Isolation and Characterization of Culturable Osmotolerant Microbiota in Hypersaline and Hypergypsic Soils as New Treatment for Osmotic Stress in Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Isolation, Identification and Analysis of the Culturable Bacteria Populations in Soil and Roots
2.3. Strains Growth and Plant Growth-Promoting Traits Performance under Osmotic Stress
2.3.1. Strains Growth under High Osmotic Pressure
2.3.2. Nitrogen Fixing Activity
2.3.3. Phosphate and Potassium Solubilization
2.3.4. Sulfur-Oxidizing Activity
2.3.5. Siderophores Production
2.3.6. Biofilm Production
2.3.7. Auxins Production
2.3.8. Production of 1-Aminocyclopropane-1-Carboxylate Deaminase (ACCd)
2.3.9. Antioxidants Production
2.4. Plant Material: Medicago Species
2.5. Germination and In Vitro Colonization Tests under High Osmotic Preassure
2.6. Evaluation Plant Osmotic-Tolerance Enhancement
2.7. Statistics
3. Results
3.1. Population Analysis
3.2. Characterization and Selection of Candidate Strains
3.3. Strains Selected for Osmotic Stress Tolerance Treatment
3.4. Evaluation of In Vitro Colonization and Germination Traits under Osmotic Stress Conditions
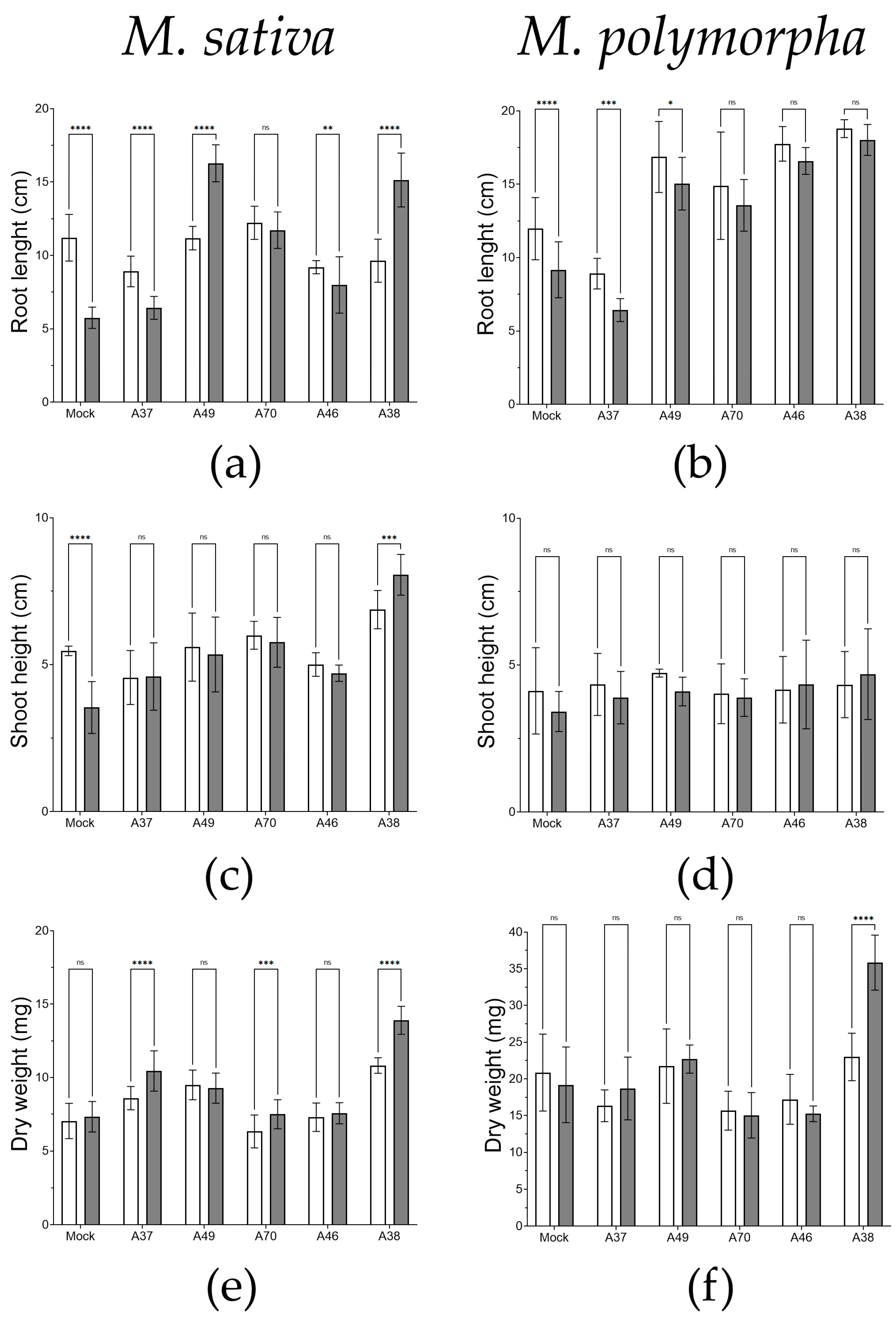
3.5. Biotreatment Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, L.; Zhu, J.-K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.U.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, H.; Sahoo, L.; Panda, S.K. Molecular Physiology of Osmotic Stress in Plants. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 179–192. [Google Scholar]
- Ayangbenro, A.S.; Babalola, O.O. Reclamation of arid and semi-arid soils: The role of plant growth-promoting archaea and bacteria. Curr. Plant Biol. 2021, 25, 100173. [Google Scholar] [CrossRef]
- Sajid, H.; Muhammad, S.; Muhammad, A.; Chunquan, Z.; Qianyu, J.; Junhua, Z. Salinity Stress in Arid and Semi-Arid Climates: Effects and Management in Field Crops. In Climate Change and Agriculture; Saddam, H., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 12. [Google Scholar]
- Jorda, H.; Perelman, A.; Lazarovitch, N.; Vanderborght, J. Exploring Osmotic Stress and Differences between Soil–Root Interface and Bulk Salinities. Vadose Zone J. 2018, 17, 170029. [Google Scholar] [CrossRef]
- Canfora, L.; Vendramin, E.; Vittori Antisari, L.; Lo Papa, G.; Dazzi, C.; Benedetti, A.; Iavazzo, P.; Adamo, P.; Jungblut, A.D.; Pinzari, F. Compartmentalization of gypsum and halite associated with cyanobacteria in saline soil crusts. FEMS Microbiol. Ecol. 2016, 92, fiw080. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, H.; Stoops, G.; Abtahi, A. SEM morphologies of halite (NaCl) in soils. J. Microsc. 1980, 120, 343–352. [Google Scholar] [CrossRef]
- Herrero, J.; Porta, J. The terminology and the concepts of gypsum-rich soils. Geoderma 2000, 96, 47–61. [Google Scholar] [CrossRef]
- Howari, F.M.; Goodell, P.C.; Miyamoto, S. Spectral properties of salt crusts formed on saline soils. J. Environ. Qual. 2002, 31, 1453–1461. [Google Scholar] [CrossRef]
- Van Alphen, J.; de los Ríos Romero, F. Gypsiferous Soils: Notes on Their Characteristics and Management; ILRI: Wageningen, The Netherlands, 1971. [Google Scholar]
- Ramos, T.B.; Castanheira, N.; Oliveira, A.R.; Paz, A.M.; Darouich, H.; Simionesei, L.; Farzamian, M.; Gonçalves, M.C. Soil salinity assessment using vegetation indices derived from Sentinel-2 multispectral data. application to Lezíria Grande, Portugal. Agric. Water Manag. 2020, 241, 106387. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, A.; Sharma, V. Microbes mediated plant stress tolerance in saline agricultural ecosystem. Plant Soil 2019, 442, 1–22. [Google Scholar] [CrossRef]
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 2011, 4, 2669–2681. [Google Scholar] [CrossRef]
- Sanchez Lopez, J.; Curt, M.; Fernandez, J. Approach to the potential production of giant reed in surplus saline lands of Spain. GCB Bioenergy 2015, 9, 105–108. [Google Scholar] [CrossRef]
- Herrero, J.; Castañeda, C.; Gómez-Báguena, R. A Heritage Agronomic Study as a Database for Monitoring the Soil Salinity of an Irrigated District in NE Spain. Agronomy 2022, 12, 126. [Google Scholar] [CrossRef]
- Ramos, T.B.; Darouich, H.; Šimůnek, J.; Gonçalves, M.C.; Martins, J.C. Soil salinization in very high-density olive orchards grown in southern Portugal: Current risks and possible trends. Agric. Water Manag. 2019, 217, 265–281. [Google Scholar] [CrossRef]
- Akpulat, H.A.; Celik, N. Flora of gypsum areas in Sivas in the eastern part of Cappadocia in Central Anatolia, Turkey. J. Arid Environ. 2005, 61, 27–46. [Google Scholar] [CrossRef]
- Álvarez, D.; Antúnez, M.; Porras, S.; Rodríguez-Ochoa, R.; Olarieta, J.R.; Poch, R.M. Quantification of Gypsum in Soils: Methodological Proposal. Span. J. Soil Sci. 2022, 12, 10669. [Google Scholar] [CrossRef]
