Exploring the Influence of Diverse Viticultural Systems on Soil Health Metrics in the Northern Black Sea Region
Abstract
:1. Introduction
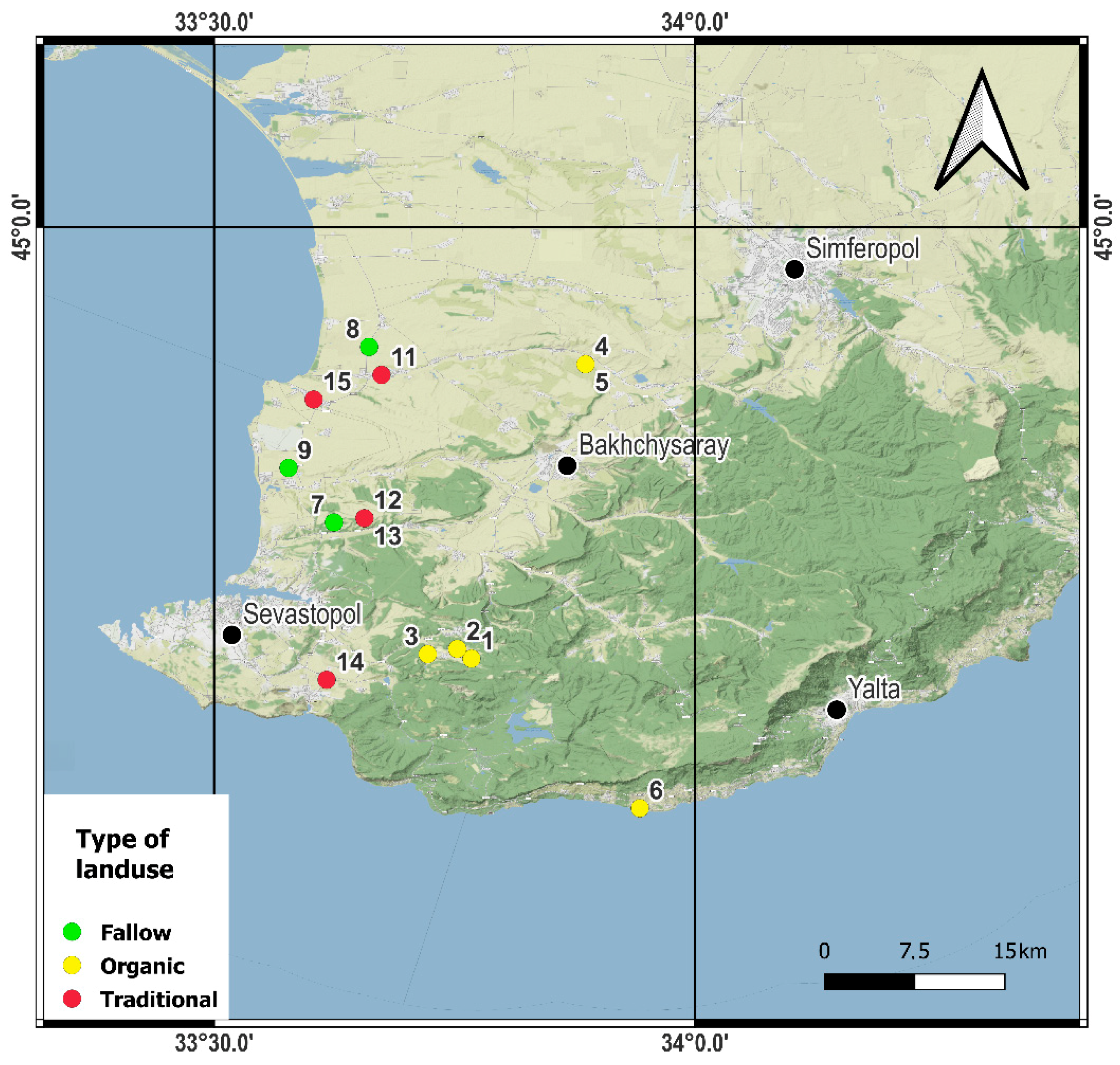
2. Materials and Methods
3. Results
3.1. Agrochemical Properties of Soils under Vineyards
3.2. Parameters of Soil Respiration in the Ampelocenoses of the Southern Part of Crimea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yena, A. Carlos Cordova, Crimea and the Black Sea: An Environmental History. I.B. Tauris & Co. Ltd: London, New York, 2015; 235 pp.: ISBN 9781784530013, Reviewed by: A. V. Yena, V. I. Vernadsky Crimean Federal University. Holocene 2016, 26, 1517–1518. [Google Scholar]
- Gorbunov, R.; Gorbunova, T.; Drygval, A.; Tabunshchik, V.A. Change of Air Temperature in Crimea. Environ. Hum. Ecol. Stud. 2020, 10, 370–383. (In Russian) [Google Scholar] [CrossRef]
- Ergina, E.I.; Zhuk, V.O. Spatiotemporal Variability of the Climate and Dangerous Hydrometeorological Phenomena on the Crimean Peninsula. Meteorol. Hydrol. 2019, 44, 494–500. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Focus OIV. The World Organic Vineyard. 2021. Available online: https://www.oiv.int/sites/default/files/2022-09/en-focus-the-world-organic-vineyard.pdf (accessed on 1 July 2022).
