Abstract
Salt-affected soils have detrimental effects on agriculture and ecosystems. However, these soils can still be used for halophyte (salt-tolerant plants) cultivation using brackish and/or saline water. In this study, we employed soil technologies and mutualistic microorganisms as a sustainable strategy to improve the growth and reproduction of the halophyte Limonium algarvense Erben’s growth and reproduction under saline conditions. A microcosm assay was conducted under controlled greenhouse conditions to cultivate L. algarvense using a saline Fluvisol (FLU) amended—or not—with a Technosol (TEC). Plants were inoculated with the arbuscular mycorrhizal fungus (AMF) Rhizoglomus irregulare and/or a consortium of plant growth-promoting bacteria (PGPB), and they were irrigated with estuarine water. Soil enzyme analysis and physicochemical characterisation of the soils, collected at the beginning and at the end of the assay, were carried out. The physiological status of non-inoculated and inoculated plants was monitored during the assay for 4 months, and AMF root colonisation was evaluated. In FLU, only plants inoculated with the AMF survived. These plants had lower number of leaves, and shoot and root dry biomass than the ones grown in the TEC by the end of the assay. In the TEC, PGPB inoculation led to higher NDVI and PRI values, and AMF inoculation promoted higher reproductive development but not pollen fertility. The findings show that the combined use of soil and microbial technologies can be successfully applied to cultivate L. algarvense, suggesting their generalized use for other Limonium species with economic interest, while contributing to the sustainable use of marginal lands.
1. Introduction
Soil salinization, which results from the presence of soluble salts in the soil and/or irrigation water, is currently one of the main causes of soil degradation [1]. The main contributors to this situation include the mineralogical and chemical properties of parent materials, topography, specific climate types (particularly arid and semi-arid climates), groundwater composition, sea/tidal water levels, windblown salt particles, and the influx of salt-laden floodwaters and runoff from affected regions [1,2]. During certain periods of the year, saline soils contain elevated concentrations of soluble salts, such as chlorides, as well as sulphates of Ca, Mg, Na, and K. This salinity gives rise to what is known as a salic horizon, as seen in Solonchaks [3]. Additionally, other soil types found in low-lying regions, influenced by marine tides, can also display considerable salinity [4]. This is the case for saline Fluvisols found in alluvial areas, which are formed through the accumulation of marine and/or fluvial sediments, as seen in saltmarshes [4,5,6]. These areas, located at the interface between land and estuarine waters, experience the influence of bi-daily tides in European estuaries [7].
Saltmarshes are predominantly inhabited by halophyte species, which are plants capable of tolerating high salinity levels [8]. These unique plants make up approximately 1% of the world’s flora, and they are relatively uncommon among angiosperms [8]. To adapt to the saline stress in their environment, these species have developed various mechanisms at the biochemical, physiological, anatomical, and morphological levels [8,9,10,11,12]. Another survival strategy involves forming associations with different halotolerant soil microorganisms, such as plant growth-promoting bacteria (PGPB) and arbuscular mycorrhizal fungi (AMF). These microorganisms play a vital role in activating several mechanisms and pathways within their host plants, aiding them in coping with salinity stress and enabling their survival in saline environments [13,14]. Both PGPB and AMF play a crucial role in promoting plant growth and development through various mechanisms. They improve nutrient uptake, produce phytohormones, such as auxins, gibberellins, and abscisic acid, and enhance the plant’s tolerance to saline stress. Among the ways they achieve this is by activating membrane transporters, such as Na+/H+ antiporters, which help the plant cope with high salt levels and contribute to improving plant–water relations [15,16,17,18,19]. Moreover, AMF and PGPB can also lead to an increase in photosynthetic pigments and glutathione levels in their hosts [20], which, in turn, results in a decrease in ROS levels and lipid peroxidation [21,22].
The major uses of halophytes are related to their applications as fodder, forage, grazing, ornamental, and landscape plants, as well as for bioremediation purposes and their potential for food and medicine [23]. Halophilic species of the Limonium genus (Plumbaginaceae) [3], such as Limonium sinuatum, a native Mediterranean species found in saline regions, are widely used as dried flowers [24] and extensively cultivated [25]. Limonium algarvense Erben, native to the Iberian–Moroccan (west Mediterranean) coast [26], is a recretohalophyte with salt glands meant for excreting excess Na+ out of the plants to avoid salt stress [10]. This species is very attractive for the nutraceutical industry due to high phenolic compound (phenolic acids, flavonoids, and tannins) content, as well as to a nutritional profile rich in polyunsaturated acids, and it can be cultivated using saline soil and saline water [27,28]. However, this and other Mediterranean endemic halophytes with a protection status are threatened due to estuary degradation (e.g., anthropic pressures) and invasive species competition (e.g., Carpobrotus edulis (L.) N.E.Br.) [5,29]. Therefore, the sustainable cultivation of these halophytes is crucial to decrease these species’ overexploitation in their natural habitats [30].
Saline soils are unsuitable for non-halophytes, which include most crops. However, they provide a unique opportunity for cultivating salt-tolerant plants [31]. Most of these species can thrive under conditions with NaCl concentrations around 200 mmol/dm3 or approximately 20 dS/m electrical conductivity (EC) [8], and their growth can be stimulated within a salinity range of 15–25 dS/m [32]. The abundance of Na in the available soil fraction complex leads to the dispersion of clay and organic matter, as well as the rupture of aggregates. Consequently, these soils exhibit a poor structure, primarily characterized by microporosity and low hydraulic conductivity (infiltration and percolation), resulting in limited aeration [33].
Although the use of marginal saline soils can be promising for halophyte cultivation, the improvement of some soil characteristics and the development of agronomic techniques adapted to this soil–plant system are required. In this context, the construction of Technosols, an ecotechnology based on a pedo-engineering approach, can be used as a salinity mitigation strategy. These tailor-made soils are developed from inorganic and organic waste materials, and they serve the purpose of environmental rehabilitation [34]. They have been used for the recovery of degraded/sediments, dredged fluvial/marine sediment management [27,35], mining soils and tailings [34,36,37,38,39], industrial soils [40,41], and saline soil management [27,30]. They present a strong anthropic influence with more than 20% (V:V) of the artefacts in the upper 100 cm [4]. Previous studies on L. algarvense employed tailor-made soils using aquaculture sediments that accumulate at the bottom of ponds, and these sediments were further disposed on the land adjacent to these ponds (slopes, marachas), in saltmarshes [27].The aim of the current study was to evaluate the use of a saline Fluvisol, amended with a mixture of organic and inorganic wastes (referred to as a Technosol), as well as the inoculation of beneficial microorganisms (PGPB and AMF) for promoting the growth and reproduction of the valued marine halophyte L. algarvense when irrigated with estuarine water. The underlying hypothesis was that the combination of PGPB, AMF, and a Technosol could synergistically improve plant growth, as well as vegetative and reproductive development.
2. Materials and Methods
2.1. Microcosm Assay
A microcosm experiment was conducted using a Fluvisol (FLU) obtained from the Sobralinho salt-marsh area (38°54′16.1″ N, 9°01′09.0″ W; Vila Franca de Xira, west coast of Portugal; ICNF, 2017). The salt tide in this area reaches approximately 50 km upstream from the river mouth [42]. To create a Technosol, a mixture of organic and inorganic wastes was manually combined and then added, as an amendment, to the FLU. This amended soil is referred to as TEC, and it consists of 85% FLU and 15% Technosol, as described in Cortinhas et al. [30]. The organic/inorganic waste mixture used for the Technosol was composed of sludge and waste kieselguhr from breweries, as well as medium sand (0.25 mm < Ø < 0.5 mm), gravel limestone (2 mm < Ø < 5 mm), and residual biomass obtained from pruning. The proportions used were 1.5:0.5:3:2:3 (by mass), respectively, as detailed in Cortinhas et al. [30]. Both the FLU and TEC were placed in pots and incubated in the dark, at 70% of their maximum water-holding capacity, for a period of 28 days. After incubation and before transplanting the seedlings, four composite soil samples (0–15 cm depth) were collected from each pot and subjected to analysis.
For the microcosm assay, L. algarvense seeds collected from plants grown in saltmarshes in Castro Marim (Guadiana estuary, Algarve, Portugal) were germinated in transparent boxes containing wet filter paper in a growth chamber (Rumed), as described previously [43]. After 2 weeks, seedlings were transferred to individual pots with sterilized sand (120 °C for 1 h, in 2 alternate days) as substrate. At that time, soils of half of the pots were inoculated with an AMF (see Section 2.3). The plantlets grew under controlled temperature, humidity, and photoperiod conditions (20–25 °C, 60% relative humidity and 16/8 h day/night photoperiod), and they were irrigated with deionised water. Every 2 weeks, 10 mL of Hoaglands solution [44] was applied to each plant.
After 3 months, plants with ~10 cm were transplanted to 4 experimental conditions (non-inoculation, inoculated with PGPB, inoculated with AMF, and double-inoculated with AMF and PGPB) in FLU or TEC soils, with 5 replicates each (40 plants in total), and left under greenhouse conditions with natural light from June to October (2021). From that moment on, inoculation with PGPB was conducted twice a month, in the corresponding treatments, until the end of the experiment (see Section 2.3). Plants were irrigated with estuarine water (VF) collected from a channel located in the Tagus estuary, keeping the soils at 70% of the maximum water-holding capacity. The experiment remained under those conditions for 4 months. At the end of the experiment, soil samples were collected from each pot for physicochemical and soil enzymatic activity analyses.
2.2. Soils and Estuarine Water Analyses
Soil samples of both FLU and TEC were characterized for physicochemical parameters and enzymatic properties at the beginning and the end of the experiment. The FLU and the TEC (fraction < 2 mm) were physicochemically characterised for the same parameters and methods described in Cortinhas et al. [30]: pH and EC in a water suspension (1:2.5 m/V), extractable K (Kextract; Egner–Riehm method) and P (Pextract; Olsen method), total N (Ntotal; Kjeldahl method), organic C by wet combustion (Sauerland method, [45]), as well as macro- and micro-nutrients [46]. In FLU and TEC enzymatic activities (dehydrogenase, β-glucosidase, celullase, acid phosphatase and urease) were also analysed as biological soil parameters [47,48,49,50].