- Escudero, A.; Palacio, S.; Maestre, F.; Luzuriaga, A. Plant life on gypsum: A review of its multiple facets. Biol. Rev. 2014, 90, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Safarnejad, A. Morphological and biochemical response to osmotic stress in alfalfa (Medicago sativa L.). Pak. J. Bot. 2008, 40, 735–746. [Google Scholar]
- Sun, W.; Xu, X.; Zhu, H.; Liu, A.; Liu, L.; Li, J.; Hua, X. Comparative transcriptomic profiling of a salt-tolerant wild tomato species and a salt-sensitive tomato cultivar. Plant Cell Physiol. 2010, 51, 997–1006. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Zhao, M.; Shen, H.; Xu, L.; Luo, Y.; Liu, Y.; Xing, A.; Kang, J.; Jing, H.; et al. Yield and quality properties of alfalfa (Medicago sativa L.) and their influencing factors in China. Eur. J. Agron. 2022, 141, 126637. [Google Scholar] [CrossRef]
- Radovic, J.; Sokolović, D.; Marković, J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, F.; Jiang, X.; Yang, X.; He, F.; Wang, Z.; Long, R.; Chen, L.; Yang, T.; Wang, C.; et al. Identification of Genetic Loci Associated with Crude Protein Content and Fiber Composition in Alfalfa (Medicago sativa L.) Using QTL Mapping. Front. Plant Sci. 2021, 12, 608940. [Google Scholar] [CrossRef] [PubMed]
- Suriyagoda, L.D.; Ryan, M.H.; Renton, M.; Lambers, H. Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus-and moisture-limited environments. Ann. Bot. 2010, 105, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Tolerance of some potential forage grasses from arid regions of Pakistan to salinity and drought. In Proceedings of the Biosaline Agriculture and Salinity Tolerance in Plants; Birkhäuser: Basel, Switzerland, 2006; pp. 15–27. [Google Scholar]
- Friesen, M.L.; von Wettberg, E.J.B.; Badri, M.; Moriuchi, K.S.; Barhoumi, F.; Chang, P.L.; Cuellar-Ortiz, S.; Cordeiro, M.A.; Vu, W.T.; Arraouadi, S.; et al. The ecological genomic basis of salinity adaptation in Tunisian Medicago truncatula. BMC Genom. 2014, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Kuhlmann, A.U.; Hoffmann, T.; Bursy, J.; Jebbar, M.; Bremer, E. Ectoine and hydroxyectoine as protectants against osmotic and cold stress: Uptake through the SigB-controlled betaine-choline-carnitine transporter-type carrier EctT from Virgibacillus pantothenticus. J. Bacteriol. 2011, 193, 4699–4708. [Google Scholar] [CrossRef]
- Vílchez, J.I.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant Drought Tolerance Enhancement by Trehalose Production of Desiccation-Tolerant Microorganisms. Front. Microbiol. 2016, 7, 1577. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Duan, J.; DiBernardo, M.; Zetter, E.; Campos-García, J.; Glick, B.R.; Santoyo, G. The Production of ACC Deaminase and Trehalose by the Plant Growth Promoting Bacterium Pseudomonas sp. UW4 Synergistically Protect Tomato Plants against Salt Stress. Front. Microbiol. 2019, 10, 1392. [Google Scholar] [CrossRef]
- Metoui Ben Mahmoud, O.; Hidri, R.; Talbi-Zribi, O.; Taamalli, W.; Abdelly, C.; Djébali, N. Auxin and proline producing rhizobacteria mitigate salt-induced growth inhibition of barley plants by enhancing water and nutrient status. S. Afr. J. Bot. 2020, 128, 209–217. [Google Scholar] [CrossRef]
- Amaresan, N.; Patel, K.; Jinal, H. Inoculation of Cucumber (Cucumis sativus L.) Seedlings with Salt-Tolerant Plant Growth Promoting Bacteria Improves Nutrient Uptake, Plant Attributes and Physiological Profiles. J. Plant Growth Regul. 2021, 40, 1728–1740. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.M.; Kim, K.M.; Lee, I.J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2022, 13, 1101862. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm forming rhizobacteria enhance growth and salt tolerance in sunflower plants by stimulating antioxidant enzymes activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Mosharaf, M.K.; Khatun, M.; Haque, M.A.; Biswas, M.S.; Islam, M.S.; Islam, M.M.; Shozib, H.B.; Miah, M.M.U.; Molla, A.H.; et al. Biofilm Producing Rhizobacteria with Multiple Plant Growth-Promoting Traits Promote Growth of Tomato Under Water-Deficit Stress. Front. Microbiol. 2020, 11, 542053. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Jan, M.; Afreen, M.; Anees, M.; Rehman, S.; Daud, M.K.; Malook, I.; Jamil, M. Halophilic bacteria mediated phytoremediation of salt-affected soils cultivated with rice. J. Geochem. Explor. 2017, 174, 59–65. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Shurigin, V.; Egamberdieva, D.; Li, L.; Davranov, K.; Panosyan, H.; Birkeland, N.-K.; Wirth, S.; Bellingrath-Kimura, S.D. Endophytic bacteria associated with halophyte Seidlitzia rosmarinus Ehrenb. ex Boiss. from saline soil of Uzbekistan and their plant beneficial traits. J. Arid Land 2020, 12, 730–740. [Google Scholar] [CrossRef]
- Azucena Gándara, L.; Marco Antonio Gutiérrez, C. Efecto de microorganismos promotores de crecimiento vegetal y yeso agrícola en el cultivo de higo. CIBA Rev. Iberoam. Cienc. Biol. Agropecu. 2023, 12, 1–21. [Google Scholar] [CrossRef]
