, H.-B.; Chen, C.-Y. Management of phosphorus in salinity-stressed agriculture for sustainable crop production by salt-tolerant phosphate-solubilizing bacteria—A review. Agronomy 2021, 11, 1552. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purakayastha, T.; Savita Kumari, J.; Pathak, H. Characterisation, stability, and microbial effects of four biochars produced from crop residues. Geoderma 2015, 239–240, 293–303. [Google Scholar] [CrossRef]
- Reibe, K.; Götz, K.P.; Ross, C.L.; Doering, T.F.; Ellmer, F.; Ruess, L. Impact of quality and quantity of biochar and hydro-char on soil collembola and growth of spring wheat. Soil Biol. Biochem. 2015, 8, 84–87. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimalization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenol phosphate for the assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Phosphorus cycle enzymes. In Methods of Soil Enzymology; Dick, R.P., Ed.; SSSA: Madison, WI, USA, 2011; pp. 161–183. [Google Scholar]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat Treatment of Bacteria: A Simple Method of DNA Extraction for Molecular Techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Jinneman, K.C.; Wetherington, J.H.; Hill, W.E.; Adams, A.M.; Johnson, J.M.; Tenge, B.I.; Dang, N.L.; Manger, R.L.; Wekell, M.M. Differentiation of Cyclospora sp. and Eimeria spp. by using the polymerase chain reaction amplification products and restriction fragment length polymorphisms. Food Drug Adm. Lab. Inf. Bull. 1996, 4, 4044. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 9, 114–121. [Google Scholar] [CrossRef]
- Saifullah, S.D.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Li, L.; Fu, Q.; Li, Q.; Naeem, M.A.; He, X.; Zhang, Z.; Jacobsen, S.E. Biochar mitigates combined effects of drought and salinity stress in quinoa. Agronomy 2020, 10, 912. [Google Scholar] [CrossRef]
- Pietikainen, J.; Kiikkila, O.; Fritze, H. Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.S.; Ali, M.G.M.; Ali, O.A.M.; Abdelaal, A.I.N.; Lin, X.; Zhou, Z.; Wang, B.; Liu, B.; He, Z. The integrated effect of salinity, organic amendments, phosphorus fertilizers, and deficit irrigation on soil properties, phosphorus fractionation and wheat productivity. Sci. Rep. 2020, 10, 2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves Lopes, E.M.; Reis, M.M.; Frazão, L.A.; da Mata Terra, L.E.; Lopes, E.F.; dos Santos, M.M.; Fernandes, L.A. Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environ. Technol. Innov. 2021, 21, 101270. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, G.; Liang, J.; Chen, J.; Xu, J.; Dai, J.; Li, X.; Chen, M.; Xu, P.; Zhou, X.; et al. Responses of bac-terial community and functional marker genes of nitrogen cycling to biochar, compost and combined amendments in soil. Appl. Microbiol. Biotechnol. 2016, 100, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Tian, I.; Nasir, F.; Bahadur, F.; Batool, A.; Luo, S.; Yang, F.; Wang, Z.; Tian, C. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2019, 135, 16–24. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Zhao, Y.; Zhang, C.; Jin, Z.; Shan, S.; Ping, L. Effects of Biochar Application on Enzyme Activities in Tea Garden Soil. Front. Bioeng. Biotechnol. 2021, 9, 728530. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Han, F.; Ren, L.; Zhang, X.C. Effect of biochar on the soil nutrients about different grasslands in the Loess Plateau. Catena 2016, 137, 554–562. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, M.C. Thermodynamic parameters of enzymes in grassland soils from Galicia, NW Spain. Soil Biol. Biochem. 2007, 39, 311–319. [Google Scholar] [CrossRef]
- Qian, Z.H.U.; Kong, L.; Shan, Y.; Yao, X.; Zhang, H.; Xie, F.; Xue, A.O. Effect of biochar on grain yield and leaf photosynthetic physiology of soybean cultivars with different phosphorus efficiencies. J. Integr. Agric. 2019, 18, 2242–2254. [Google Scholar]
- Mahmood, S.; Finlay, R.D.; Fransson, A.M.; Wallander, H. Effects of hardened wood ash on microbial activity, plant growth and nutrient uptake by ectomycorrhizal spruce seedlings. FEMS Microbiol. Ecol. 2003, 43, 121–131. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi—Current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Thakur, D.; Kaushal, R.; Shyam, V. Phosphate solubilising microorganisms: Role in phosphorus nutrition of crop plants—A review. Agric. Rev. 2014, 35, 159–171. [Google Scholar] [CrossRef]
- Stella, M.; Halimi, M.S. Gluconic acid production by bacteria to liberate phosphorus from insoluble phosphate complexes. J. Trop. Agric. Food Sci. 2015, 43, 41–53. [Google Scholar]