- Volkov, Y.; Volkova, M. Methodological Recommendations on Cultivation of Technical Grape Varieties in the System of Organic Farming on the Example of D.V. Shelayev’s Farm, Republic of Crimea. Union of Organic Agriculture. 2021. Available online: https://soz.bio/wp-content/uploads/2021/11/shelaev-metodicheskie-rekomendacii-volkov-soz-2021-1.pdf (accessed on 1 July 2022).
- Fedorova, N.; Egorova, A.; Rodionov, A.; Grechina, M. Control of copper-containing pesticide residues in plant products. Veg. Russ. 2020, 3, 57–62. (In Russian) [Google Scholar]
- Fernández-Calviño, D.; Rodríguez-Suárez, J.A.; López-Periago, E.; Arias-Estévez, M.; Simal-Gándara, J. Copper content of soils and river sediments in a winegrowing area, and its distribution among soil or sediment components. Geoderma 2008, 145, 91–97. [Google Scholar] [CrossRef]
- Kurnik, V.; Gaberšek, V.; Unuk, T.; Tojnko, S.; Vogrin, A.; Vajs, S.; Lešnik, M. Influence of alternative copper fungicide formulations on copper content in apple fruits. Erwerbs-Obstbau 2012, 54, 161–170. [Google Scholar] [CrossRef]
- Ghiglieno, I.; Simonetto, A.; Facciano, L.; Tonni, M.; Donna, P.; Valenti, L.; Gilioli, G. Comparing the Carbon Footprint of Conventional and Organic Vineyards in Northern Italy. Sustainability 2023, 15, 5252. [Google Scholar] [CrossRef]
- Luttikholt, L.W.M. Principles of organic agriculture as formulated by the International Federation of Organic Agriculture Movements. NJAS-Wagening. J. Life Sci. 2007, 54, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Litskas, V.; Mandoulaki, A.; Vogiatzakis, I.N.; Tzortzakis, N.; Stavrinides, M. Sustainable Viticulture: First Determination of the Environmental Footprint of Grapes. Sustainability 2020, 12, 8812. [Google Scholar] [CrossRef]
- Probst, B.; Schüler, C.; Joergensen, R.G. Vineyard Soils under Organic and Conventional Management-Microbial Biomass and Activity Indices and Their Relation to Soil Chemical Properties. Biol. Fertil. Soils 2008, 44, 443–450. [Google Scholar] [CrossRef]
- Perez-Rodriguez, P.; Soto-Gomez, D.; Lopez-Periago, J.E.; Paradelo, M. Modeling Raindrop Strike Performance on Copper Wash-off from Vine Leaves. J. Environ. Manag. 2015, 150, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Mahlungulu, A.; Kambizi, L.; Akinpelu, E.A.; Nchu, F. Levels of Heavy Metals in Grapevine Soil and Leaf Samples in Response to Seasonal Change and Farming Practice in the Cape Winelands. Toxics 2023, 11, 193. [Google Scholar] [CrossRef]
- Dobrovolskaya, T.G.; Zvyagintsev, D.G.; Chernov, I.Y.; Golovchenko, A.V.; Zenova, G.M.; Lysak, L.V.; Manucharova, N.A.; Marfenina, O.E.; Polyanskaya, L.M.; Stepanov, A.L.; et al. The role of microorganisms in ecological functions of soils. Soil Sci. 2015, 9, 1087–1096. [Google Scholar] [CrossRef]
- Adhikari, K.; Hartemink, A. Linking soils to ecosystem services—A global review. Geoderma 2016, 262, 101–111. [Google Scholar] [CrossRef]
- Saccá, M.L.; Caracciolo, A.B.; Lenola, M.; Grenni, P. Ecosystem Services Provided By Soil Microorganisms. In Soil Biological Communities and Ecosystem Resilience; Springer: Berlin/Heidelberg, Germany, 2017; pp. 9–24. [Google Scholar]