The estuarine water was analysed for pH, EC, and concentrations of chloride (Mohr method), as well as hydrogencarbonate (titration method using HCl solution methyl orange as indicator), P (molybdenum blue method [51]), Na, Ca, Mg, K, Fe, Zn, Mn, and Cu (atomic absorption spectrometry).
The concentration of mycorrhizal-infective propagules in FLU and TEC was analysed by the Most Probable Number Technique-MPN [52,53], with soil dilutions from 10−1 to 10−5 and the use of leeks as trap plants. After 5 months of growth in the greenhouse, leek roots from each soil dilution were stained with 0.05% Trypan blue in lactic acid, following the protocols of [54,55]. Root systems were observed under an optical microscope, and the presence/absence of mycorrhizal structures in each root system was annotated. The program of [56] was used to calculate the concentration of mycorrhizal infective propagules per gram of soil.
2.3. Microbial Inocula
Arbuscular mycorrhizal fungal (AMF) inoculum was provided by the Agrifood Institute of Research and Technology (IRTA, Cabrils, Barcelona, Spain), and it was composed by 100 mycorrhizal propagules of Rhizoglomus irregulare BEG72 per gram. Inoculation was done by placing a layer of inoculum (7.5 g in total) in the middle of two sand layers in each pot.
Bacterial inoculum was composed of Vibrio kanaloae RA1, Pseudoalteromonas sp. RA8, Pseudoalteromonas rhizosphaerae RA15, and Staphylococcus warneri RA18 [57,58]. This inoculum was prepared, as described, by [59]. Briefly, each bacterial strain was incubated separately in Tryptic Soy Broth-TBS (Liofilchem, Roseto degli Abruzzi, Italy) overnight at 28 °C by shaking (115 rpm). Then, cultures with 108 cells/mL were washed with 0.9% sterile NaCl solution, centrifuged, and pellets were resuspended in 5 mL of the same sterile saline solution. The process was repeated, and the 4 bacteria were mixed in a 50 mL Falcon tube. Limonium algarvense plants were watered with the consortium diluted in the irrigation water, and the process was repeated fortnightly until the end of the experiment.
2.4. Plant Performance and Root Colonization Evaluation
Along the microcosm assay, the photochemical reflectance index (PRI) and the normalized difference vegetation index (NDVI) were measured with a PlantPen model PRI 200 and NDVI 300, respectively, as indicators of the plant performance [30]. Measurements were performed once a month in three random leaves per plant.
At the end of the experiment, the number of leaves per plant was counted, and shoots, roots, and inflorescences were separated to determine the fresh biomass using a digital balance. Then, root systems were stained following the protocols of [54,55]. There were 10 stained root segments (1 cm long) per experimental treatment placed in slides, and the presence/absence of mycorrhizal structures was annotated in each one. Since those roots also appeared to be colonized by dark septate endophytes (DSE), their presence/absence was also annotated, and the percentage of root colonization by AMF and DSE was calculated for each root system.
2.5. Pollen Fertility
Pollen fertility was estimated by analysing pollen tube germination, as in [43]. Three flowers per plant (five fresh anthers per flower) and three plants per experimental treatment were used to analyse tube germination in vitro.
Pollen grains were collected from plants immediately after anther dehiscence and placed in a culture medium containing 20 mmol/dm3 boric acid, 6 mmol/dm3 calcium nitrate, 0.1% casein hydrolysate, and 7% sucrose [60]. To create suitable physical conditions for pollen germination, a dialysis tubing and filter paper support, combined with 23% polyethylene glycol-20,000 as an osmoticum, were used in the medium. The incubation of pollen grains took place at 37 °C in the dark, lasting either 48 h or 72 h.
Pollen grains were considered germinated when pollen tube length equalled or exceeded the diameter of the pollen grain. To analyse the germinated pollen tubes, ten random samples were selected from each treatment. The measurements were taken on micrographs using a 63× objective on a Zeiss Axioskop 2 fluorescence microscope, and the images were captured with an AxioCam MRc5 digital camera (Zeiss, Jena, Germany).
2.6. Statistical Analyses
At the beginning of the experiment, soil physicochemical characteristics and soil enzymatic activities between FLU and TEC were compared by a t-test or by a Mann–Whitney U test when data did not follow a normal distribution.At the end of the experiment, the data collected from different soils under various microbial inoculation treatments were analysed separately for the TEC and FLU conditions. The soil type factor could not be included in a factorial ANOVA due to the non-survival of plants from two experimental groups in the FLU (non-inoculated and PGPB-inoculated plants).
In TEC, a two-way ANOVA was conducted to determine the effects of AMF and PGPG inoculation, as well as of their interaction, on soil physicochemical characteristics, soil enzymatic activities, the number of leaves, shoot and root fresh biomass, dark septate endophyte (DSE) colonization percentage, and the monthly collected NDVI and PRI data. Data from FLU were analysed using a t-test, comparing AMF and AMF + PGPB treatments. Additionally, subsequent t-tests were performed to compare plant parameters in FLU and TEC for both the AMF and AMF + PGPB treatments. Mycorrhizal colonization data in AMF-inoculated plants were compared by a one-way ANOVA test, followed by Duncan’s post hoc test.
All analyses were performed using SPSS Statistics vs. 23 (IBM) program.
3. Results
3.1. Characterisation of Irrigation Water and Soils
The estuarine water had neutral pH, presenting high concentrations of chloride, hydrogencarbonate, Na, K, Ca, and Mg (Table 1).

Table 1.
Chemical characteristics of the estuarine water used in the experiment. Data correspond to the average value of three technical repetitions ± standard error. EC: Electric Conductivity. DL—Detection limit.
At the beginning of the experiment, the initial analyses of the soils showed that FLU presented slight alkaline pH and very high salinity (Table 2). Although total N concentrations can be considered as medium, organic C and extractable P and N (N-NH4 and N-NO3) concentrations, important to plant and microorganism growth, were low (Table 1). Nonetheless, extractable K concentration was high. In the TEC, the pH values were also slightly alkaline, but the application of the waste mixture to the FLU contributed to an increase in organic C and some elements’ concentrations in the available fraction (e.g., N-NO3, P, Ca, Na, Cu, and Zn; Table 1). The case of extractable P concentration, which was more than 30-fold higher in TEC compared to FLU (Table 2), is noteworthy. Acid phosphatase, β-glucosidase, urease, and dehydrogenase activities were also significantly higher in the TEC than in the FLU (Table 2). The largest difference was found in dehydrogenase activity, which was 9.8 times higher in the TEC. However, the number of propagules in the TEC (0.7 propagules per gram) tended to be lower than in the FLU (2.2 per gram).

Table 2.
Soil physicochemical characteristics, soil enzymatic activities, and the concentration of mycorrhizal propagules at the beginning of the experiment. Data correspond to the average value of four technical repetitions ± standard error. Different letters indicate significant differences according to t-test or to Mann–Whitney U test.
After 4 months of plant growth in FLU and TEC, soil properties were analysed again. We found a significant increase in EC, as well as in soil Na and Mg concentrations, in both FLU and TEC compared to the initial values. The EC was 3 times higher in both soils, and Na concentration was 6.5 times higher in FLU and 2.5 times higher in TEC, compared to the initial concentration. In contrast, FLU and TEC had lower concentrations of N-NH4, N-NO3, and Fe than the initial soils (Table 2 and Table 3).

Table 3.
Physicochemical and biological characteristics of Fluvisols (FLU) and amended Fluvisols (TEC) after 4 months of Limonium algarvense plants’ growth under four inoculation treatments (non-inoculated, inoculation with bacterial consortium-PGPB, inoculation with the mycorrhizal fungus-AMF, and AMF + PGPB). Data represent mean values ± standard error. Different lower-case letters indicate statistical differences between means in the TEC, according to the Duncan post hoc test (or Dunn test when data did not fulfil normality or variance homogeneity conditions), and different capital letters indicate significant differences between means in the FLU, according to the t-student test. The asterisk represents plants that did not survive.
When L. algarvense plants were transplanted to those soils, non-mycorrhizal plants did not survive in the FLU. Therefore, the pots containing those plants were discarded, and the soils were not analysed at the end of the experiment. However, although we could not study the effect of the soil type factor in a full factorial ANOVA, several trends could be observed between FLU and TEC. The most remarkable ones were the higher C, P, K, total N and NH4, and Zn concentrations in the TEC compared to the FLU. The case of P, where TEC had 337.32 g of P/kg on average and FLU had an average of 10.78 g/Kg, is noteworthy.
In the TEC, extractable P was significantly affected by AMF inoculation (p = 0.02; Table 4), and pots with AMF-inoculated plants tended to have lower soil P concentration than the ones with non-mycorrhizal plants (Table 3). Total N was significantly affected by both AMF and PGPB inoculations (p = 0.02 and p = 0.02, respectively; Table 4), but the mean comparison test did not show any significant differences among the experimental groups (Table 3). Nitrate (NO3) and NH4 concentrations were not affected by any of the microbial inoculation types, but in both cases, the non-inoculated treatment tended to have the highest vales. Soil Na and Ca concentrations showed significant interactions between AMF and PGPB factors (Table 4), with Na concentrations being significantly higher in pots with non-inoculated plants than in the other ones, and Ca concentrations being significantly higher in pots with double-inoculated plants than in the rest of the experimental groups (Table 4). Organic C, Mg, and K did not show an effect of the inoculant type.

Table 4.
p-values of the two-way ANOVA test conducted to study the effect of the inoculation with the arbuscular mycorrhizal fungus (AMF), the consortium of plant growth promoting bacteria (PGPB), as well as of their interaction in TEC (Fluvisol amended with Technosol).
Concerning micronutrients, in the TEC, all of them showed significant interactions between AMF and PGPB (Table 4). Iron, Zn, and Cu concentrations followed the same pattern: pots with double-inoculated plants had significantly lower concentrations than the other pots. Contrastingly, soil Mn concentrations were highest in AMF-inoculated plants and lowest in the AMF + PGPB-inoculated ones (Table 3).
As said before, in FLU, only AMF-inoculated plants (with and without PGPB) survived; therefore, data analysis by a two-way ANOVA was not possible. The t-test conducted to compare AMF and AMF + PGPB-inoculated plants indicated that organic C, as well as total N, Mg, and Zn, were significantly lower in pots with double-inoculated plants than in the AMF-inoculated ones.
