Soil Carbon Stock and Indices in Sandy Soil Affected by Eucalyptus Harvest Residue Management in the South of Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment and Treatments
- AR: all forest residues remained on the soil (i.e., bark, branches, leaves, and the litter layer from the previous rotation), and only trunk wood was removed.
- NB: where bark was also removed.
- NBr: in which branches were also removed.
- NR: in which all eucalyptus residues were removed.
- NRs: same as NR, but a shade net was also used to prevent litter from the new plantation from reaching the soil surface.
2.2. Estimation of Carbon Input and Biochemical Composition Analysis of Eucalyptus Harvesting Residue Components
2.3. Soil Organic C Stock and Fractionation
2.4. Carbon Management Index
2.5. Annual Soil C Retention Rates
2.6. Estimate of the Organic Matter Humification (k1) and Decomposition (k2) Coefficients
2.7. Statistical Analysis
3. Results
3.1. C Contribution and Composition of Eucalyptus Harvest Residues, Litter, and Roots
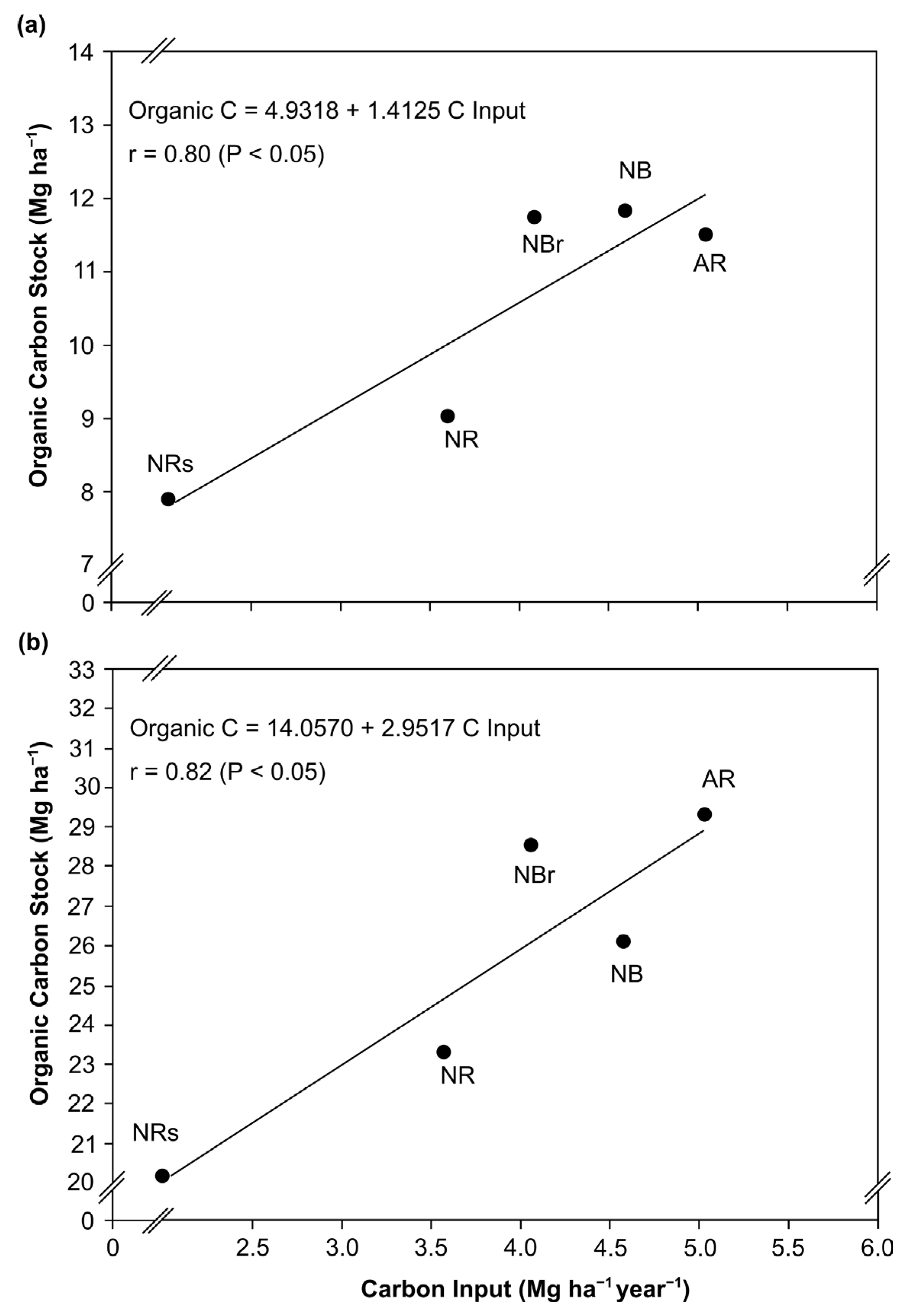
3.2. Effect of Eucalyptus Harvest Residue Management on Soil Organic C Content and Stocks
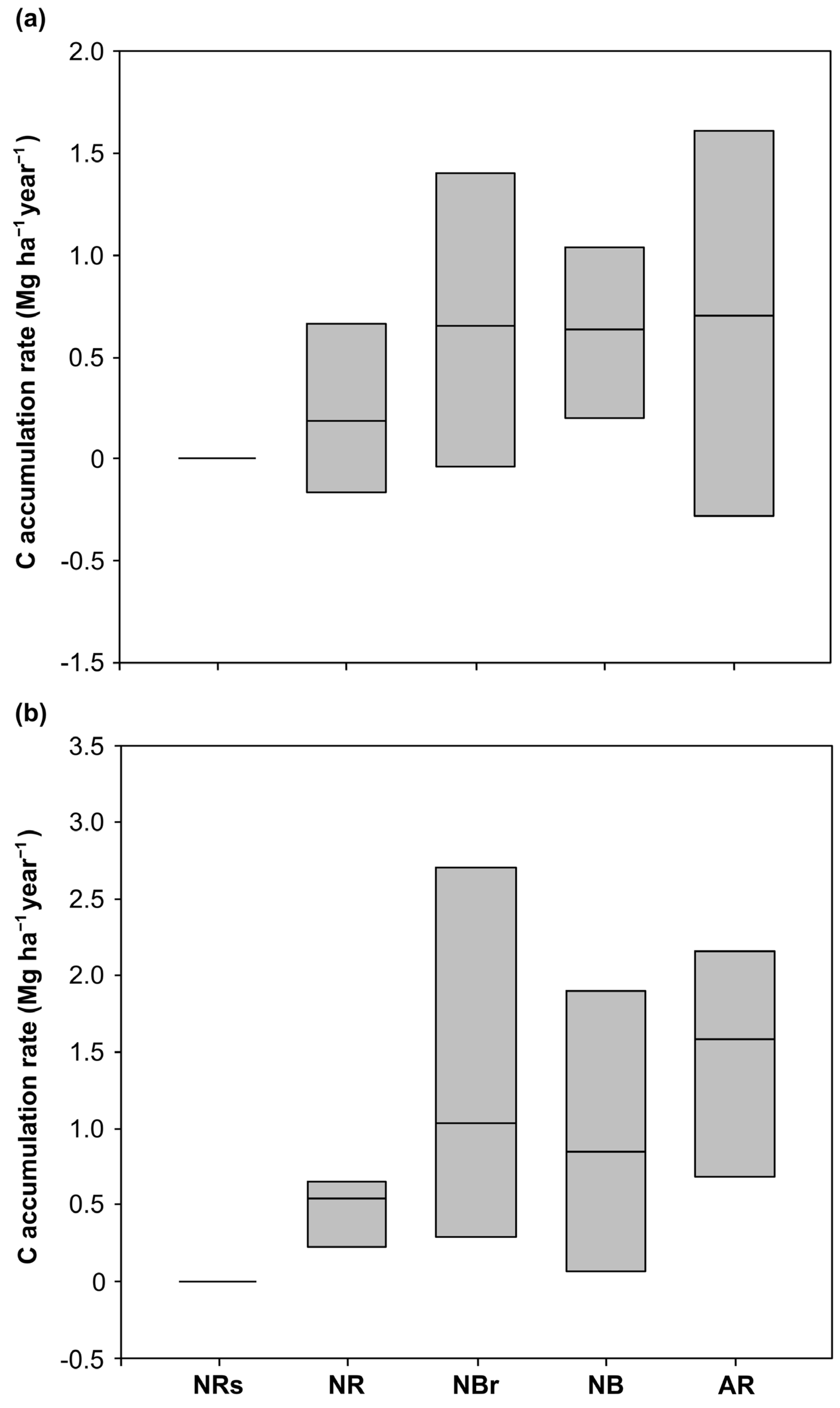
3.3. Annual Soil C Retention Rates
3.4. Estimate of the Humification (k1) and Decomposition (k2)
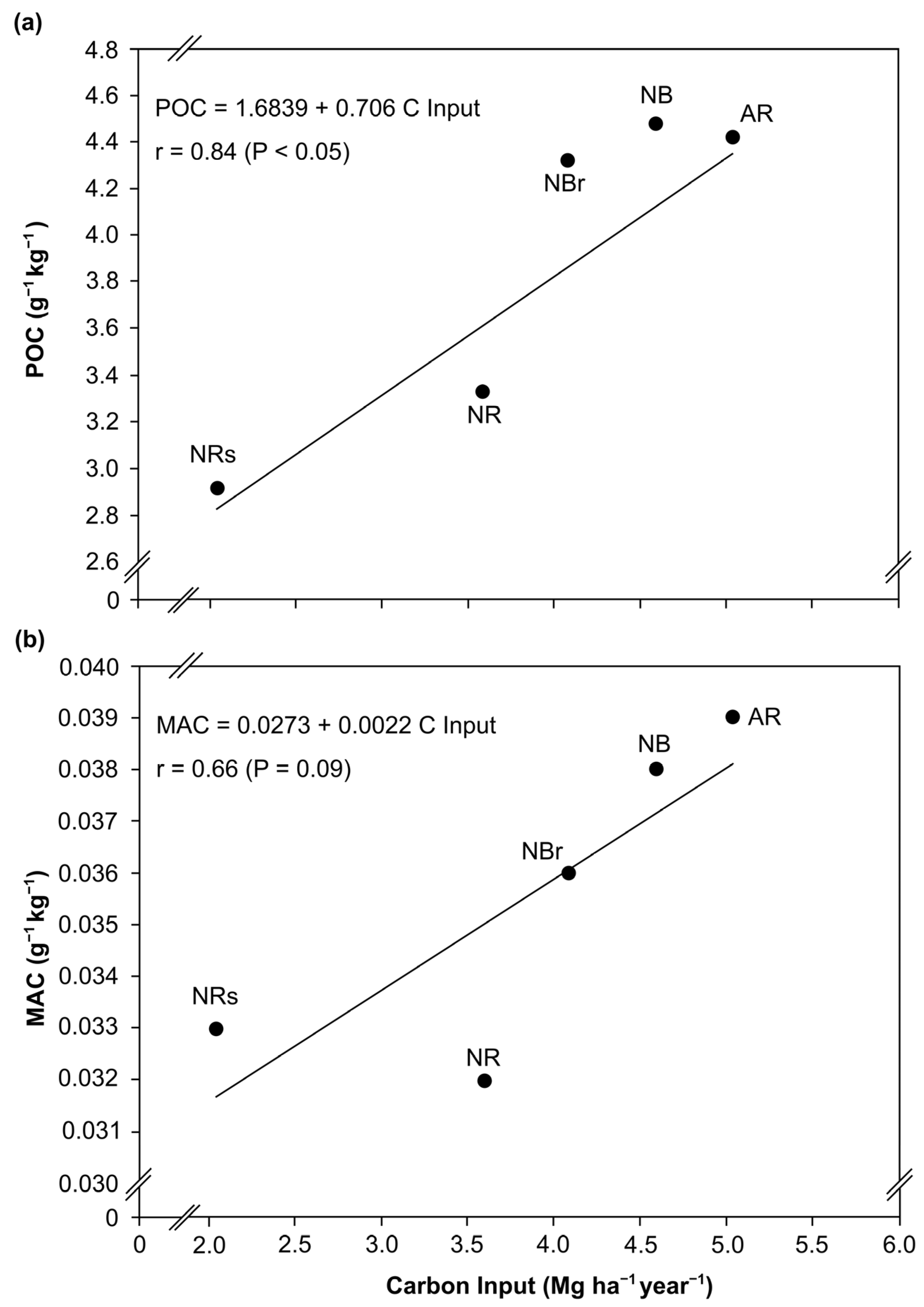