- Chaudhary, A.; Chaudhary, P.; Upadhyay, A.; Kumar, A.; Rani, A. Effect of Gypsum on Plant Growth Promoting Rhizobacteria. Environ. Ecol. 2022, 39, 1248–1256. [Google Scholar]
- Breakwell, D.; Woolverton, C.; MacDonald, B.; Smith, K.; Robison, R. Colony Morphology Protocol. In Proceedings of the 14th American Society for Microbiology Conference for Undergraduate Educators (ASMCUE), Buffalo, NY, USA, 29 September 2007. [Google Scholar]
- Dashti, A.; Jadaon, M.; Abdulsamad, A.; Dashti, H. Heat Treatment of Bacteria: A Simple Method of DNA Extraction for Molecular Techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Wang, T.; Liu, H.; Tian, D.; Zhang, Y. Melatonin Application Improves Salt Tolerance of Alfalfa (Medicago sativa L.) by Enhancing Antioxidant Capacity. Plants 2020, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.; Zarza, X.; Antoniou, C.; Obata, T.; Villarroel, C.; Ganopoulos, I.; Harokopos, V.; Gohari, G.; Aidinis, V.; Madesis, P.; et al. Systems biology reveals key tissue-specific metabolic and transcriptional signatures involved in the response of Medicago truncatula plant genotypes to salt stress. Comput. Struct. Biotechnol. J. 2021, 19, 2133–2147. [Google Scholar] [CrossRef]
- Sulistiyani, T.; Meliah, S. Isolation and Characterization of Nitrogen Fixing Endophytic Bacteria Associated with Sweet Sorghum (Sorghum bicolor). In Proceedings of the SATREPS Conference, Bogor City, Indonesia, 14 November 2017. [Google Scholar]
- Iwata, K.; Azlan, A.; Yamakawa, H.; Omori, T. Ammonia accumulation in culture broth by the novel nitrogen-fixing bacterium, Lysobacter sp. E4. J. Biosci. Bioeng. 2010, 110, 415–418. [Google Scholar] [CrossRef]
- Hartono, H.; Nurfitriani; Asnawati, F.; Citra, H.; Handayani, N.I.; Junda, M.; Ali, A.; Hala, Y.; Jumadi, O. Ability of ammonium excretion, indol acetic acid production, and phosphate solubilization of nitrogen-fixing bacteria isolated from crop rhizosphere and their effect on plant growth. ARPN J. Eng. Appl. Sci. 2016, 11, 11735–11741. [Google Scholar]
- Zheng, B.-X.; Ibrahim, M.; Zhang, D.-P.; Bi, Q.-F.; Li, H.-Z.; Zhou, G.-W.; Ding, K.; Peñuelas, J.; Zhu, Y.-G.; Yang, X.-R. Identification and characterization of inorganic-phosphate-solubilizing bacteria from agricultural fields with a rapid isolation method. AMB Express 2018, 8, 47. [Google Scholar] [CrossRef]
- Rajawat, M.V.S.; Singh, S.; Tyagi, S.P.; Saxena, A.K. A Modified Plate Assay for Rapid Screening of Potassium-Solubilizing Bacteria. Pedosphere 2016, 26, 768–773. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Halimi, M.; Samsudin, A.A. Isolation and Characterisation of Sulphur Oxidizing Bacteria Isolated from Hot Spring in Malaysia for Biological Deodorisation of Hydrogen Sulphide in Chicken Manure. Media Peternak. 2017, 40, 178–187. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of Blue Agar CAS Assay for Siderophore Detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 381. [Google Scholar] [CrossRef]
- Coffey, B.M.; Anderson, G.G. Biofilm formation in the 96-well microtiter plate. In Pseudomonas Methods and Protocols; Methods in Molecular Biology; Humana: New York, NY, USA, 2014; Volume 1149, pp. 631–641. [Google Scholar] [CrossRef]
- Ambrosini, A.; Passaglia, L.M.P. Plant Growth–Promoting Bacteria (PGPB): Isolation and Screening of PGP Activities. Curr. Protoc. Plant Biol. 2017, 2, 190–209. [Google Scholar] [CrossRef]
- Niza-Costa, M.; Rodríguez-dos Santos, A.S.; Rebelo-Romão, I.; Ferrer, M.V.; Sequero López, C.; Vílchez, J.I. Geographically Disperse, Culturable Seed-Associated Microbiota in Forage Plants of Alfalfa (Medicago sativa L.) and Pitch Clover (Bituminaria bituminosa L.): Characterization of Beneficial Inherited Strains as Plant Stress-Tolerance Enhancers. Biology 2022, 11, 1838. [Google Scholar] [CrossRef]
- Reiner, K. Catalase Test Protocol; American Society for Microbiology: Washington, DC, USA, 2010. [Google Scholar]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Takao, T.; Kitatani, F.; Watanabe, N.; Yagi, A.; Sakata, K. A Simple Screening Method for Antioxidants and Isolation of Several Antioxidants Produced by Marine Bacteria from Fish and Shellfish. Biosci. Biotechnol. Biochem. 1994, 58, 1780–1783. [Google Scholar] [CrossRef]
- Vílchez, J.I.; Yang, Y.; Yi, D.; Zhang, H. Measurements of Root Colonized Bacteria Species. Bio-protocol 2021, 11, e3976. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Zambaux, K.; Hills, A.; Sulpice, R.; Pattison, R.J.; Blatt, M.R.; Amtmann, A. EZ-Rhizo: Integrated software for the fast and accurate measurement of root system architecture. Plant J. 2009, 57, 945–956. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Vilchez, J.I. Population and Screening Analysis of Salt/Gypsum Strains. 2023. Available online: https://figshare.com/articles/dataset/Population_and_screening_analysis_of_Salt_gypsum_strains/22927658 (accessed on 9 July 2023).