- Gugino, B.K.; Idowu, O.J.; Schindelbeck, R.R.; van Es, H.M.; Wolfe, D.W.; Thies, J.E. Cornell Soil Health Assessment Training Manual; Cornell University College of Agriculture and Life Sciences: Geneva, NY, USA, 2009. [Google Scholar]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Amaral, H.F.; Schwan-Estrada KR, F.; Sena JO, A.D.; Colozzi-Filho, A.; Andrade, D.S. Seasonal variations in soil chemical and microbial indicators under conventional and organic vineyards. Acta Scientiarum. Agron. 2022, 45, 1–13. [Google Scholar]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Ohtonen, R.; Aikio, S.; Väre, H. Ecological theories in soil biology. Soil Biol. Biochem. 1997, 29, 1613–1619. [Google Scholar] [CrossRef]
- Fründ, H.C.; Bossung, C.; Bravin, M.; Emmerling, C.; Hinsinger, P.; Mensh, M. Is Copper Stabilizing Organic Matter in Soils? A Survey of Vineyards and Contaminated Sites in Germany and France. In Proceedings of the International Symposium on Organic Matter Dynamics in Agro-Ecosystems, Poitiers, France, 16–19 July 2007; p. 458. [Google Scholar]
- Ananyeva, N.D.; Ivashchenko, K.V.; Sushko, S.V. Microbial indicators of urban soils and their role in the assessment of ecosystem services (review). Soil Sci. 2021, 54, 1517–1531. [Google Scholar] [CrossRef]
- Raubuch, M.; Beese, F. Pattern of microbial indicators in forest soils along an European transect. Biol. Fertil. Soils 1995, 19, 362–368. [Google Scholar] [CrossRef]
- Ruess, R.W.; Seagle, S.W. Landscape Patterns in Soil Microbial Processes in the Serengeti National Park, Tanzania. Ecology 1994, 75, 892–904. [Google Scholar] [CrossRef]
- Yan, T.; Yang, L.; Campbell, C.D. Microbial biomass and metabolic quotient of soils under different land use in the Three Gorges Reservoir area. Geoderma 2003, 115, 129–138. [Google Scholar] [CrossRef]
- Zak, D.R.; Tilman, D.; Parmenter, R.R.; Rice, C.V.; Fisher, F.M.; Vose, J.; Milchunas, D.; Martin, C.W. Plant Production and Soil Microorganisms in Late-Successional Ecosystems: A Continental-Scale Study. Ecology 1994, 75, 2333–2347. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Dilly, O. Microbial Energetics in Soils. In Microorganisms in Soils: Roles in Genesis and Functions; Springer: Berlin/Heidelberg, Germany, 2005; pp. 123–138. [Google Scholar] [CrossRef]
- Zavarzin, G.A.; Kudeyarov, V.N. Soil as the main source of carbon dioxide and organic carbon reservoir in Russia. Bull. Russ. Acad. Sci. 2006, 76, 12–26. [Google Scholar]
- Stepanov, A.L. Microbial Transformation of Greenhouse Gases in Soils; Lomonosov Moscow State University, Faculty of Soil Science, GEOS: Moscow, Russia, 2011; pp. 179–191. [Google Scholar]
- Zibilske, L.M. Carbon Mineralization. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; SSSA Book Series 5.2; Wiley: Hoboken, NJ, USA, 1994; Chapter 38. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 1995; 608p. [Google Scholar]
- Vershinin, A.A.; Petrov, A.M.; Karimullin, L.K.; Ignatiev Yu, A. Influence of oil pollution on the ecological and biological state of various types of soils. Bull. Kazan Technol. Univ. 2012, 207–211. [Google Scholar]