3.5. Carbon Lability, Carbon Management Index, and Relationship with Eucalyptus Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, H.; Wen, Y.; Goodale, U.M.; Zhu, Y.; Yu, S.; Li, C.; Li, X. Intensive management and declines in soil nutrients lead to serious exotic plant invasion in Eucalyptus plantations under successive short-rotation regime. Land Degrad. Dev. 2020, 31, 297–310. [Google Scholar] [CrossRef]
- Brazilian Tree Industry: Annual Report. 2022. Available online: https://www.iba.org/datafiles/publicacoes/relatorios/relatorio-anual-iba2022-compactado.pdf (accessed on 7 December 2022).
- Ordinance No. 323, of October 21, 2021, of the Ministry of Agriculture, Livestock and Supply of Brazil. Available online: https://in.gov.br/en/web/dou/-/portaria-mapa-n-323-de-21-de-outubro-de-2021-353829462 (accessed on 20 June 2023).
- Mayer, S.; Wiesmeier, M.; Sakamoto, E.; Hübner, R.; Cardinael, R.; Kühnel, A.; Kögel-Knabner, I. Soil organic carbon sequestration in temperate agroforestry systems—A meta-analysis. Agric. Ecosyst. Environ. 2022, 323, 107689. [Google Scholar] [CrossRef]
- Gonçalves, J.L.M.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; Ferraz, S.F.B.; Lima, W.P.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- U.S. Energy Information Admnistration. Internaltional Energy Outlook 2021 with Projections to 2050. 2021. Available online: https://www.eia.gov/outlooks/ieo/ (accessed on 8 November 2022).