When soil enzymatic activity was analysed after 4 months of plant growth in FLU and TEC, we observed a significant decrease in soil dehydrogenase activity in both soils, with a 4 and 6.5 times decrease, respectively (Table 2 and Table 3). Soil β-glucosidase and cellulase activities also decreased by 2.2 and 2.3 times, respectively, in TEC, but they remained at similar levels in FLU (Table 2 and Table 3).
On the other hand, at the end of the experiment, FLU had lower levels of β-glucosidase (56%), acid phosphatase (71%), urease (55%), and dehydrogenase activities (83%) compared to the TEC (Table 3).
When soil enzyme activity data were analysed individually for each type of soil in TEC, although the two-way ANOVA did not show a significant effect for AMF or PGPB-inoculation factors, the multiple comparison test showed that PGPB-inoculated treatment (without AMF) had significantly higher β-glucosidase and acid phosphatase activity values than the AMF-inoculated treatment (without PGPB) (Table 3). No significant differences were found between AMF and AMF + PGPB treatments in FLU.
3.2. Evolution of Physiological Parameters of Plants with Microbial Inoculations Grown in Fluvisols (FLU) and Amended Fluvisols (TEC)
As previously said, 1 week after L. algarvense transplantation into FLU (July), both non-inoculated and PGPB-inoculated plants did not tolerate the new conditions, and all the individuals died. In the remaining plants, the vegetative indices, NDVI and PRI, were monitored every month.
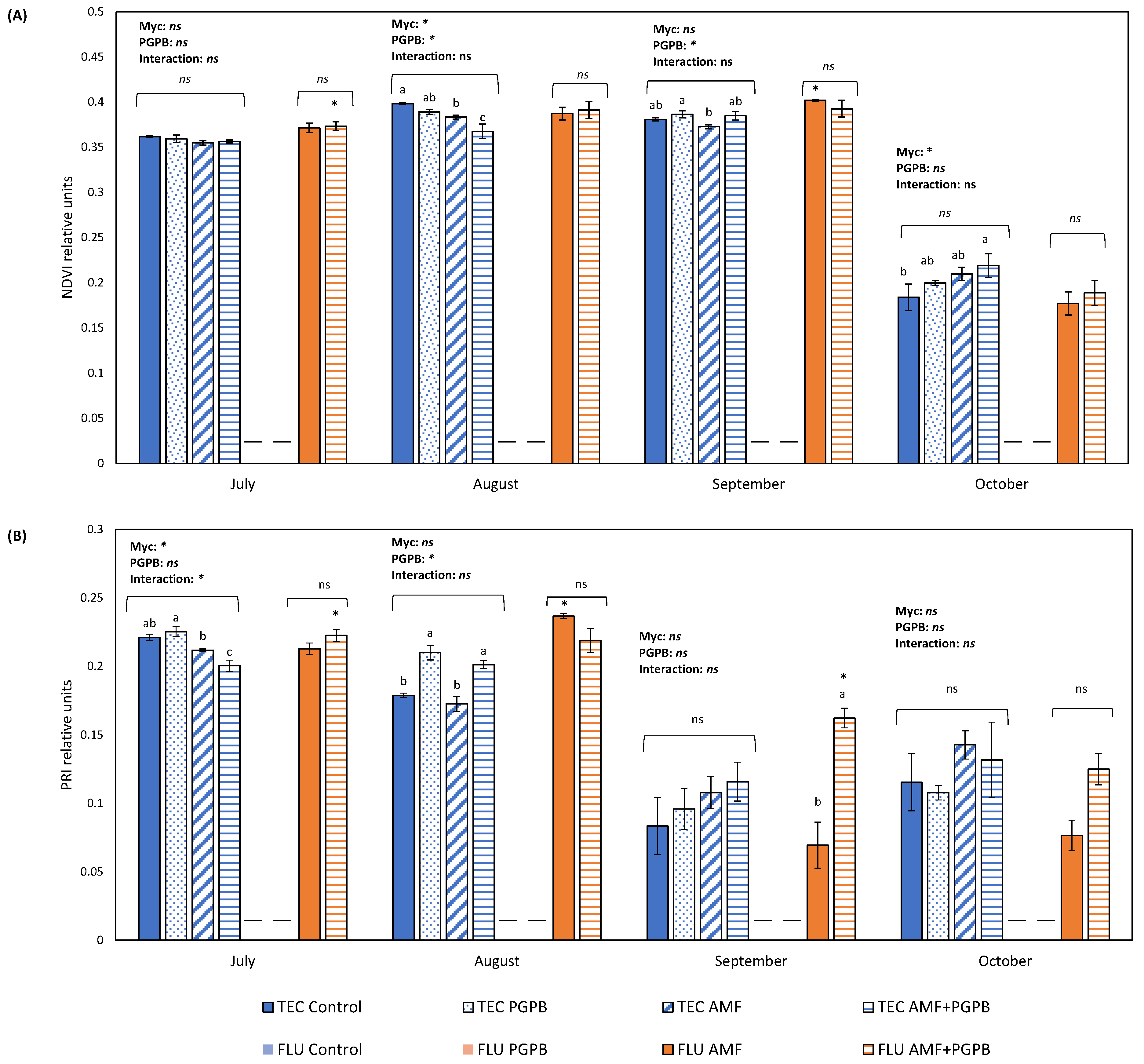
In TEC, both PGPB and AMF inoculations had a significant effect on this parameter. Two months after transplant (August), PGPB inoculation had a significant negative effect in NDVI, but in September, 3 months after transplant, the effect became positive (Figure 1A). Mycorrhizal inoculation also had a negative effect in NDVI in August, but this trend changed 4 months after transplant (October), and AMF-inoculated plants were the ones with the highest NDVI values (Figure 1A). Contrastingly, in the FLU, no significant differences were observed between AMF-inoculated and PGPB + AMF-inoculated plants.
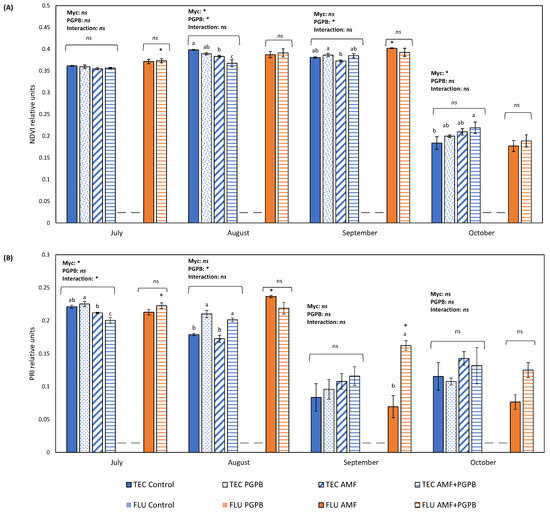
Figure 1.
(A) Normalized difference vegetation index (NDVI) and (B) photochemical reflectance index (PRI) of Limonium algarvense plants during 4 months in 2 different substrates (FLU—Fluvisol and TEC—Fluvisol amended with Technosol) and with 4 inoculation treatments (non-inoculated, inoculation with bacterial consortium-PGPB, inoculation with the mycorrhizal fungus-AMF, and AMF + PGPB). Bars represent mean values ± standard error. Different letters indicate statistical differences between means, and “ns” indicates non-significant differences. The asterisk above the bars indicates significant differences in AMF and AMF + PGPB-treated plants between TEC and FLU. The dashes within the chart indicate that all plants from the respective experimental group died. On the top of the bars, the results of the two-way ANOVA, conducted to study the effect of the mycorrhizal inoculation (AMF) and plant growth promoting bacteria inoculation (PGPB), as well as of their interaction, are indicated. The asterisk represents a significant effect at p = 0.05.
When comparing this parameter between TEC and FLU, we observed differences in July for AMF + PGPB-inoculated plants and in September for AMF-inoculated plants. In both cases, NDVI values were higher in FLU than in TEC.
Regarding PRI, when the inoculation treatments were analysed separately for each type of soil, in the TEC, a significant negative effect of AMF inoculation was found 1 month after transplant (July) (Figure 1B), and 1 month later, and a significant positive effect was observed for PGPB inoculation (Figure 1B). However, the different microbial inoculations no longer had a significant effect on PRI in September and October. In FLU, 3 months after transplant (September), plants inoculated with AMF and PGPB had significantly higher values than plants inoculated with just AMF. A month later, the trend was still the same, but it was not statistically significant (p = 0.07) (Figure 1B).