- Mastrocicco, M.; Colombani, N. The Issue of Groundwater Salinization in Coastal Areas of the Mediterranean Region: A Review. Water 2021, 13, 90. [Google Scholar] [CrossRef]
- Kharroubi, A.; Tlahigue, F.; Agoubi, B.; Azri, C.; Bouri, S. Hydrochemical and statistical studies of the groundwater salinization in Mediterranean arid zones: Case of the Jerba coastal aquifer in southeast Tunisia. Environ. Earth Sci. 2012, 67, 2089–2100. [Google Scholar] [CrossRef]
- Melendez-Pastor, I.; Hernández, E.; Navarro-Pedreño, J.; Gómez Lucas, I. Mapping Soil Salinization of Agricultural Coastal Areas in Southeast Spain. In Remote Sensing Applications; IntechOpen: London, UK, 2012; Volume 6. [Google Scholar]
- Günal, H.; Korucu, T.; Birkas, M.; Özgöz, E.; Halbac-Cotoara-Zamfir, R. Threats to Sustainability of Soil Functions in Central and Southeast Europe. Sustainability 2015, 7, 2161–2188. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef]
- Rilling, J.I.; Acuña, J.J.; Nannipieri, P.; Cassan, F.; Maruyama, F.; Jorquera, M.A. Current opinion and perspectives on the methods for tracking and monitoring plant growth-promoting bacteria. Soil Biol. Biochem. 2019, 130, 205–219. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, J.; Arora, N.K. Plant Growth-Promoting Rhizobacteria: Diversity and Applications. In Environmental Biotechnology: For Sustainable Future; Sobti, R.C., Arora, N.K., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 129–173. [Google Scholar]
- Sharma, A.; Dev, K.; Sourirajan, A.; Choudhary, M. Isolation and characterization of salt-tolerant bacteria with plant growth-promoting activities from saline agricultural fields of Haryana, India. J. Genet. Eng. Biotechnol. 2021, 19, 99. [Google Scholar] [CrossRef]
- Kumawat, C.; Kumar, A.; Parshad, J.; Sharma, S.S.; Patra, A.; Dogra, P.; Yadav, G.K.; Dadhich, S.K.; Verma, R.; Kumawat, G.L. Microbial Diversity and Adaptation under Salt-Affected Soils: A Review. Sustainability 2022, 14, 9280. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, C.; Wang, Y.; Xia, Y.; Xiao, W.; Cui, X. Salt-tolerant and plant-growth-promoting bacteria isolated from high-yield paddy soil. Can. J. Microbiol. 2018, 64, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Siddikee, M.A.; Chauhan, P.S.; Anandham, R.; Han, G.H.; Sa, T. Isolation, characterization, and use for plant growth promotion under salt stress, of ACC deaminase-producing halotolerant bacteria derived from coastal soil. J. Microbiol. Biotechnol. 2010, 20, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F.; Demirci, A. Salt Stress Mitigating Potential of Halotolerant/Halophilic Plant Growth Promoting. Geomicrobiol. J. 2020, 37, 663–669. [Google Scholar] [CrossRef]