- Hernández, J.; del Pino, A.; Hitta, M.; Lorenzo, M. Management of forest harvest residues affects soil nutrient availability during reforestation of Eucalyptus grandis. Nutr. Cycl. Agroecosyst. 2016, 105, 141–155. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Marques, E.R.G.; Gonçalves, J.L.M.; Hübner, A.; Brandani, C.B.; Ferraz, A.V.; Moreira, R.M. Decomposition rates of forest residues and soil fertility after clear-cutting of Eucalyptus grandis stands in response to site management and fertilizer application. Soil Use Manag. 2016, 32, 289–302. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Ferreira, G.W.; Dungait, J.A.; Araújo, E.F.; Soares, E.M.; Silva, I. R Eucalypt harvest residue management influences microbial community structure and soil organic matter fractions in an afforested grassland. Soil Tillage Res. 2021, 205, 104787. [Google Scholar] [CrossRef]
- São José, J.F.B.; Cherubin, M.R.; Vargas, L.K.; Araújo, E.F.; Bayer, C. A soil quality index for subtropical sandy soils under different Eucalyptus harvest residue managements. J. For. Res. 2022, 34, 243–255. [Google Scholar] [CrossRef]
- São José, J.F.B.; Vargas, L.K.; Bayer, C.; Lisboa, B.B.; Araújo, E.F.D. Initial growth and nutrition of eucalyptus under different management of harvest residues. Floresta Ambiente 2020, 27, e20180161. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Gonçalves, J.L.M.; Gava, J.L.; Godinho, T.O.; Melo, E.A.S.C.; Bazani, J.H.; Hubner, A.; Arthur, J.C.; Wichert, M.P. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. For. Ecol. Manag. 2016, 379, 1–10. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Gonçalves, J.L.M.; Gava, J.L.; Godinho, T.O.; Melo, E.A.S.C.; Bazani, J.H.; Hubner, A.; Arthur Junior, J.C.; Wichert, M.P. Forest residue removal decreases soil quality and affects wood productivity even with high rates of fertilizer application. For. Eco. Manag. 2018, 430, 188–195. [Google Scholar] [CrossRef]
- Epron, D.; Mouanda, C.; Mareschal, L.; Koutika, L. Impacts of organic residue management on the soil C dynamics in a tropical eucalypt plantation on a nutrient-poor sandy soil after three rotations. Soil Biol. Biochem. 2015, 85, 183–189. [Google Scholar] [CrossRef]
- Menegale, M.L.C.; Rocha, J.H.T.; Harrison, R.; Goncalves, J.L.D.M.; Almeida, R.F.; Piccolo, M.D.C.; Hubner, A.; Arthur Junior, J.C.; De Vicente Ferraz, A.; James, J.N.; et al. Effect of timber harvest intensities and fertilizer application on stocks of soil C, N, P, and S. Forests 2016, 7, 319–333. [Google Scholar] [CrossRef]
- Demolinari, M.S.M.; Sousa, R.N.; Silva, I.R.; Teixeira, R.S.; Neves, J.C.L.; Mendes, G.O. Effect of mineral nitrogen on transfer of 13C-carbon from Eucalyptus harvest residue components to soil organic matter fractions. Rev. Bras. Cienc. Solo 2017, 41, e0160203. [Google Scholar] [CrossRef]
- Mendham, D.S.; Ogden, G.N.; Short, T.; O’Connell, T.M.; Grove, T.S.; Rance, S.J. Repeated harvest residue removal reduces E. globulus productivity in the 3rd rotation in south-western Australia. For. Ecol. Manag. 2014, 329, 279–286. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Mendham, D.S.; Grove, T.S.; O’Connell, A.M.; Sankaran, K.V.; Rance, S.J. Harvest residue effects on soil organic matter, nutrients and microbial biomass in eucalypt plantations in Kerala, India. For. Ecol. Manag. 2014, 328, 140–149. [Google Scholar] [CrossRef]
- Kramer, M.G.; Chadwick, O.A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale. Nat. Clim. Chang. 2018, 8, 1104–1108. [Google Scholar] [CrossRef]
- Wan, X.; Xiao, L.; Vadeboncoeur, M.A.; Johnson, C.E.; Huang, Z. Response of mineral soil carbon storage to harvest residue retention depends on soil texture: A meta-analysis. For. Ecol. Manag. 2018, 408, 9–15. [Google Scholar] [CrossRef]