3.3. Effects of Microbial Inoculations and Technosols in L. algarvense Vegetative and Reproductive Growth
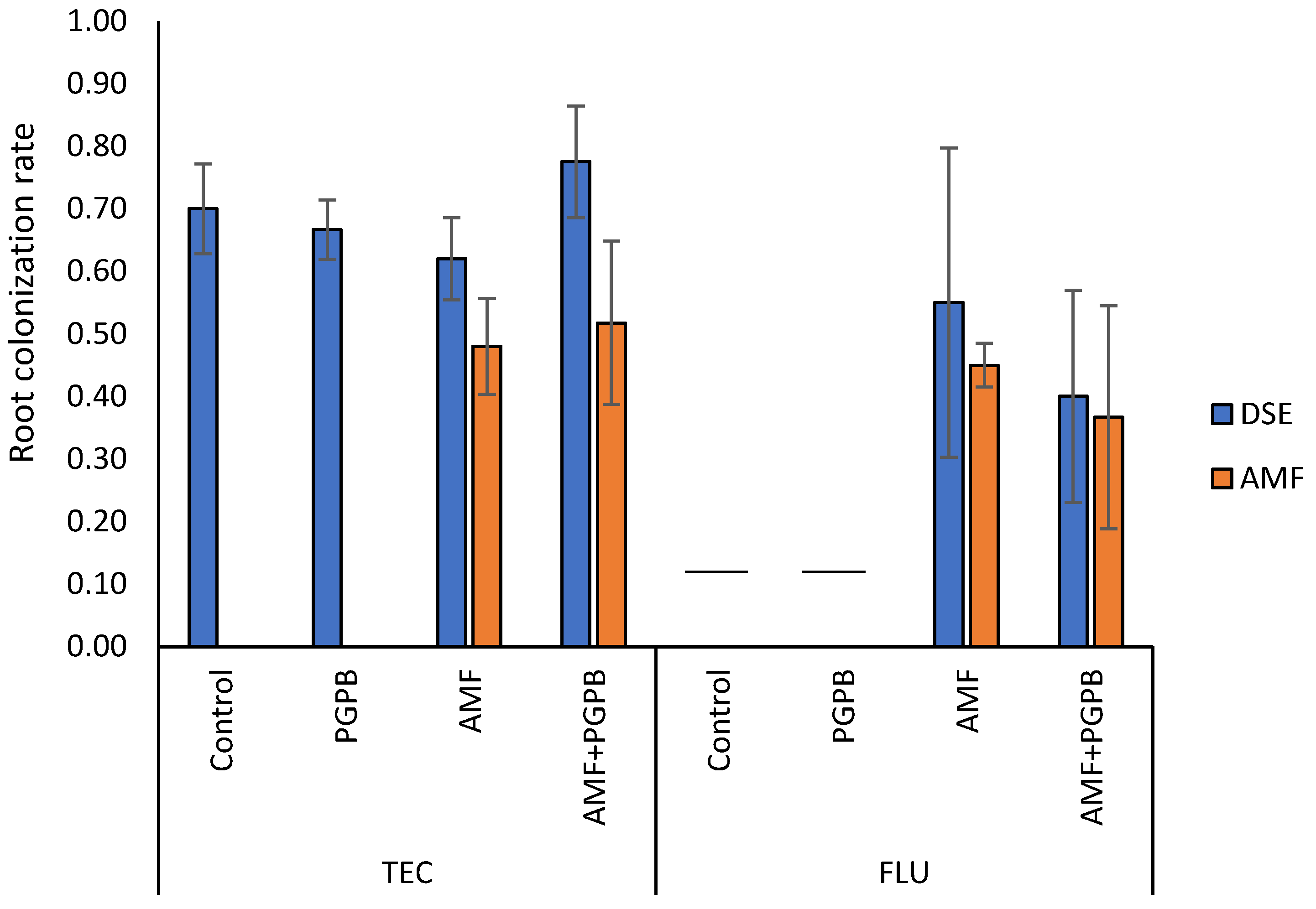
In TEC, after 4 months of plant growth, by the end of the experiment, no mycorrhizal colonization was observed in plants that were not inoculated with AMF. In FLU and TEC, root colonization was around 50% in both AMF-inoculated and AMF + PGPB-inoculated plants (Figure 2). Remarkably, all plants presented root colonization by DSE (Figure 2, Supplementary Figure S1), which was between 62% and 78%. No significant differences were found in this parameter between the experimental treatments (Figure 2).
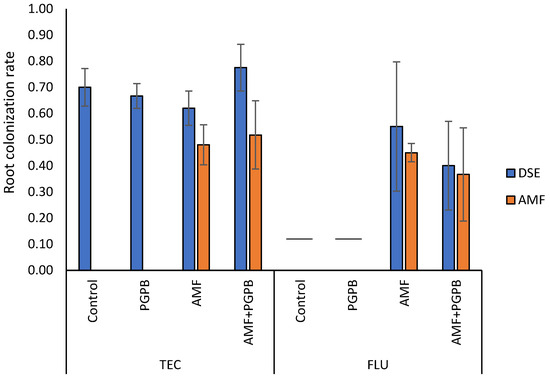
Figure 2.
Root colonization rate by arbuscular mycorrhizal fungi (AMF) and dark septated endophytes (DSE). Bars represent mean values ± standard error. Missing bars (the two dashes within the chart) indicate that all plants from the respective experimental group died. FLU—Fluvisol; TEC—Fluvisol amended with Technosol.
When plant growth parameters were analysed, in TEC, the two-way ANOVA, conducted to study the effects of AMF and PGPB inoculations, did not show any significant effect for any of the factors. Nevertheless, the number of leaves tended to be lower in PGPB-inoculated plants (p = 0.06), and shoot biomass tended to be higher in AMF-inoculated plants (p = 0.09) (Table 5A). In FLU, no differences in the number of leaves and shoot and root dry biomass between AMF-inoculated and AMF + PGPB-inoculated plants were found.

Table 5.
(A) p-values of the two-way ANOVA test conducted to study the effect of the inoculation with the AMF, the consortium of PGPB, as well as of their interaction in TEC. (B) Average values ± standard error of the n° of leaves, shoot fresh weight, and root fresh biomass. The asterisk indicates significant differences in AMF and AMF + PGPB-treated plants between TEC and FLU.
When the number of leaves, as well as shoot and root biomass, in both AMF-inoculated and AMF + PGPB-inoculated plants were compared between FLU and TEC, significant differences were observed. In all cases, the values were higher in TEC (Table 5B).
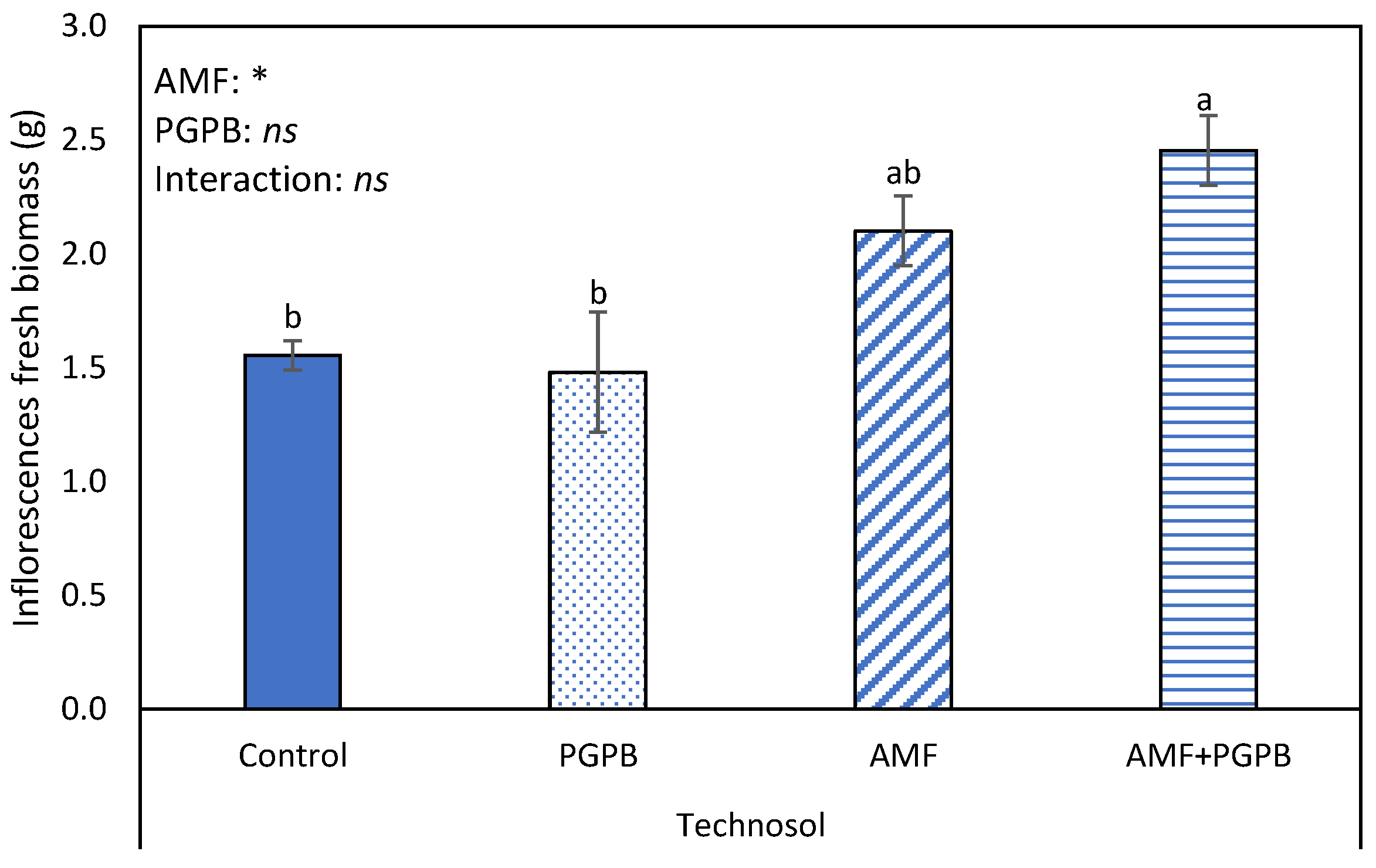
Regarding the reproductive growth in FLU, only one plant belonging to the AMF inoculation treatment developed an inflorescence. By contrast, in TEC, all plants had inflorescences (one or two scapes), and AMF inoculation had a positive significant effect on their fresh biomass (Figure 3).
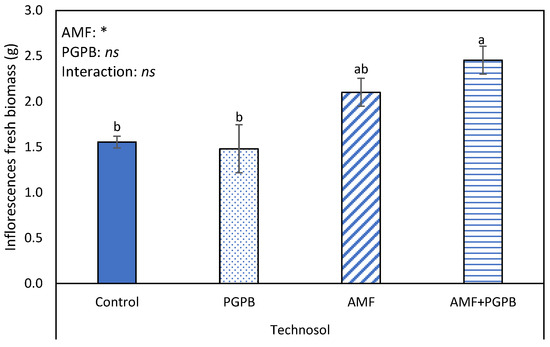
Figure 3.
Total fresh weight of Limonium algarvense inflorescences, inoculated or not, with an arbuscular mycorrhizal fungi (AMF) and a consortium of plant growth promoting bacteria (PGPB), growing in the amended Fluvisol (TEC). Bars represent mean values ± standard error. Different letters indicate statistical differences between means (p = 0.05). On the top of the bars, the result of the two-way ANOVA, conducted to study the effect of the AMF and PGPB, as well as of their interaction, is indicated. The asterisk represents a significant effect at p = 0.05, while “ns” indicates no significant effect.