- Jhuma, T.A.; Rafeya, J.; Sultana, S.; Rahman, M.T.; Karim, M.M. Isolation of Endophytic Salt-Tolerant Plant Growth-Promoting Rhizobacteria from Oryza sativa and Evaluation of Their Plant Growth-Promoting Traits under Salinity Stress Condition. Front. Sustain. Food Syst. 2021, 5, 687531. [Google Scholar] [CrossRef]
- Orhan, F. Potential of Halophilic/Halotolerant Bacteria in Enhancing Plant Growth Under Salt Stress. Curr. Microbiol. 2021, 78, 3708–3719. [Google Scholar] [CrossRef]
- Rincón, A.; Arenal, F.; González, I.; Manrique, E.; Lucas, M.M.; Pueyo, J.J. Diversity of Rhizobial Bacteria Isolated from Nodules of the Gypsophyte Ononis tridentata L. Growing in Spanish Soils. Microb. Ecol. 2008, 56, 223–233. [Google Scholar] [CrossRef]
- Neshat, M.; Abbasi, A.; Hosseinzadeh, A.; Sarikhani, M.R.; Dadashi Chavan, D.; Rasoulnia, A. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants 2022, 28, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Ahmad, I.; Shahid, M.; Shah, G.M.; Farooq, A.B.U.; Akram, M.; Tabassum, S.A.; Naeem, M.A.; Khalid, U.; Ahmad, S.; et al. Regulation of antioxidant production, ion uptake and productivity in potato (Solanum tuberosum L.) plant inoculated with growth promoting salt tolerant Bacillus strains. Ecotoxicol. Environ. Saf. 2019, 178, 33–42. [Google Scholar] [CrossRef]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; Malek, R.A.; Ilyas, N.; Suriani, N.L.; Khan, N.; El Enshasy, H.A. Production of Plant Beneficial and Antioxidants Metabolites by Klebsiella variicola under Salinity Stress. Molecules 2021, 26, 1894. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 2022, 13, 1069053. [Google Scholar] [CrossRef] [PubMed]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Sarmiento-López, L.G.; López-Meyer, M.; Maldonado-Mendoza, I.E.; Quiroz-Figueroa, F.R.; Sepúlveda-Jiménez, G.; Rodríguez-Monroy, M. Production of indole-3-acetic acid by Bacillus circulans E9 in a low-cost medium in a bioreactor. J. Biosci. Bioeng. 2022, 134, 21–28. [Google Scholar] [CrossRef]
- Qin, L.; Tian, P.; Cui, Q.; Hu, S.; Jian, W.; Xie, C.; Yang, X.; Shen, H. Bacillus circulans GN03 Alters the Microbiota, Promotes Cotton Seedling Growth and Disease Resistance, and Increases the Expression of Phytohormone Synthesis and Disease Resistance-Related Genes. Front. Plant Sci. 2021, 12, 644597. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-S.; Song, H.-G. Growth promotion of tomato plant under drought conditions by treatment of rhizobacteria producing ACC deaminase and phytohormones. Korean J. Microbiol. 2013, 49, 46–50. [Google Scholar] [CrossRef]
- Bhutani, N.; Maheshwari, R.; Sharma, N.; Kumar, P.; Dang, A.S.; Suneja, P. Characterization of halo-tolerant plant growth promoting endophytic Bacillus licheniformis MHN 12. J. Genet. Eng. Biotechnol. 2022, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Lin, W.; Gong, K.; Han, Z.; Ma, H.; Zhang, M.; Zhang, Q.; Gao, Y.; Li, J.; Zhang, X. Bacillus Co-Inoculation Alleviated Salt Stress in Seedlings Cucumber. Agronomy 2021, 11, 966. [Google Scholar] [CrossRef]