- Martens, R. Current methods for measuring microbial biomass C in soil: Potentials and limitations. Biol. Fertil. Soils 1995, 19, 87–99. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.G.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.R.; Scheu, S. An Inter-Laboratory Comparison of Ten Different Ways of Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Lin, Q.; Brookes, P.C. Comparison of substrate induced respiration, selective inhibition and biovolume measurements of microbial biomass and its community structure in unamended, ryegrass-amended, fumigated and pesticide-treated soils. Soil Biol. Biochem. 1999, 31, 1999–2014. [Google Scholar] [CrossRef]
- Martens, R. Estimation of microbial biomass in soil by the respiration method: Importance of soil pH and flushing methods for the measurement of respired CO2. Soil Biol. Biochem. 1987, 19, 77–81. [Google Scholar]
- Sparling, G.P.; Feltham, C.W.; Reynolds, J.; West, A.W.; Singleton, P. Estimation of soil microbial C by a fumigation-extraction method: Use on soils of high organic matter content, and a reassessment of the kEC-factor. Soil Biol. Biochem. 1990, 22, 301–307. [Google Scholar] [CrossRef]
- Grodnitskaya, I.D.; Sorokin, N.D. Application of microbes to the soils of Siberian tree nurseries. Degradation, Rehabilitation, and Conservation of Soils. Eurasian Soil Sci. 2007, 40, 329–334. [Google Scholar] [CrossRef]
- Dilly, O.; Blume, H.-P.; Sehy, U.; Jimenez, M.; Munch, J.C. Variation of stabilised, microbial and biologically active carbon and nitrogen in soil under contrasting land use and agricultural management practices. Chemosphere 2003, 52, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Moreno, J.L.; Hernández, T.; García, C. Microbiological activity in a soil 15 years after its devegetation. Soil Biol. Biochem. 2006, 38, 2503–2507. [Google Scholar] [CrossRef]
- Ananyeva, N.D. Microbiological Aspects of Self-Purification and Sustainability of Soils; Nauka: Moscow, Russia, 2003; 226p, ISBN 5-02-006451-3. [Google Scholar]
- Fließbach, A.; Martens, R.; Reber, H.H. Soil microbial biomass and microbial activity in soils treated with heavy metal contaminated sewage sludge. Soil Biol. Biochem. 1994, 26, 1201–1205. [Google Scholar] [CrossRef]
- Heilmann, B.; Lebuhn, M.; Beese, F. Methods for the investigation of metabolic activities and shifts in the microbial community in a soil treated with a fungicide. Biol. Fertil. Soils 1995, 19, 186–192. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Blagodatskaya, E.; Demkina, T.S. Estimating the resistance of soil microbial complexes to natural and anthropogenic impacts. Eurasian Soil Sci. 2002, 35, 514–521. [Google Scholar] [CrossRef]
- Yuangen, Y.; Campbell, C.D.; Clark, L. Microbial indicators of heavy metal contamination in urban and rural soils. Chemosphere 2006, 63, 1942–1952. [Google Scholar] [CrossRef]
- Papa, S.; Bartoli, G.; Pellegrino, A.; Fioretto, A. Microbial activities and trace element contents in an urban soil. Environ. Monit. Assess. 2010, 165, 193–203. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Purushothaman, S.M. Efficacy of Carbosulfan against yellow stem borer, Scirpophaga incertulas Walker (Pyralidae: Lepidoptera). Indian J. Plant Prot. 2000, 28, 212–214. [Google Scholar]