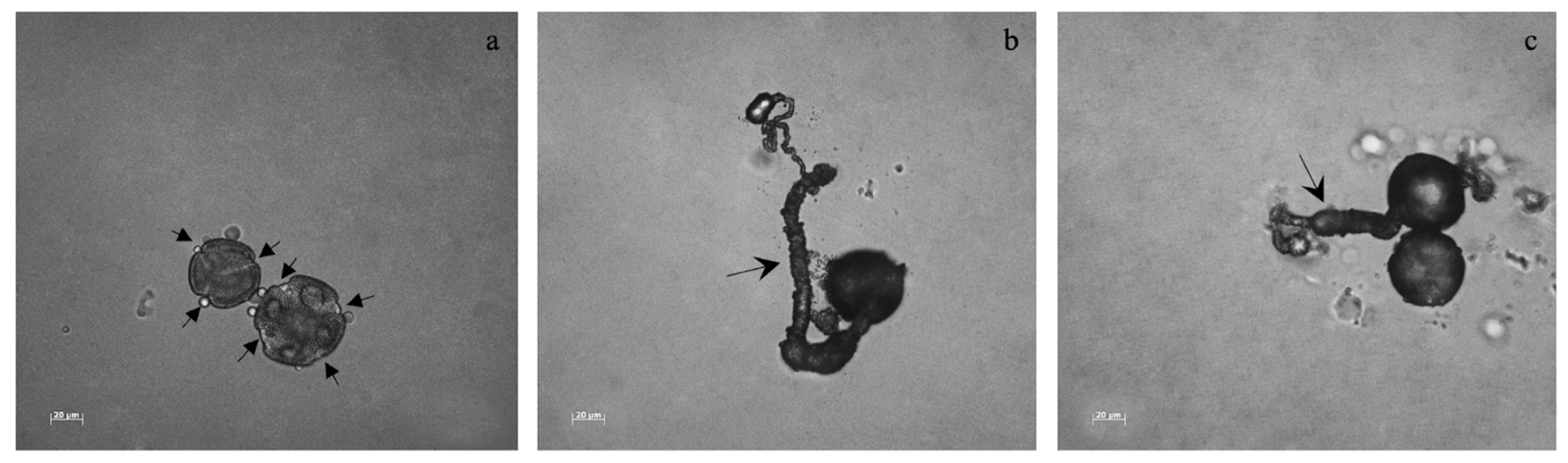
In plants bearing inflorescences, an average of 55 pollen grains per anther, with different sizes and different colpi numbers (3 to 5) were found. In general, pollen grain germination rate was low for all plants. The pollen grains that germinated (Figure 4) were usually the bigger ones with 4 colpi, with sizes ranging from 50–80 μm. Non-inoculated plants from TEC had the highest germination rate, ranging from 9% to 27% per flower, with a total of 0.60% considering all replicates, whereas the PGPB inoculated ones were the ones with the lowest pollen germination rate (1.8% per flower; 0.02% considering all the replicates).
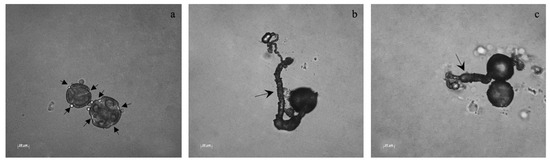
Figure 4.
Pollen grains and pollen tubes. Different pollen sizes and colpi apertures were noticed in all the treatments. The arrows in (a) indicate colpi in pollen grains. The arrow in (b) shows a pollen tube. The arrow in (c) indicate a pollen tube from a pollen grain with three colpi.
4. Discussion
4.1. Soil Technologies Improve Soil Properties
In this study on L. algarvense growth and development, using combined soil and microbial technologies, we found that the Technosol produced from organic and inorganic wastes improved the physicochemical characteristics and soil enzymatic activities of the FLU and, consequently, plant development and vegetative and reproductive growth.
The presence of wastes, particularly the organic ones, in the TEC resulted in an improvement in nutrient concentrations compared to the original FLU. At the beginning of the experiment, the TEC had higher levels of extractable P, organic C, as well as total N, N-NO3, Ca, Na, Mg, Cu, and Zn (Table 2). Over time, nutrient concentrations decreased, likely due to their absorption and utilization by plants and microorganisms to support growth and metabolic activities. However, even at the end of the experiment, the TEC treatment still exhibited higher concentrations of organic C, P, total N, N-NH4, and Zn compared to the FLU treatment (Table 3 and Table 4). This demonstrates the usefulness of this technology for improving the soil physicochemical characteristics of poor-quality soils, as also found in other works [38,61,62,63].
Regarding soil enzymes, their activities were also significantly higher in TEC than in FLU, especially acid phosphatase, β-glucosidase, and urease activities, which are associated to P, C, and N cycles, respectively [64], as well as soil dehydrogenase, which was the enzymatic activity showing the highest differences between both soil types. This enzyme activity serves as an indicator of the microbiological redox system and microbial oxidative activities in soils [65], and its activity stimulation can even occur in the absence of plants, as observed by [66]. Our results are, thus, in agreement with other authors [67] who also reported a significant stimulation of enzymatic activities in a saline soil (EC: 9.1 dS/m) with the application of different organic wastes. This demonstrates that the addition of inorganic and organic amendments stimulated the activity of soil microorganisms, leading to an improvement in soil functions in TEC when compared to the FLU.
However, after L. algarvense plant growth for 4 months, in both FLU and TEC, dehydrogenase showed a strong activity decrease in both soil types. Furthermore, notable reductions were also observed in β-glucosidase and cellulase activities, especially in the TEC. The significant increase in soil salinity may be responsible for such changes since salinity is known to be an important stress factor for microbial communities, and it can have a profound impact on their activities and overall nutrient cycling functioning [68,69,70,71,72,73]. Relevantly, at the end of the assay, an increase in EC, as well as extractable Na and Mg concentrations, were observed in both FLU and TEC, which became extremely saline (>3.2 dS/m; [74]). This could be attributed to the high salinity of the irrigation water (Table 1).
Some microorganisms, such as PGPB, are involved in soil nutrient cycles (e.g., by fixing atmospheric N, solubilizing inorganic phosphates, degrading organic matter through exo-enzyme excretion) [75]. At the end of the experiment, in pots with FLU and AMF + PGPB-inoculated plants, significantly higher organic C, total N, Mg, and Zn concentrations were found when compared to pots with AMF-inoculated plants (without PGPB). This could be attributed to the lytic enzymes produced by those bacteria that could contribute to the degradation of organic matter and the release of some nutrients into the soil [76,77]. Contrastingly, in TEC, no significant effects of either PGPB or AMF were found on soil enzyme activities. Since this soil was much richer in organic matter, the effect of PGPB in soil nutrient concentrations may have not been as evident as in the FLU, with significantly lower organic C concentrations.
Interestingly, in the TEC, inoculation with AMF + PGPB led to lower EC and Na values compared to the non-inoculated treatment. The microorganisms used in our study are halotolerant, as demonstrated by previous studies [78,79]. This kind of microorganism has evolved a series of mechanisms to tolerate salinity conditions, among which are the formation of biofilms [76], which may have contributed to reduce the concentration of salts in the soil. In addition, the mutualistic symbiosis of L. algarvense with those microorganisms may have led to changes in the root exudation of some compounds (e.g., polysaccharides, organic acids) that may also affect the growth, composition, and activity of microorganisms and improve soil properties [80]. This might, ultimately, lead to a decrease in EC and Na concentration. Another interesting hypothesis is that PGPB and AMF might have enhanced Na uptake, translocation, and further excretion in L. algarvense leaves by salt glands [10], contributing to a decrease in soil Na concentration.
4.2. Microbial and Soil Technologies Improve Limonium algarvense Development
In our study, considering that the applied wastes were rich in macro and micro-nutrients [27] and lead to soil fertility enhancement, the observed growth improvement in plants grown in the TEC was also highly expected. Accordingly, a greater number of leaves, as well as increased shoot and root dry biomass, were found in AMF and AMF + PGPB-inoculated plants grown in the TEC compared to those grown in the saline FLU (Table 5B).
Regarding microbial inoculations, to our knowledge, this is the first report showing beneficial effects of L. algarvense’s inoculation by AMF and PGPB, although some previous studies indicated successful mycorrhizal colonization in other species of the same genus, such as in Limonium echioides L. (Mill.) and Limonium sinuatum (L.) Mill [81,82]. The mycorrhizal fungus used in this study (R. irregulare BEG 72) was already tested in saline soils and halophyte species, with positive results found in improving plant growth under such conditions [78]. On the other hand, root colonization by different PGPB (species from the genera Bacillus, Glutamicibacter, Streptomyces, Pseudomonas, Klebsiella, Serratia, Arthrobacter, Isoptericola, and Microbacterium) has also been reported in Limonium sinense (Girard) Kuntze and Limonium vulgare Mill [83,84,85,86,87,88,89]. Furthermore, the inoculation of L. sinense plants with halotolerant PGPB has demonstrated to be a suitable strategy to improve plant growth under saline conditions [84].
In our work, we also show, for the first time, Limonium species root colonization by DSE. However, several previous works have dealt with the characterization of these endophytic fungi [90,91,92,93]. Dark septate endophytes integrate a polyphyletic fungal group within the Ascomycota. They colonize plant roots inter and intracellularly, forming septate (cross-walled), and mostly melanised hyphae and microsclerotia [94,95]. Despite their ubiquitous distribution and wide diversity of plant hosts [94], DSE commonly associate with plants growing in stressful environments affected by drought, soil salinity, potentially toxic elements, contamination, or poor fertility, supporting the hypothesis of “habitat-adapted symbiosis”, i.e., plant–DSE mutualism generally occurs under stressful conditions [96,97]. Although the exact ecological role of DSE is still not well-understood [94], their potential benefits in promoting plant growth, particularly in enhancing the host’s tolerance to environmental stress factors, have been proposed [96,98].
A month after L. algarvense plant transplantation into FLU, only the ones inoculated with AMF (with or without PGPB) survived. Arbuscular mycorrhizal fungi are known to alleviate transplant shock in crop plants (grapevine, riverhemp, avocado) [99,100,101] due to their capacity to enhance water absorption and plant–water status [99]. This mechanism may be crucial in saline Fluvisols, where plants have impaired water relations due to osmotic stress [102]. Even though L. algarvense is a halophyte, it is important to note that, prior to transplanting the seedlings into pots, all of them were irrigated with deionized water for a period of 3 months. Therefore, a sudden root environmental change from non-saline to saline conditions can cause saline stress that could lead to plant death if their osmotic adjustment to the new environment is not fast enough. Since AMF improve membrane integrity and permeability under saline conditions [103,104], in addition to stimulating ABA production, accumulating osmolytes, and promoting the uptake of osmotic equivalents such as K+ [76], mycorrhizal plants may have experienced faster adaptation to the non-amended saline soil and, potentially, a less intense transplantation shock compared to non-mycorrhizal plants.