- Pham, V.T.K.; Rediers, H.; Ghequire, M.G.K.; Nguyen, H.H.; De Mot, R.; Vanderleyden, J.; Spaepen, S. The plant growth-promoting effect of the nitrogen-fixing endophyte Pseudomonas stutzeri A15. Arch. Microbiol. 2017, 199, 513–517. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; Rocha-Granados, M.d.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Montecillo, J.A.V.; Bae, H. In silico analysis of koranimine, a cyclic imine compound from Peribacillus frigoritolerans reveals potential nematicidal activity. Sci. Rep. 2022, 12, 18883. [Google Scholar] [CrossRef]
- Sato, S.; Ichiyanagi, N.; Sugiyama, K.; Aburai, N.; Fujii, K. Production of polyglutamic acid-like mucilage protein by Peribacillus simplex strain 8h. Folia Microbiol. 2023, 68, 101–113. [Google Scholar] [CrossRef]
- Dey, G.; Banerjee, P.; Sharma, R.K.; Maity, J.P.; Etesami, H.; Shaw, A.K.; Huang, Y.-H.; Huang, H.-B.; Chen, C.-Y. Management of Phosphorus in Salinity-Stressed Agriculture for Sustainable Crop Production by Salt-Tolerant Phosphate-Solubilizing Bacteria—A Review. Agronomy 2021, 11, 1552. [Google Scholar] [CrossRef]
- Feng, K.; Cai, Z.; Ding, T.; Yan, H.; Liu, X.; Zhang, Z. Effects of potassium-solubulizing and photosynthetic bacteria on tolerance to salt stress in maize. J. Appl. Microbiol. 2019, 126, 1530–1540. [Google Scholar] [CrossRef]
- Muthuraja, R.; Muthukumar, T. Co-inoculation of halotolerant potassium solubilizing Bacillus licheniformis and Aspergillus violaceofuscus improves tomato growth and potassium uptake in different soil types under salinity. Chemosphere 2022, 294, 133718. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A.; et al. Melatonin Enhances Seed Germination and Seedling Growth of Medicago sativa Under Salinity via a Putative Melatonin Receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef] [PubMed]
- Zhanwu, G.; Hui, Z.; Jicai, G.; Chunwu, Y.; Chunsheng, M.; Deli, W. Germination responses of Alfalfa (Medicago sativa L.) seeds to various salt-alkaline mixed stress. Afr. J. Agric. Res. 2011, 6, 3793–3803. [Google Scholar]
- Ansari, M.; Shekari, F.; Mohammadi, M.H.; Juhos, K.; Végvári, G.; Biró, B. Salt-tolerant plant growth-promoting bacteria enhanced salinity tolerance of salt-tolerant alfalfa (Medicago sativa L.) cultivars at high salinity. Acta Physiol. Plant. 2019, 41, 195. [Google Scholar] [CrossRef]
- Alikooyi, M.; Surki, A.; Mobini-Dehkordi, M.; Kiyani, S. Effects of Plant Growth-Promoting Rhizobacteria on Germination and Early Growth of Alfalfa (Medicago sativa) under Salt Stress Conditions. Iran. J. Seed Res. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Cherifi, K.; Boufous, E.H.; Abdelhamid, E.M. Diversity of Salt Tolerance During Germination in Medicago ciliaris (L.) and Medicago polymorpha (L.). Atlas J. Plant Biol. 2017, 1, 6–12. [Google Scholar] [CrossRef]