- Tuomela, M.; Steffen, K.T.; Kerko, E.; Hartikainen, H.; Hofrichter, M.; Hatakka, A. Influence of Pb contamination in boreal forest soil on the growth and ligninolytic activity of litter-decomposing fungi. FEMS Microbiol. Ecol. 2005, 53, 179–186. [Google Scholar] [CrossRef]
- Vasenev, V.I.; Ananyeva, N.D.; Ivashchenko, K.V. Influence of pollutants (heavy metals, diesel fuel) on the respiratory activity of constructozems. Ecology. 2013, 44, 475–483. [Google Scholar]
- Dilly, O.; Winter, K.; Lang, A.; Munch, J.C. Energetic eco-physiology of the soil microbiota in two landscapes of southern and northern Germany. J. Plant Nutr. Soil Sci. 2001, 164, 407–413. [Google Scholar] [CrossRef]
- Miao, S.; Tang Yu Xue, H.; Qiao, Y. Soil bacterial community responses to land-use change in Mollisol of Northeast China. Ecol. Eng. 2022, 184, 50–62. [Google Scholar] [CrossRef]
- Gherghina, C.-A.; Matei, S.; Matei, G.; Daniela, R. Influence of land use on microbiological activity of sandy soils. In Proceedings of the ICAMS 2014—5th International Conference on Advanced Materials and Systems, Bucharest, Romania, 23–25 October 2014; pp. 427–432. [Google Scholar]
- Rybalko, E.A.; Baranova, N.V. Agroecological zoning of the Crimean Peninsula for growing grapes. Environ. Control. Syst. 2018, 1, 90–94. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Williams, C.H.; Steinberg, A. Soil sulphur fraction as chemical indices of available sulphur in some Australian soils. Aus. J. Agric. Res. 1959, 10, 340–352. [Google Scholar] [CrossRef]
- Chernenok, V.; Barkusky, D. Diagnosis and Optimization of Phosphorus Nutrition Conditions of Grain Crops in Northern Kazakhstan. In Novel Measurement and Assessment Tools for Monitoring and Management of Land and Water Resources in Agricultural Landscapes of Central Asia; Springer: Berlin/Heidelberg, Germany, 2014; pp. 667–679. [Google Scholar]
- ISO 16072:2002; Soil Quality—Laboratory Methods for Determination of Microbial Soil Respiration. ISO (International Organization for Standardization): New York, NY, USA, 2002.
- Ryżak, M.; Bartmiński, P.; Bieganowski, A. Methods for determination of particle size distribution of mineral soils. Acta Agrophysica 2022, 4, 1–84. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Package ‘factoextra’: Extract and Visualize the Results of Multivariate Data Analyses; The R Foundation: Ames, IA, USA, 2017; Volume 76. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 29 May 2023).
- Anderson, T.H.; Domsch, K.H. Carbon assimilation and microbial activity in soil. Z. Pflanzenernährung Bodenkd. 1986, 149, 457–468. [Google Scholar] [CrossRef]
- Kolesnikov, S.I.; Vernigorova, N.A.; Kuzina, A.A.; Kazeev KSh Kostenko, I.V.; Timoshenko, A.N.; Ter-Misakyants, T.A.; Nevedomaya, E.N.; Akimenko, Y.V. The limits of soil and ecosystems of the Crimea to heavy metal pollution. Ecol. Ind. Russ. 2019, 23, 56–60. [Google Scholar] [CrossRef]
- Aleinikova, G.Y. Phenology of grapes under local climate change. In Proceedings of the International Scientific and Practical Conference “Actual Problems of Viticulture and Winemaking: Fundamental and Applied Aspects”, Yalta, Ukraine, 23–27 October 2018. [Google Scholar]