In the FLU, the positive effect of PGPB inoculation in plants was only found 3 months after plant transplantation (in September). Plants double-inoculated with PGPB and AMF had higher PRI than the ones inoculated with the AMF alone. This trend was maintained until the end of the experiment, although it was no longer significant. Given that this parameter serves as an indicator of plant performance [30,105], our finding suggests that, in the FLU, the double inoculation with both AMF and PGPB is a viable strategy to support the development of L. algarvense.
The two vegetative indices, PRI and NDVI also showed a negative effect of the AMF inoculation 1 and 2 months after plant transplant to TEC. Although AMF colonization may have reduced transplantation shock, the AMF may drain a substantial amount of carbon from the plant, especially when they are being establishing in new roots and/or soil environments, which may cause an initial growth/performance decrease [106,107,108,109]. Nevertheless, at the end of the experiment (October), the trend inverted and both NDVI and PRI, as well as in shoot biomass, tended to be higher in AMF-inoculated plants.
Moreover, a significant positive effect of mycorrhizal inoculation was found in inflorescence fresh biomass. Several studies demonstrate that AMF may influence plant reproduction by advancing flowering time, increasing flower size, the amount of pollen, pollen germination, and pollen tube growth, as well as the seed number, biomass, and seed germination percentage [110,111]. Nevertheless, differences exist depending on the AMF species and soil P concentration [112]. In the current study, neither the AMF nor the PGPB inoculation led to a significant improvement in L. algarvense pollen fertility, as evaluated by pollen germination results. In commercial substrates, this species produced heterogeneous pollen in morphology and size, with moderate-to-no viability, which germinated poorly in vitro [113]. Since L. algarvense showed a high percentage of seeds per scape, with moderate-to-high germination [113], they are most probably originated by apomixis (asexual seed production).
Plant inoculation with PGPB in TEC also showed beneficial effects in L. algarvense development since a significant positive effect was found in NDVI by the end of the experiment (3 months after transplantation) and in PRI (2 months after transplant). However, PGPB inoculation tended to decrease the number of leaves in plants grown in the TEC. Since shoot fresh biomass did not differ from the non-inoculated plants, this result suggests that leaves were slightly larger than in the other experimental treatments. The bacteria used in the present study possessed plant growth-promoting activities, such as siderophore and auxin production, and were diazotrophic [49], which may have contributed to a better vegetative and reproductive performance of those plants.
In summary, AMF-inoculation promoted higher growth and reproductive development in TEC, while PGPB-inoculation led to higher NDVI values, supporting the use of these microbial-based technologies in salt-affected soil recovery.
5. Conclusions
This study highlights that soil technologies offer a viable option for enhancing soil characteristics and promoting the long-term development of L. algarvense while also reducing plant mortality following transplantation. Additionally, AMF and PGPB can serve as additional aids in improving plant survival, as well as in enhancing vegetative and reproductive growth.
However, despite these promising findings, it is essential to acknowledge that further validation, through field trials and collaboration with stakeholders, is an essential step towards assessing the cost-effectiveness of large-scale use and for successfully applying these combined soil and microbial technologies in real-world salt-affected soil recovery and vegetation restoration efforts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/soilsystems7030074/s1, Figure S1: Esclerotia of dark septate endophyte in a Limonium algarvense root.
Author Contributions
Conceptualization, A.D.C. and A.N.; methodology, A.N., A.C. and M.M.A.; software, A.N.; validation, A.D.C., M.M.A., A.C. and E.S.S.; investigation S.N.-T., A.S.R., R.F. and M.B.; writing—original draft preparation, A.N., S.N.-T., A.D.C., E.S.S., M.M.A., A.S.R. and R.F.; writing—review and editing, A.N., A.D.C., E.S.S. and M.M.A.; supervision, A.N. and A.D.C.; project administration, A.D.C.; funding acquisition, A.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Funds through Foundation for Science and Technology (FCT) under the Project UIDB/04129/2020 MycoHaloph funded by the Research Unit LEAF-Linking Landscape, Environment, Agriculture and Food Research Center (Instituto Superior de Agronomia). S.N.-T. thanks the Federation of European Microbiological Societies (FEMS) (FEMS-GO-2020-203), University of Sevilla (Spain; Plan Propio de Investigación y Tranferencia 2021 Ayuda A1-I.3A1) for grants to support the stays at ISA-ULisboa (Portugal).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
Authors are grateful to C. Calvet and A. Camprubí (Institute of Agrifood Research and Technology-IRTA, Cabrils, Barcelona, Spain) for kindly providing the inoculum of R. irregulare BEG 72 for the experiments), and to Ignacio D. Rodriguez-Llorente and Eloisa Pajuelo (Department of Microbiology and Parasitology, Faculty of Pharmacy, University of Seville) for providing the PGPB for the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stavi, I.; Thevs, N.; Priori, S. Soil Salinity and Sodicity in Drylands: A Review of Causes, Effects, Monitoring, and Restoration Measures. Front. Environ. Sci. 2021, 9, 330. [Google Scholar] [CrossRef]
- Omuto, C.T.; Vargas, R.R.; El Mobarak, A.M.; Mohamed, N.; Viatkin, K.; Yigini, Y. Mapping of Salt-Affected Soils—Technical Manual; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2020; Available online: https://www.fao.org/documents/card/fr/c/CA9215EN (accessed on 3 June 2023).
- Hulisz, P.; Pindral, S.; Kobierski, M.; Charzyński, P. Technogenic Layers in Organic Soils as a Result of the Impact of the Soda Industry. Eurasian Soil Sci. 2018, 51, 1133–1141. [Google Scholar] [CrossRef]
- WRB, (IUSS Working Group). World Reference Base for Soil Resources (2015) International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; WRB (IUSS Working Group): Vienna, Austria, 2019. [Google Scholar]
- Almeida, D. Ecology and Dynamics of Mediterranean Saltmarshes in a Perspective of Habitat Management and Restoration Policies: The Cases of Alvor and Arade in Portugal; Universidade de Lisboa: Lisbon, Portugal, 2016. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Global Symposium on Salt-Affected Soils: Outcome Document; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/documents/card/es/c/cb9929en/ (accessed on 3 June 2023).
- Elliott, M.; McLusky, D.S. The Need for Definitions in Understanding Estuaries. Estuar. Coast. Shelf. Sci. 2002, 55, 815–827. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Guo, J.; Shabala, S.; Wang, B. Reproductive Physiology of Halophytes: Current Standing. Front. Plant Sci. 2018, 9, 1954. [Google Scholar] [CrossRef] [PubMed]
- Caperta, A.D.; Róis, A.S.; Teixeira, G.; Garcia-Caparros, P.; Flowers, T.J. Secretory Structures in Plants: Lessons from the Plumbaginaceae on Their Origin, Evolution and Roles in Stress Tolerance. Plant Cell Environ. 2020, 43, 2912–2931. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Saeed, S.; Gul, B.; Ajmal Khan, M. Comparative Effects of NaCl and Sea Salt on Seed Germination of Arthrocnemum indicum. Pak. J. Bot. 2011, 43, 1091–1103. [Google Scholar]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant Salinity Tolerance Conferred by Arbuscular Mycorrhizal Fungi and Associated Mechanisms: A Meta-Analysis. Front. Plant Sci. 2020, 11, 588550. [Google Scholar] [CrossRef]
- Tian, C.Y.; Feng, G.; Li, X.L.; Zhang, F.S. Different Effects of Arbuscular Mycorrhizal Fungal Isolates from Saline or Non-Saline Soil on Salinity Tolerance of Plants. Appl. Soil Ecol. 2004, 26, 143–148. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Synergistic Effects of the Inoculation with Nitrogen-Fixing and Phosphate-Solubilizing Rhizobacteria on the Performance of Field-Grown Chickpea. J. Plant Nutr. Soil Sci. 2007, 170, 283–287. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity Stress Alleviation Using Arbuscular Mycorrhizal Fungi. A Review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Carro, L.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Caviedes, M.Á.; Igual, J.M.; Klenk, H.P.; Montero-Calasanz, M.D.C. Halomonas radicis sp. Nov., Isolated from Arthrocnemum macrostachyum Growing in the Odiel Marshes (Spain) and Emended Descriptions of Halomonas xinjiangensis and Halomonas zincidurans. Int. J. Syst. Evol. Microbiol. 2020, 70, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.M.; Kim, K.M.; Lee, I.J. Halotolerant Bacteria Mitigate the Effects of Salinity Stress on Soybean Growth by Regulating Secondary Metabolites and Molecular Responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved Salinity Tolerance of Arachis hypogaea (L.) by the Interaction of Halotolerant Plant-Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant Rhizobacteria Promote Growth and Enhance Salinity Tolerance in Peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef]
- Santos, J.; Al-Azzawi, M.; Aronson, J.; Flowers, T.J. EHALOPH a Database of Salt-Tolerant Plants: Helping Put Halophytes to Work. Plant Cell Physiol. 2016, 57, e10. [Google Scholar] [CrossRef]
- Lledó, M.D.; Karis, P.O.; Crespo, M.B.; Fay, M.F.; Chase, M.W. Endemism and Evolution in Macaronesian and Mediterranean Limonium Taxa. In The Biology of Island Floras; Cambridge University Press: Cambridge, UK, 2011; pp. 325–337. [Google Scholar] [CrossRef]
- Morgan, E.; Funnell, K. Limonium. In Handbook of Plant Breeding; Springer: Cham, Switzerland, 2018; pp. 513–527. [Google Scholar] [CrossRef]
- Caperta, A.D.; Castro, S.; Loureiro, J.; Róis, A.S.; Conceição, S.; Costa, J.; Rhazi, L.; Espírito Santo, D.; Arsénio, P. Biogeographical, Ecological and Ploidy Variation in Related Asexual and Sexual Limonium taxa (Plumbaginaceae). Bot. J. Linn. Soc. 2017, 183, 75–93. [Google Scholar] [CrossRef]
- Cortinhas, A.; Caperta, A.D.; Teixeira, G.; Carvalho, L.; Abreu, M.M. Harnessing Sediments of Coastal Aquaculture Ponds through Technosols Construction for Halophyte Cultivation Using Saline Water Irrigation. J. Environ. Manag. 2020, 261, 109907. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Monteiro, I.; Castañeda-Loaiza, V.; Placines, C.; Conceição Oliveira, M.; Reis, C.; Caperta, A.D.; Soares, F.; Pousão-Ferreira, P.; Pereira, C.; et al. Growth Performance, in Vitro Antioxidant Properties and Chemical Composition of the Halophyte Limonium algarvense Erben Are Strongly Influenced by the Irrigation Salinity. Ind. Crops Prod. 2020, 143, 111930. [Google Scholar] [CrossRef]
- Caperta, A.; Carapeto, A. Limonium daveaui: Ficha de Avaliação Do Risco de Extinção. In Lista Vermelha da Flora Vascular de Portugal Continental; Sociedade Portuguesa de Botânica, Associação Portuguesa de Ciência da Vegetação—PHYTOS and Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2020; Available online: https://listavermelha-flora.pt/wp-content/uploads/2020/10/Lista_Vermelha_Flora_Vascular_Portugal_Continental_2020_versao_digital.pdf (accessed on 3 June 2023).