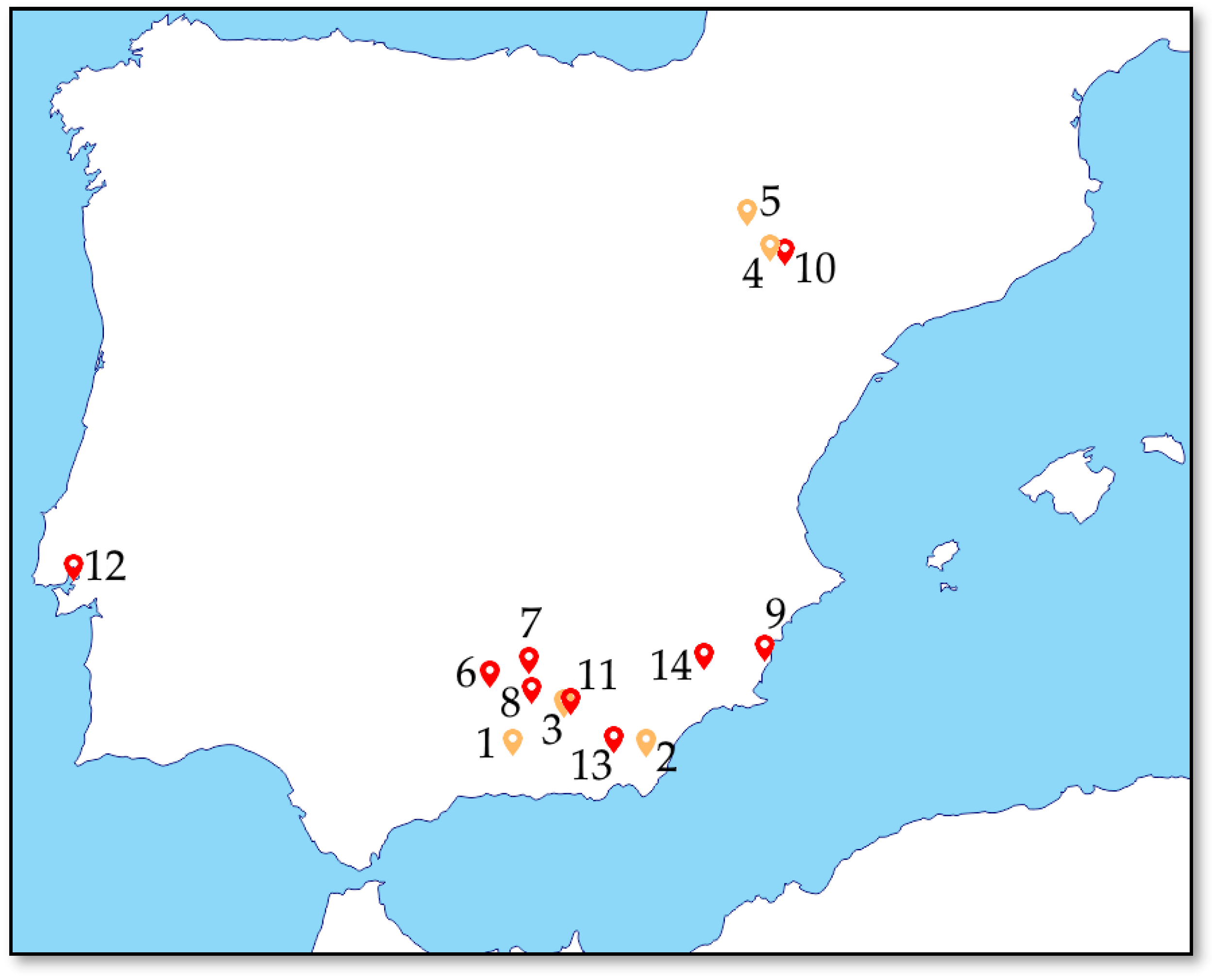
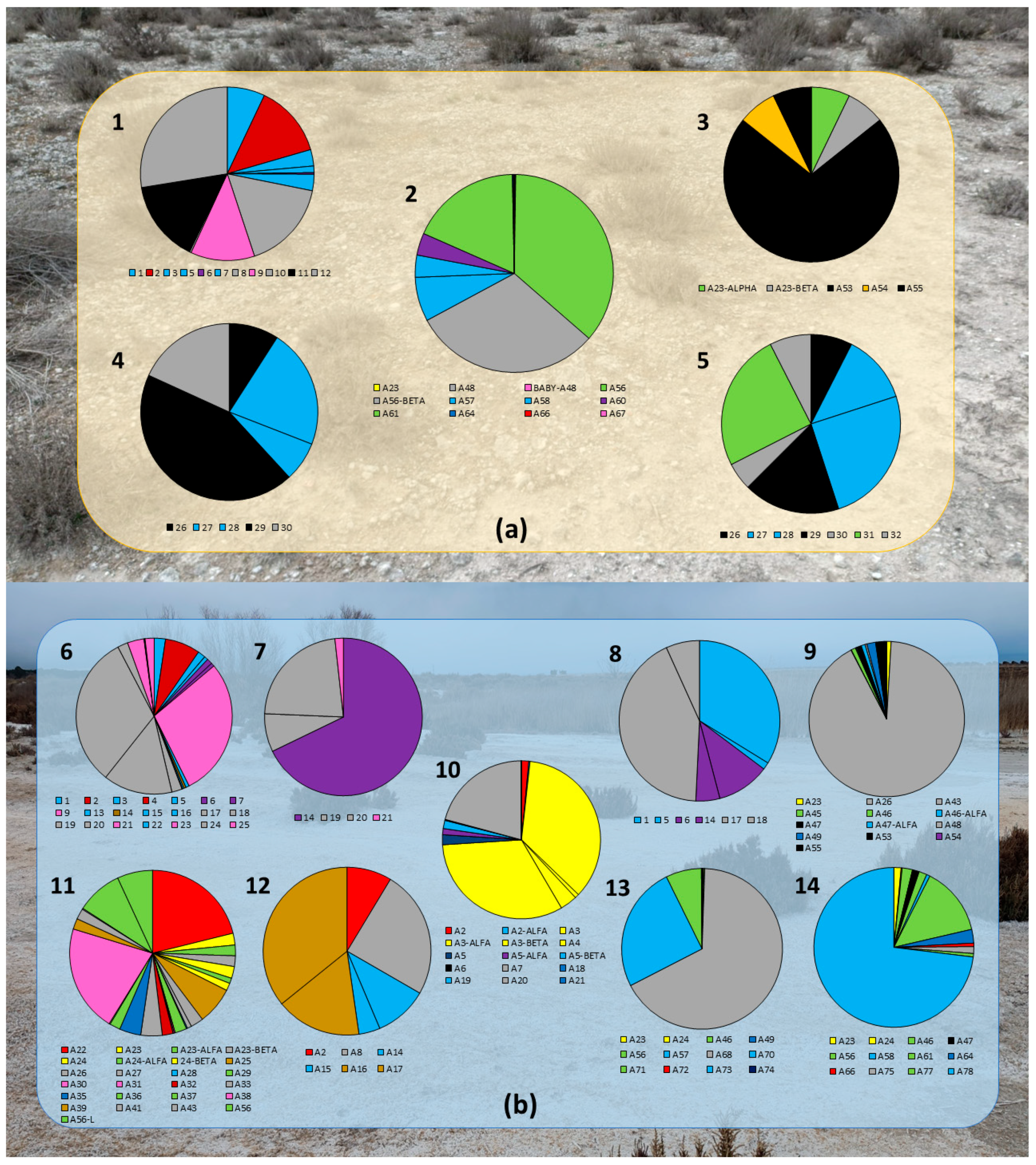


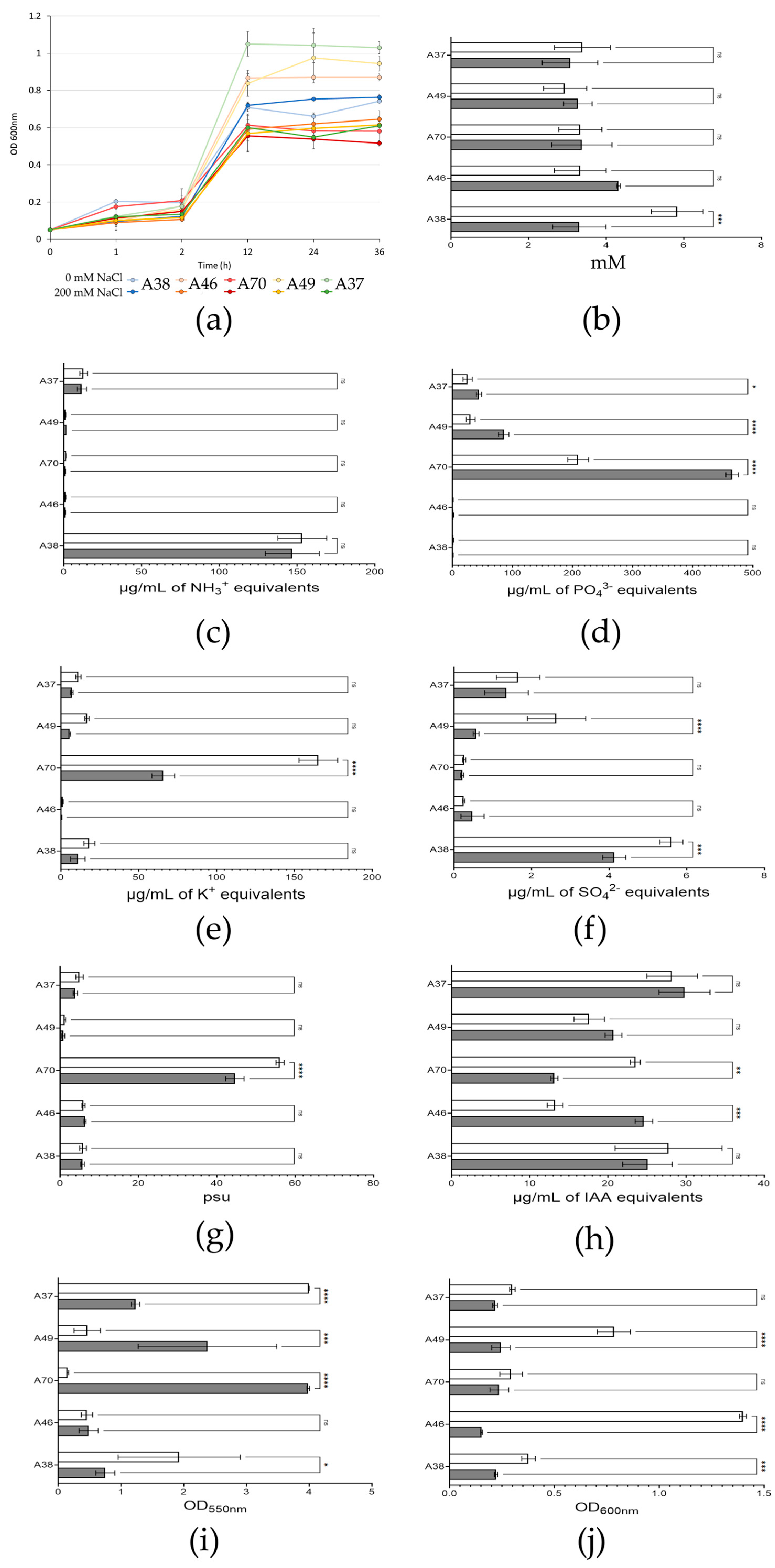
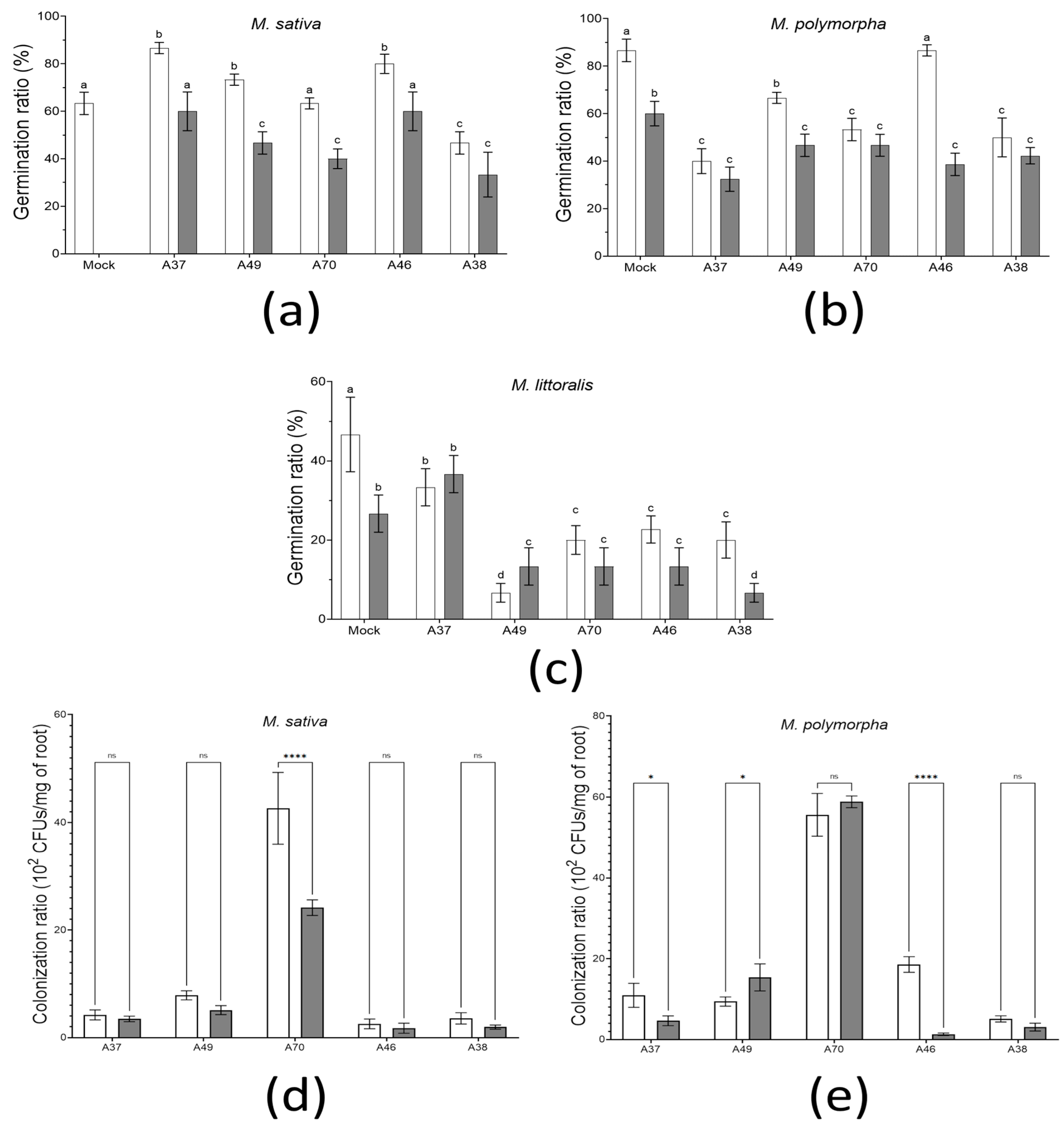

| No. | Type | Name | Location | GPS | Altitude (Mamsl) | Main Basal Lithology a | Type of Soil b | pH c | EC c,d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Gypsum | Arroyo Salado (AR) | La Malahá, Granada (SP) | 37°06′25.3″ N 3°43′14.7″ W | 726 | Dolomites and dolomitic marbles | Cambic calcisol | 8.02 | 0.31 |
| 2 | Gypsum | Rambla de la Mojonera (RM) | Sorbas, Almería (SP) | 37°05′59.5″ N 2°08′08.7″ W | 395 | Sands and gravels | Cambic calcisol | 9.79 | 0.06 |
| 3 | Gypsum | Hoya de Baza (HB) | Baza, Granada (SP) | 37°30′50.4″ N 2°45′34.5″ W | 792 | Clays with pebbles | Calcaric fluvisol | 8.52 | 0.4 |
| 4 | Gypsum | Quinto (GQ) | Quinto, Zaragoza (SP) | 41°26′51.8″ N 0°32′15.5″ W | 195 | Gypsum, marlstones limestones | Haplic calcisol | 8.46 | 0.52 |
| 5 | Gypsum | Zuera (GZ) | Zuera, Zaragoza (SP) | 41°52′56.7″ N 0°47′21.6″ W | 335 | Gypsum and clays | Haplic gypsisol | 8.56 | 0.18 |
| 6 | Salt | Salina de San José (SJ) | Torredonjimeno, Jaén (SP) | 37°45′26.8″ N 4°00′12.1″ W | 451 | Marlstones and marly siltstones | Cambic calcisol | 8.66 | 2.08 |
| 7 | Salt | Salinas de las Escuelas (SE) | Baeza, Jaén (SP) | 37°52′15.9″ N 3°31′18.1″ W | 479 | Dolomites | Cambic calcisol | 9.58 | 2.16 |
| 8 | Salt | Salinas de Las Cañadas (SC) | Montejícar, Jaén (SP) | 37°36′31.9″ N 3°30′19.9″ W | 1028 | Conglomerates, sandstones, clays | Cambic calcisol | 8.64 | 2.18 |
| 9 | Salt | Laguna Roja (LR) | Torrevieja, Alicante (SP) | 37°59′46.8″ N 0°42′11.8″ W | −2 | Siltstones | Calcaric leptosol | 9.24 | 2.68 |
| 10 | Salt | Saladas de Sástago (SB) | Bujaraloz, Zaragoza (SP) | 41°25′15.6″ N 0°11′39.6″ W | 322 | Clays and silstones | Calcaric fluvisol | 8.22 | 2.42 |
| 11 | Salt | Saladar del Baíco (EB) | El Baíco, Granada (SP) | 37°32′28.5″ N 2°43′57.1″ W | 711 | Marls, conglomerates, limestones, sandstones | Cambic calcisol | 8.97 | 5.37 |
| 12 | Salt | Salinas do Samouco (SS) | Alcochete, Setúbal (PT) | 38°44′11.6″ N 9°00′01.7″ W | 0 | Sandstones and conglomerates | Eutric regosol | 6.88 | 2.23 |
| 13 | Salt | Barranco de las Salinas (BS) | Gádor, Almería (SP) | 37°01′01.9″ N 2°26′51.7″ W | 263 | Marls, sandstones and siltstones | Cambic calcisol | 9.38 | 1.94 |
| 14 | Salt | Rambla de Librilla (RL) | Librilla, Murcia (SP) | 37°54′23.7″ N 1°22′15.8″ W | 205 | Conglomerates and sandstones | Cambic calcisol | 9.21 | 3.78 |
| Species Isolated | Location |
|---|---|
| Rhizobium zeae | 2 |
| Pseudarthrobacter oxydans | 1, 6 |
| Arthrobacter globiformis | 11 |
| Arthrobacter agilis | 6 |
| Pseudomonas sp. | 1, 2, 6 |