- Lisetsky, F.N.; Smekalova, T.N. Ampelopedological and ecological features of viticulture in the rural district of Kalos Limena. In Proceedings of the III International Scientific-Practical Conference, Kalos Limen, Simferopol, Russia, 29–31 May 2017; pp. 110–117. [Google Scholar]
- Matskul, A.V.; Korotkova, T.G. Ecological Safety of Wine Products in the System “Soil-Grape-Wine”. Sci. Proc. Kuban State Technol. Univ. 2019, 3, 853–863. [Google Scholar]
- Kovalevskaya, N.P.; Zavyalova, N.E.; Sharavin, D.Y.; Fomin, D.S. Biological activity of sod-podzolic soil in a long-term experiment with different agronomic practices. Russ. Agric. Sci. 2019, 38–41. [Google Scholar] [CrossRef]
- Aristarkhov, A. Sulfur in Russian agroecosystems: Monitoring the content in soils and the effectiveness of its application. Int. Agric. J. 2016, 39–47. [Google Scholar]
- Susyan, E.A.; Wirth, S.; Ananyeva, N.D.; Stolnikova, E.V. Forest succession on abandoned arable soils in European Russia—Impacts on microbial biomass, fungal-bacterial ratio, and basal CO2 respiration activity. Eur. J. Soil Biol. 2011, 47, 169–174. [Google Scholar] [CrossRef]
- Stoev, K. Physiology of Grape and Bases of Its Cultivation: Volume 2 Growth and Development of Grapevine; Stoev, K., Ed.; Book on Demand: Moscow, Russia, 2013; 386p. [Google Scholar]


| Farm Number | Depth, cm | Land-Use System | pH (H2O) | Sand | Clay | Silt | Corg | P2O5 | K2O | S |
|---|---|---|---|---|---|---|---|---|---|---|
| pH Unit | % | % | % | % | mg kg−1 | mg kg−1 | mg kg−1 | |||
| 1 | 0–10 | Organic | 7.5 | 20 | 15 | 65 | 1.48 | 84.8 | 521 | 28.1 |
| 10–20 | 7.8 | 2.47 | 48.8 | 551 | 16.3 | |||||
| 2 | 0–10 | 7.9 | 40 | 28 | 32 | 3.53 | 21.3 | 575 | 29.2 | |
| 10–20 | 7.8 | 2.48 | 3.80 | 168 | 17.1 | |||||
| 3 | 0–10 | 6.5 | 35 | 30 | 35 | 2.28 | 14.6 | 428 | 10.7 | |
| 10–20 | 6.7 | 2.21 | 8.10 | 433 | 9.40 | |||||
| 4 | 0–10 | 7.9 | 35 | 20 | 45 | 2.78 | 14.3 | 478 | 13.7 | |
| 10–20 | 7.9 | 2.48 | 1.94 | 328 | 12.3 | |||||
| 5 | 0–10 | 7.2 | 20 | 5 | 75 | 3.48 | 43.8 | 521 | 10.6 | |
| 10–20 | 7.3 | 3.84 | 46.9 | 514 | 7.60 | |||||
| 6 | 0–10 | 7.2 | 40 | 35 | 25 | 1.93 | 31.4 | 359 | 37.8 | |
| 10–20 | 7.4 | 1.74 | 35.2 | 410 | 26.3 | |||||
| 7 | 0–10 | Fallow | 8.3 | 40 | 25 | 35 | 3.31 | 29.0 | 511 | 6.40 |
| 10–20 | 8.4 | 3.09 | 17.9 | 408 | 5.70 | |||||
| 8 | 0–10 | 8.2 | 35 | 30 | 35 | 3.64 | 50.0 | 475 | 3.60 | |
| 10–20 | 8.2 | 3.83 | 19.0 | 485 | 3.10 | |||||
| 9 | 0–10 | 8.3 | 45 | 35 | 20 | 3.17 | 10.7 | 556 | 7.80 | |
| 10–20 | 8.3 | 3.08 | 4.70 | 374 | 6.40 | |||||
| 10 | 0–10 | Traditional | 7.9 | 35 | 30 | 35 | 3.53 | 21.3 | 575 | 39.2 |
| 10–20 | 7.9 | 3.41 | 17.6 | 475 | 4.70 | |||||
| 11 | 0–10 | 7.9 | 30 | 25 | 45 | 3.49 | 20.9 | 578 | 3.60 | |
| 10–20 | 8.1 | 3.28 | 11.5 | 547 | 3.40 | |||||
| 12 | 0–10 | 8.1 | 35 | 25 | 40 | 4.34 | 49.2 | 1109 | 2.10 | |
| 10–20 | 8.2 | 4.13 | 60.7 | 1152 | 2.10 | |||||
| 13 | 0–10 | 7.5 | 40 | 20 | 40 | 2.04 | 138 | 709 | 5.70 | |
| 10–20 | 7.6 | 1.96 | 57.4 | 729 | 4.20 | |||||