- Cortinhas, A.; Ferreira, T.C.; Abreu, M.M.; Caperta, A.D. Conservation of a Critically Endangered Endemic Halophyte of West Portugal: A Microcosm Assay to Assess the Potential of Soil Technology for Species Reintroduction. Front. Ecol. Evol. 2021, 9, 604509. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline Soils Worldwide: Identifying the Most Promising Areas for Saline Agriculture. J. Arid. Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Rozema, J.; Schat, H. Salt Tolerance of Halophytes, Research Questions Reviewed in the Perspective of Saline Agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil Chemistry Factors Confounding Crop Salinity Tolerance—A Review. Agronomy 2016, 6, 53. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Macías, F.; de Varennes, A. Chemical Quality of Leachates and Enzymatic Activities in Technosols with Gossan and Sulfide Wastes from the São Domingos Mine. J. Soils Sediments 2016, 16, 1366–1382. [Google Scholar] [CrossRef]
- Macías, F. Recuperación de Suelos Degradados, Reutilización de Residuos y Secuestro de Carbono. Una Alternativa Integral de Mejora de La Calidad Ambiental. Recur. Rurais 2004, 1, 49–56. [Google Scholar]
- Macías García, F.; Marta Camps Arbestain, F.; Vázquez, F.M. Utilización de Tecnosoles Derivados de Residuos En Procesos de Restauración de Suelos en La Mina de Touro. In Proceedings of the Minería Sostenible: Conferencia internacional 09, Santiago de Compostela, Spain, 15–18 April 2009; Cámara Oficial Mineira de Galicia: La Coruña, Spain, 2009; pp. 651–662.
- Moreno-Barriga, F.; Díaz, V.; Acosta, J.A.; Muñoz, M.Á.; Faz, Á.; Zornoza, R. Organic Matter Dynamics, Soil Aggregation and Microbial Biomass and Activity in Technosols Created with Metalliferous Mine Residues, Biochar and Marble Waste. Geoderma 2017, 301, 19–29. [Google Scholar] [CrossRef]
- Arán, D.; Santos, E.S.; Abreu, M.M.; Antelo, J.; Macías, F. Use of Combined Tools for Effectiveness Evaluation of Tailings Rehabilitated with Designed Technosol. Environ. Geochem. Health 2022, 44, 1857–1873. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Macías, F.; de Varennes, A. Improvement of Chemical and Biological Properties of Gossan Mine Wastes Following Application of Amendments and Growth of Cistus ladanifer L. J. Geochem. Explor. 2014, 147, 173–181. [Google Scholar] [CrossRef]
- Hafeez, F.; Spor, A.; Breuil, M.C.; Schwartz, C.; Martin-Laurent, F.; Philippot, L. Distribution of Bacteria and Nitrogen-Cycling Microbial Communities along Constructed Technosol Depth-Profiles. J. Hazard. Mater. 2012, 231–232, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Villenave, C.; Séré, G.; Schwartz, C.; Watteau, F.; Jimenez, A.; Cortet, J. Rapid Changes in Soil Nematodes in the First Years after Technosol Construction for the Remediation of an Industrial Wasteland. Eurasian Soil Sci. 2018, 51, 1266–1273. [Google Scholar] [CrossRef]
- Guerreiro, M.; Fortunato, A.B.; Freire, P.; Rilo, A.; Taborda, R.; Freitas, M.C.; Andrade, C.; Silva, T.; Rodrigues, M.; Bertin, X.; et al. Evolution of the Hydrodynamics of the Tagus Estuary (Portugal) in the 21st Century. J. Integr. Coast. Zone Manag. 2015, 15, 65–80. [Google Scholar] [CrossRef]
- Róis, A.S.; Teixeira, G.; Sharbel, T.F.; Fuchs, J.; Martins, S.; Espírito-Santo, D.; Caperta, A.D. Male Fertility versus Sterility, Cytotype, and DNA Quantitative Variation in Seed Production in Diploid and Tetraploid Sea Lavenders (Limonium sp., Plumbaginaceae) Reveal Diversity in Reproduction Modes. Sex. Plant Reprod. 2012, 25, 305–318. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. In Circular; California Agricultural Experiment Station: Berkeley, CA, USA, 1950; Volume 347. [Google Scholar]
- Springer, U.; Klee, J. Prüfung der Leistungsfähigkeit von Einigen Wichtigeren Verfahren zur Bestimmung des Kohlenstoffs Mittels Chromschwefelsäure Sowie Vorschlag Einer Neuen Schnellmethode. Z. Pflanz. Düngung Bodenkd. 1954, 64, 1–26. [Google Scholar] [CrossRef]
- Lakanen, E.; Ervio, R. A Comparison of Eight Extractants for the Determination of Plant Available Micronutrients in Soils. Acta Agral. Fenn. 1971, 123, 223–232. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. ISBN 9780891188650. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Hope, C.F.A.; Burns, R.G. Activity, Origins and Location of Cellulases in a Silt Loam Soil. Biol. Fertil. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J. A modified single Solution for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31. [Google Scholar] [CrossRef]
- Porter, W. The “most Probable Number” Method for Enumerating Infective Propagules of Vesicular Arbuscular Mycorrhizal Fungi in Soil. Aust. J. Soil Res. 1979, 17, 515. [Google Scholar] [CrossRef]
- Powell, C.L. Mycorrhizal Infectivity of Eroded Soils. Soil Biol. Biochem. 1980, 12, 247–250. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. A Modified Procedure for Staining Roots to Detect VA Mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Jarvis, B.; Wilrich, C.; Wilrich, P.-T. Reconsideration of the Derivation of Most Probable Numbers, Their Standard Deviations, Confidence Bounds and Rarity Values. J. Appl. Microbiol. 2010, 109, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Torre, S.; Barcia-Piedras, J.M.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Camacho, M.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Assessing the Role of Endophytic Bacteria in the Halophyte Arthrocnemum Macrostachyum Salt Tolerance. Plant Biol. 2016, 19, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Torre, S.; Carro, L.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Caviedes, M.Á.; Igual, J.M.; Klenk, H.P.; Montero-Calasanz, M.D.C. Pseudoalteromonas rhizosphaerae sp. Nov., a Novel Plant Growthpromoting Bacterium with Potential Use in Phytoremediation. Int. J. Syst. Evol. Microbiol. 2020, 70, 3287–3294. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Barcia-Piedras, J.M.; Caviedes, M.A.; Pajuelo, E.; Redondo-Gómez, S.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Bioaugmentation with Bacteria Selected from the Microbiome Enhances Arthrocnemum Macrostachyum Metal Accumulation and Tolerance. Mar. Pollut. Bull. 2017, 117, 340–347. [Google Scholar] [CrossRef]
- Zhang, C.; Fountain, D.W.; Morgan, E.R. In Vitro Germination of the Trinucleate Pollen of Limonium perezii. Grana 1997, 36, 284–288. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C. Use of Organic Amendments to Reclaim Saline and Sodic Soils: A Review. Arid. Land Res. Manag. 2018, 33, 1498038. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Santos, E.S.; Saraiva, J.A.; Magalhães, M.C.F.; Macías, F.; Abreu, M.M. The Potential of Cistus salviifolius L. to Phytostabilize Gossan Mine Wastes Amended with Ash and Organic Residues. Plants 2022, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Betancur-Corredor, B.; Loaiza-Usuga, J.C.; Denich, M.; Borgemeister, C. Changes of Technosol Properties and Vegetation Structure along a Chronosequence of Dredged Sediment Deposition in Areas with Alluvial Gold Mining in Colombia. J. Soils Sediments 2020, 20, 2377–2394. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The Biological Activities of β-Glucosidase, Phosphatase and Urease as Soil Quality Indicators: A Review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z.; Wolińska, A.; Stępniewska, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Santos, E.S.; Arán, D.; Abreu, M.M.; de Varennes, A. Engineered Soils Using Amendments for In Situ Rehabilitation of Mine Lands. In Bio-Geotechnologies for Mine Site Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 131–146. [Google Scholar] [CrossRef]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Use of Organic Amendment as a Strategy for Saline Soil Remediation: Influence on the Physical, Chemical and Biological Properties of Soil. Soil Biol. Biochem. 2006, 38, 1413–1421. [Google Scholar] [CrossRef]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of Salinity on Soil Microbial Communities and the Decomposition of Maize in Acidic Soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Wang, Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of Soil Physicochemical Properties and Enzyme Activities to Long-Term Reclamation of Coastal Saline Soil, Eastern China. Sci. Total Environ. 2017, 607–608, 1419–1427. [Google Scholar] [CrossRef]
- Boyrahmadi, M.; Raiesi, F. Plant Roots and Species Moderate the Salinity Effect on Microbial Respiration, Biomass, and Enzyme Activities in a Sandy Clay Soil. Biol. Fertil. Soils 2018, 54, 509–521. [Google Scholar] [CrossRef]
- Sritongon, N.; Sarin, P.; Theerakulpisut, P.; Riddech, N. The Effect of Salinity on Soil Chemical Characteristics, Enzyme Activity and Bacterial Community Composition in Rice Rhizospheres in Northeastern Thailand. Sci. Rep. 2022, 12, 20360. [Google Scholar] [CrossRef]
- Kang, Y.H.; Liu, S.H.; Wan, S.Q.; Wang, R.S. Assessment of Soil Enzyme Activities of Saline-Sodic Soil under Drip Irrigation in the Songnen Plain. Paddy Water Environ. 2013, 11, 87–95. [Google Scholar] [CrossRef]