| Pseudomonas fragi | 6 |
| Pseudoclavibacter helvolus | 6, 7, 8 |
| Stutzerimonas stutzeri | 6, 7, 11 |
| Pantoea agglomerans | 10 |
| Leclercia adecarboxylata | 1, 6, 8 |
| Bhargavaea beijingensis/cecembensis | 14 |
| Streptomyces spiroverticillatus | 3, 9 |
| Niallia circulans | 11 |
| Niallia nealsonii | 11 |
| Bacillus cereus | 13 |
| Bacillus pumilus | 9, 11, 13 |
| Bacillus subtilis | 5, 10 |
| Bacillus thuringiensis/toyonensis | 2, 14 |
| Bacillus thuringiensis | 10 |
| Bacillus atrophaeus | 2, 9, 10, 11, 13, 14 |
| Bacillus mojavensis | 3, 11 |
| Bacillus safensis | 9 |
| Bacillus sonorensis | 13 |
| Bacillus licheniformis | 2, 9, 11, 13, 14 |
| Peribacillus frigoritolerans/simplex | 1, 2, 4, 5, 6, 8, 9, 10, 11, 12, 13, 14 |
| Peribacillus muralis | 6 |
| Paenibacillus amylolyticus | 2, 14 |
| Paenibacillus taichungensis | 10,12 |
| Paenibacillus urinalis | 11 |
| Paenibacillus polymyxa | 2, 13, 14 |
| Priestia endophytica | 11 |
| Priestia megaterium | 12 |
| Priestia flexa | 11 |
| Metabacillus idriensis | 2, 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, T.; Teixeira, R.; Sousa, A.; d’Oliveira Palmeiro, M.A.; Cruz Coimbra de Matos, A.; Niza Costa, M.; Ferrer, M.V.; Rodrígues dos Santos, A.S.; Sequero López, C.; Rebelo Romão, I.; et al. Isolation and Characterization of Culturable Osmotolerant Microbiota in Hypersaline and Hypergypsic Soils as New Treatment for Osmotic Stress in Plants. Soil Syst. 2023, 7, 86. https://doi.org/10.3390/soilsystems7040086
Gil T, Teixeira R, Sousa A, d’Oliveira Palmeiro MA, Cruz Coimbra de Matos A, Niza Costa M, Ferrer MV, Rodrígues dos Santos AS, Sequero López C, Rebelo Romão I, et al. Isolation and Characterization of Culturable Osmotolerant Microbiota in Hypersaline and Hypergypsic Soils as New Treatment for Osmotic Stress in Plants. Soil Systems. 2023; 7(4):86. https://doi.org/10.3390/soilsystems7040086
Chicago/Turabian StyleGil, Tatiana, Raquel Teixeira, André Sousa, Maria Alice d’Oliveira Palmeiro, Alice Cruz Coimbra de Matos, Marla Niza Costa, María Victoria Ferrer, Ana Sofía Rodrígues dos Santos, Cristina Sequero López, Inês Rebelo Romão, and et al. 2023. "Isolation and Characterization of Culturable Osmotolerant Microbiota in Hypersaline and Hypergypsic Soils as New Treatment for Osmotic Stress in Plants" Soil Systems 7, no. 4: 86. https://doi.org/10.3390/soilsystems7040086
APA StyleGil, T., Teixeira, R., Sousa, A., d’Oliveira Palmeiro, M. A., Cruz Coimbra de Matos, A., Niza Costa, M., Ferrer, M. V., Rodrígues dos Santos, A. S., Sequero López, C., Rebelo Romão, I., & Vílchez, J. I. (2023). Isolation and Characterization of Culturable Osmotolerant Microbiota in Hypersaline and Hypergypsic Soils as New Treatment for Osmotic Stress in Plants. Soil Systems, 7(4), 86. https://doi.org/10.3390/soilsystems7040086