- Egamberdiyeva, D. Characterisation of Pseudomonas species isolated from the rhizosphere of plants grown in serozem soil, semi arid region of Uzbekistan. Sci. World J. 2005, 5, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Behera, B.; Yadav, H.; Singh, S.; Mishra, R.; Sethi, B.; Dutta, S.; Thatoi, H. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Vargas, A.; Rodríguez-Gacha, L.M.; Sánchez-Castro, N.; Garzón-Jaramillo, R.; Pedroza-Camacho, L.D.; Poutou-Piñales, R.A.; Rivera-Hoyos, C.M.; Díaz-Ariza, L.A.; Pedroza-Rodríguez, A.M. Phosphate-solubilizing Pseudomonas sp., and Serratia sp., co-culture for Allium cepa L. growth promotion. Heliyon 2020, 6, e05218. [Google Scholar] [CrossRef]
- Li, L.; Abu Al-Soud, W.; Bergmark, L.; Riber, L.; Hansen, L.H.; Magid, J.; Sørensen, S.J. Investigating the diversity of Pseudomonas spp. in soil using culture dependent and independent techniques. Curr. Microbiol. 2013, 67, 423–430. [Google Scholar] [CrossRef] [PubMed]
- De Amaral Leite, A.; de Souza Cardoso, A.A.; de Almeida Leite, R.; De Oliveira-Longatti, S.M.; Lustosa Filho, J.F.; de Souza Moreira, F.M.; Melo, L.C.A. Selected bacterial strains enhance phosphorus availability from biochar-based rock phosphate fertilizer. Ann. Microbiol. 2020, 70, 6. [Google Scholar] [CrossRef]
- Estrada, G.A.; Baldani, V.L.D.; de Oliveira, D.M.; Urquiaga, S.; Baldani, J.I. Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 2013, 369, 115–129. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Castro, P.M.L. Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol. Eng. 2014, 73, 526–535. [Google Scholar] [CrossRef]

| Plant Dry Weight | Soil FDA | Soil AKP | Soil ACP | Soil C | Soil N | Soil P | |
|---|---|---|---|---|---|---|---|
| Biochar | ** | ** | ns | ** | ** | ** | ** |
| Salinity | ** | * | * | * | ** | ** | ** |
| Biochar × Salinity | ** | ns | ns | * | ns | ns | * |
| Isolated Strains Deposited to GenBank | Closest Match (16S Ribosomal RNA Genes) (GenBank) | ||||
|---|---|---|---|---|---|
| Strain | Length (bp) | Accession Number | Reference Strains | Accession Number | Percent Identity |
| Port 1 | 1439 | MT825595 | Variovorax boronicumulans | NR_041588.1 | 99.65 |
| Port 2 | 1439 | MT825596 | Pseudomonas baetica | NR_116899.1 | 99.44 |
| Port 3 | 1441 | MT825597 | Pseudomonas helmanticensis | NR_126220.1 | 99.65 |
| Port 4 | 1438 | MT825598 | Pseudomonas silesiensis | NR_156815.1 | 99.86 |
| Port 5 | 1459 | MT825599 | [Brevibacterium] frigoritolerans | NR_115064.1 | 99.52 |
| Port 6 | 1456 | MT825600 | Bacillus aryabhattai | NR_115953.1 | 99.59 |
| Port 7 | 1453 | MT825601 | Bacillus megaterium | NR_112636.1 | 99.52 |
| Port 8 | 1450 | MT825602 | Pseudomonas grimontii | NR_025102.1 | 99.38 |
| Port 9 | 1463 | MT825603 | Rahnella aquatilis | NR_025337.1 | 98.91 |
| Port 10 | 1456 | MT825604 | Pseudomonas kilonensis | NR_028929.1 | 99.66 |
| Port 11 | 1439 | MT825605 | Pseudomonas frederiksbergensis | NR_117177.1 | 99.79 |
| Port 12 | 1427 | MT825606 | Stenotrophomonas rhizophila | NR_121739.1 | 99.65 |
| Port 13 | 1424 | MT825607 | Streptomyces mediolani | NR_112465.1 | 99.79 |
| Port 14 | 1458 | MT825608 | Staphylococcus warneri | NR_025922.1 | 99.38 |
| Port 15 | 1399 | MT825609 | Rothia endophytica | NR_109752.1 | 99.50 |
| Port 16 | 1439 | MT825610 | Pseudomonas azotoformans | NR_113600.1 | 99.65 |
| Port 17 | 1446 | MT825611 | Pseudomonas lini | NR_029042.2 | 99.38 |
| Port 18 | 1450 | MT825612 | Pseudomonas reinekei | NR_042541.1 | 99.10 |
| Port 19 | 1457 | MT825613 | Pseudomonas moraviensis | NR_043314.1 | 99.11 |
| Port 20 | 1446 | MT825614 | Pseudomonas marginalis | NR_117821.1 | 99.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egamberdieva, D.; Ma, H.; Shurigin, V.; Alimov, J.; Wirth, S.; Bellingrath-Kimura, S.D. Biochar Additions Alter the Abundance of P-Cycling-Related Bacteria in the Rhizosphere Soil of Portulaca oleracea L. under Salt Stress. Soil Syst. 2022, 6, 64. https://doi.org/10.3390/soilsystems6030064
Egamberdieva D, Ma H, Shurigin V, Alimov J, Wirth S, Bellingrath-Kimura SD. Biochar Additions Alter the Abundance of P-Cycling-Related Bacteria in the Rhizosphere Soil of Portulaca oleracea L. under Salt Stress. Soil Systems. 2022; 6(3):64. https://doi.org/10.3390/soilsystems6030064
Chicago/Turabian StyleEgamberdieva, Dilfuza, Hua Ma, Vyacheslav Shurigin, Jakhongir Alimov, Stephan Wirth, and Sonoko Dorothea Bellingrath-Kimura. 2022. "Biochar Additions Alter the Abundance of P-Cycling-Related Bacteria in the Rhizosphere Soil of Portulaca oleracea L. under Salt Stress" Soil Systems 6, no. 3: 64. https://doi.org/10.3390/soilsystems6030064
APA StyleEgamberdieva, D., Ma, H., Shurigin, V., Alimov, J., Wirth, S., & Bellingrath-Kimura, S. D. (2022). Biochar Additions Alter the Abundance of P-Cycling-Related Bacteria in the Rhizosphere Soil of Portulaca oleracea L. under Salt Stress. Soil Systems, 6(3), 64. https://doi.org/10.3390/soilsystems6030064