| 14 | 0–10 | 8.1 | 20 | 10 | 70 | 3.24 | 5.20 | 373 | 7.80 | |
| 10–20 | 8.2 | 3.19 | 6.10 | 431 | 7.40 |
| Farm Number | Depth, cm | Land-Use System | BR | SIR | Cmic | qCO2 | Cmic/Corg | qCO2/Corg | QR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0–10 | Organic | 0.59 | 9.05 | 363 | 1.63 | 2.45 | 110 | 0.07 |
| 10–20 | 0.67 | 8.57 | 344 | 1.94 | 1.39 | 78.5 | 0.08 | ||
| 2 | 0–10 | 0.48 | 6.27 | 251 | 1.90 | 0.71 | 53.8 | 0.08 | |
| 10–20 | 0.24 | 8.47 | 340 | 0.69 | 1.37 | 27.8 | 0.03 | ||
| 3 | 0–10 | 0.74 | 22.7 | 907 | 0.82 | 3.98 | 36.0 | 0.05 | |
| 10–20 | 0.53 | 21.4 | 856 | 0.61 | 3.87 | 27.6 | 0.02 | ||
| 4 | 0–10 | 0.74 | 15.6 | 624 | 1.20 | 2.24 | 43.2 | 0.05 | |
| 10–20 | 0.20 | 2.13 | 86.0 | 2.16 | 0.35 | 87.1 | 0.09 | ||
| 5 | 0–10 | 1.50 | 19.9 | 797 | 1.90 | 2.29 | 54.6 | 0.08 | |
| 10–20 | 0.92 | 7.20 | 288 | 3.21 | 0.75 | 83.6 | 0.13 | ||
| 6 | 0–10 | 0.25 | 8.30 | 333 | 0.73 | 1.73 | 37.8 | 0.03 | |
| 10–20 | 0.50 | 9.00 | 359 | 1.41 | 2.06 | 81.0 | 0.06 | ||
| 7 | 0–10 | Fallow | 0.45 | 2.03 | 82 | 5.57 | 0.26 | 168 | 0.22 |
| 10–20 | 0.11 | 3.19 | 128 | 0.84 | 0.41 | 27.2 | 0.03 | ||
| 8 | 0–10 | 0.21 | 3.10 | 125 | 1.70 | 0.34 | 46.7 | 0.07 | |
| 10–20 | 0.06 | 4.46 | 179 | 0.33 | 0.47 | 8.60 | 0.01 | ||
| 9 | 0–10 | 0.29 | 1.98 | 80.0 | 3.61 | 0.25 | 114 | 0.15 | |
| 10–20 | 0.31 | 2.50 | 100 | 3.14 | 0.32 | 102 | 0.12 | ||
| 10 | 0–10 | Traditional | 0.48 | 6.27 | 251 | 1.90 | 0.71 | 53.8 | 0.08 |
| 10–20 | 0.10 | 3.87 | 155 | 0.63 | 0.45 | 18.5 | 0.03 | ||
| 11 | 0–10 | 0.30 | 3.52 | 141 | 2.15 | 0.40 | 61.6 | 0.09 | |
| 10–20 | 0.18 | 7.52 | 302 | 0.58 | 0.92 | 17.7 | 0.02 | ||
| 12 | 0–10 | 0.21 | 2.11 | 85.0 | 2.44 | 0.19 | 56.2 | 0.10 | |
| 10–20 | 0.13 | 3.04 | 122 | 1.11 | 0.30 | 26.9 | 0.04 | ||
| 13 | 0–10 | 0.54 | 5.89 | 236 | 2.31 | 1.16 | 113 | 0.09 | |
| 10–20 | 0.09 | 4.85 | 194 | 0.45 | 0.99 | 23.0 | 0.02 | ||
| 14 | 0–10 | 0.11 | 2.28 | 92.0 | 1.23 | 0.28 | 38.0 | 0.05 | |
| 10–20 | 0.33 | 2.13 | 86.0 | 4.01 | 0.27 | 126 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabechaya, V.; Andreeva, I.; Morev, D.; Yaroslavtsev, A.; Neaman, A.; Vasenev, I. Exploring the Influence of Diverse Viticultural Systems on Soil Health Metrics in the Northern Black Sea Region. Soil Syst. 2023, 7, 73. https://doi.org/10.3390/soilsystems7030073
Gabechaya V, Andreeva I, Morev D, Yaroslavtsev A, Neaman A, Vasenev I. Exploring the Influence of Diverse Viticultural Systems on Soil Health Metrics in the Northern Black Sea Region. Soil Systems. 2023; 7(3):73. https://doi.org/10.3390/soilsystems7030073
Chicago/Turabian StyleGabechaya, Valeria, Irina Andreeva, Dmitriy Morev, Alexis Yaroslavtsev, Alexander Neaman, and Ivan Vasenev. 2023. "Exploring the Influence of Diverse Viticultural Systems on Soil Health Metrics in the Northern Black Sea Region" Soil Systems 7, no. 3: 73. https://doi.org/10.3390/soilsystems7030073
APA StyleGabechaya, V., Andreeva, I., Morev, D., Yaroslavtsev, A., Neaman, A., & Vasenev, I. (2023). Exploring the Influence of Diverse Viticultural Systems on Soil Health Metrics in the Northern Black Sea Region. Soil Systems, 7(3), 73. https://doi.org/10.3390/soilsystems7030073