- Singh, K. Microbial and Enzyme Activities of Saline and Sodic Soils. Land Degrad. Dev. 2016, 27, 706–718. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Weil, R.R., Brady, N.C., Eds.; Pearson Education Limited: Edinburgh, UK, 2017; ISBN 0133254488. [Google Scholar]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Bacteria as Inoculants in Agricultural. Genet. Mol. Biol. 2015, 38, 401. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable Agricultural Management of Saline Soils in Arid and Semi-Arid Mediterranean Regions through Halophytes, Microbial and Soil-Based Technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B. Potential PGPR Properties of Cellulolytic, Nitrogen-Fixing, Phosphate-Solubilizing Bacteria in Rehabilitated Tropical Forest Soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Camprubi, A.; Abril, M.; Estaun, V.; Calvet, C. Contribution of Arbuscular Mycorrhizal Symbiosis to the Survival of Psammophilic Plants after Sea Water Flooding. Plant Soil 2012, 351, 97–105. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Mateos-Naranjo, E.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Isolation of Plant-Growth-Promoting and Metal-Resistant Cultivable Bacteria from Arthrocnemum Macrostachyum in the Odiel Marshes with Potential Use in Phytoremediation. Mar. Pollut. Bull. 2016, 110, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Lu, X.B.; Li, Z.H.; Tian, C.Y.; Song, J. The Role of Root-Associated Microbes in Growth Stimulation of Plants under Saline Conditions. Land Degrad. Dev. 2021, 32, 3471–3486. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Pérez-Corona, M.E.; Manrique, E. Impacts of Simulated N Deposition on Plants and Mycorrhizae from Spanish Semiarid Mediterranean Shrublands. Ecosystems 2013, 16, 838–851. [Google Scholar] [CrossRef]
- Sheikh-Assadi, M.; Khandan-Mirkohi, A.; Alemardan, A.; Moreno-Jiménez, E. Mycorrhizal Limonium sinuatum (L.) Mill. Enhances Accumulation of Lead and Cadmium. Int. J. Phytoremed. 2015, 17, 556–562. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, Y.J.; Yuan, B.; Xu, P.Y.; Xing, K.; Wang, J.; Jiang, J.H. Isolation of ACC Deaminase-Producing Habitat-Adapted Symbiotic Bacteria Associated with Halophyte Limonium sinense (Girard) Kuntze and Evaluating Their Plant Growth-Promoting Activity under Salt Stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.W.; Wang, T.T.; Ding, P.; Xing, K.; Jiang, J.H. Plant Growth-Promoting Effect and Genomic Analysis of the Beneficial Endophyte streptomyces sp. KLBMP 5084 Isolated from Halophyte Limonium sinense. Plant Soil 2017, 416, 117–132. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.-W.; Zhang, Y.-J.; Wang, T.-T.; Xiong, Y.-W.; Xing, K. Diversity of Bacterial Microbiota of Coastal Halophyte Limonium sinense and Amelioration of Salinity Stressdamage. Appl. Environ. Microbiol. 2018, 84, e01533-18. [Google Scholar] [PubMed]
- Feng, W.W.; Wang, T.T.; Bai, J.L.; Ding, P.; Xing, K.; Jiang, J.H.; Peng, X.; Qin, S. Glutamicibacter halophytocola sp. Nov., an Endophytic Actinomycete Isolated from the Roots of a Coastal Halophyte, Limonium sinense. Int. J. Syst. Evol. Microbiol. 2017, 67, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Ding, P.; Chen, P.; Xing, K.; Bai, J.L.; Wan, W.; Jiang, J.H.; Qin, S. Complete Genome Sequence of Endophyte Bacillus flexus KLBMP 4941 Reveals Its Plant Growth Promotion Mechanism and Genetic Basis for Salt Tolerance. J. Biotechnol. 2017, 260, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, E.; Liu, C.; Jousset, A.; Salles, J.F. Functionality of Root-Associated Bacteria along a Salt Marsh Primary Succession. Front. Microbiol. 2017, 8, 2102. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Li, X.W.; Wang, T.T.; Gong, Y.; Zhang, C.M.; Xing, K.; Qin, S. Root Exudates-Driven Rhizosphere Recruitment of the Plant Growth-Promoting Rhizobacterium Bacillus flexus KLBMP 4941 and Its Growth-Promoting Effect on the Coastal Halophyte Limonium sinense under Salt Stress. Ecotoxicol. Environ. Saf. 2020, 194, 110374. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Clements, C.; Edrada-Ebel, R.; Orlikova, B.; Diederich, M.; Wray, V.; Lin, W.; Proksch, P. NF Kappa B Inhibitors and Antitrypanosomal Metabolites from Endophytic Fungus penicillium sp. Isolated from Limonium tubiflorum. Bioorg. Med. Chem. 2011, 19, 414–421. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Kim, H.; Nam, Y.J.; Oh, Y.; Jeong, M.J.; Choi, H.R.; You, Y.H.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; et al. Diversity and Plant Growth Promoting Capacity of Endophytic Fungi Associated with Halophytic Plants from the West Coast of Korea. Mycobiology 2015, 43, 373–383. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Choi, D.H.; Woo, J.R.; Jeong, M.J.; Oh, Y.; Kim, Y.G.; Lee, I.J.; Choo, Y.S.; Kim, J.G. Diversity and Plant Growth-Promoting Effects of Fungal Endophytes Isolated from Salt-Tolerant Plants. J. Microbiol. Biotechnol. 2020, 30, 1680–1687. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Choi, D.H.; Kim, J.G.; Lee, I.S. Endophytic Fungi of Salt-Tolerant Plants: Diversity and Ability to Promote Plant Growth. J. Microbiol. Biotechnol. 2021, 31, 1526–1532. [Google Scholar] [CrossRef]
- Mandyam, K.G.; Jumpponen, A. Mutualism-Parasitism Paradigm Synthesized from Results of Root-Endophyte Models. Front. Microbiol. 2014, 5, 776. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal Endophytes: Diversity and Functional Roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Malicka, M.; Magurno, F.; Piotrowska-Seget, Z. Plant Association with Dark Septate Endophytes: When the Going Gets Tough (and Stressful), the Tough Fungi Get Going. Chemosphere 2022, 302, 134830. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress Tolerance in Plants via Habitat-Adapted Symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the Role of Dark Septate Endophytes in the Plant Resistance to Abiotic and Biotic Stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef]
- Menge, J.; Davis, R. Mycorrhizal Fungi Increase Growth and Reduce Transplant Injury in Avocado. Calif. Agric. 1978, 32, 6–7. [Google Scholar]
- Subhan, S.; Sharmila, P.; Pardha Saradhi, P. Glomus Fasciculatum Alleviates Transplantation Shock of Micropropagated Sesbania Sesban. Plant Cell Rep. 1998, 17, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Krishna, H.; Singh, S.K.; Minakshi; Patel, V.B.; Khawale, R.N.; Deshmukh, P.S.; Jindal, P.C. Arbuscular-Mycorrhizal Fungi Alleviate Transplantation Shock in Micropropagated Grapevine (Vitis vinifera L.). J. Hortic. Sci. Biotechnol. 2006, 81, 259–263. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic Adjustment and Plant Adaptation to Environmental Changes Related to Drought and Salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Hameed, A.; Dilfuza, E.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Ahmad, P. Salinity Stress and Arbuscular Mycorrhizal Symbiosis in Plants. In Beyond the Dance Floor; Springer: New York, NY, USA, 2014; pp. 139–159. ISBN 9781461494669. [Google Scholar]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, T.; Liao, J.; Zhang, W.; Huang, C. Do Halophytes and Glycophytes Differ in Their Interactions with Arbuscular Mycorrhizal Fungi under Salt Stress? A Meta-Analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Sembiring, H.; Raun, W.R.; Johnson, G.V.; Stone, M.L.; Solie, J.B.; Phillips, S.B. Detection of Nitrogen and Phosphorus Nutrient Status in Winter Wheat Using Spectral Radiance. J. Plant Nutr. 1998, 21, 1207–1233. [Google Scholar] [CrossRef]
- Jakobsen, I. Transport of Phosphorus and Carbon in Arbuscular Mycorrhizas. In Mycorrhiza; Springer: Berlin/Heidelberg, Germany, 2016; pp. 305–332. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Is There a Role for Arbuscular Mycorrhizal Fungi in Production Agriculture? Plant Soil 2002, 244, 263–271. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Archer, E.; Valentine, A.J. Mycorrhizal C Costs and Nutritional Benefits in Developing Grapevines. Mycorrhiza 2005, 15, 159–165. [Google Scholar] [CrossRef]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct Evidence for Modulation of Photosynthesis by an Arbuscular Mycorrhiza-induced Carbon Sink Strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Wang, S.; Du, Y.; Zhang, D.; Tang, Z. Effects of Arbuscular Mycorrhizal Symbiosis on the Growth and Reproduction of Cherry Tomato Can Be Persistent to the next Generation. Eur. J. Soil Biol. 2022, 112, 103429. [Google Scholar] [CrossRef]
- Zhu, B.; Gao, T.; Zhang, D.; Ding, K.; Li, C.; Ma, F. Functions of Arbuscular Mycorrhizal Fungi in Horticultural Crops. Sci. Hortic. 2022, 303, 111219. [Google Scholar] [CrossRef]
- Liu, S.; Guo, H.; Xu, J.; Song, Z.; Song, S.; Tang, J.; Chen, X. Arbuscular Mycorrhizal Fungi Differ in Affecting the Flowering of a Host Plant under Two Soil Phosphorus Conditions. J. Plant Ecol. 2018, 11, 623–631. [Google Scholar] [CrossRef]
- Conceição, S.I.R.; Róis, A.S.; Caperta, A.D. Nonreduction via Meiotic Restitution and Pollen Heterogeneity May Explain Residual Male Fertility in Triploid Marine Halophyte Limonium algarvense (Plumbaginaceae). Caryologia 2019, 72, 53–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).