- Almeida, L.F.J.; Hurtarte, L.C.C.; Souza, I.F.; Soares, E.M.B.; Vergütz, L.; Silva, I.R. Soil organic matter formation as affected by eucalypt litter biochemistry—Evidence from an incubation study. Geoderma 2018, 312, 121–129. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Xu, Z.; Abdullah, K.M.; Zhou, Q. Effects of changed litter inputs on soil labile carbon and nitrogen pools in a eucalyptus-dominated forest of southeast Queensland, Australia. J. Soils Sediments 2019, 19, 1661–1671. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2967. [Google Scholar] [CrossRef]
- García, A.; Alriols, M.G.; Labidi, J. Evaluation of different lignocellulosic raw materials as potential alternative feedstocks in biorefinery processes. Ind. Crops Prod. 2014, 53, 102–110. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Dalal, R.C.; Mayer, R.J. Long-term trends in fertility of soils under continuous cultivation and cereal cropping in southern Queensland: II Total organic carbon and its rate of loss from the soil profile. Aust. J. Soil Res. 1986, 24, 265–279. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Vieira, F.C.B.; Bayer, C.; Zanatta, J.A.; Dieckow, J.; Mielniczuk, J.; He, Z.L. Carbon management index based on physical fractionation of soil organic matter in an Acrisol under long-term no-till cropping systems. Soil Tillage Res. 2007, 96, 195–204. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Meena, V.S.; Alam, N.M.; Dogra, P.; Bhattacharyya, R.; Sharma, N.K.; Mishra, P.K. Impact of conservation practices on soil aggregation and the carbon management index after seven years of maize–wheat cropping system in the Indian Himalayas. Agric. Ecosyst. Environ. 2016, 216, 247–257. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharyya, R.; Meena, M.C.; Dwivedi, B.S.; Singh, G.; Agnihotri, R.; Sharma, C. Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Tillage Res. 2018, 177, 134–144. [Google Scholar] [CrossRef]
- Sá, J.C.M.; Gonçalves, D.R.P.; Ferreira, L.A.; Mishra, U.; Inagaki, T.M.; Furlan, F.J.F.; Morog, R.S.; Florianih, N.; Briedisf, C.; Ferreira, A.O. Soil carbon fractions and biological activity based indices can be used to study the impact of land management and ecological successions. Ecol. Indic. 2018, 84, 96–105. [Google Scholar] [CrossRef]
- Reichert, J.M.; Amado, T.J.C.; Reinert, D.J.; Rodrigues, M.F.; Suzuki, L.E.A.S. Land use effects on subtropical, sandy soil under sandization/desertification processes. Agric. Ecosyst. Environ. 2016, 233, 370–380. [Google Scholar] [CrossRef]
- Witschoreck, R. Recomendação de Fertilizantes Para Eucalyptus saligna Sm. Com Base no Balanço Nutricional na Região de GUAÍBA-RS. Ph.D. Thesis, Universidade Federal de Santa Maria, Santa Maria, Brazil, 2014. [Google Scholar]
- Londero, E.K.; Schumacher, M.V.; Szymczak, D.A.; Araújo, E.F. Calibração do modelo 3-PG para Eucalyptus saligna Smith na região de Guaíba-RS. Cienc. Florest 2015, 25, 293–305. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Soares, C.P.B.; Fehrmann, L.; Jacovine, L.A.G.; von Gadow, K. Aboveground and belowground biomass and carbon estimates for clonal eucalyptus trees in southeast Brazil. Rev. Arvore 2015, 39, 353–363. [Google Scholar] [CrossRef]
- Van Soest, P.J. Development of a comprehensive system of feed analysis and its application to forages. J. Anim. Sci. 1967, 26, 119–128. [Google Scholar] [CrossRef]
- EMBRAPA. Manual de métodos de análise de solo. In Empresa Brasileira de Pesquisa Agropecuária, 2nd ed.; EMBRAPA: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Dieckow, J.; Mielniczuk, J.; Knicker, H.; Bayer, C.; Dick, D.P.; Kögel-Knabner, I. Carbon and nitrogen stocks in physical fractions of a subtropical Acrisol as influenced by long-term no-till cropping systems and N fertilisation. Plant Soil 2005, 268, 319–328. [Google Scholar] [CrossRef]
- Bayer, C.; Lovato, T.; Dieckow, J.; Zanatta, J.A.; Mielniczuk, J. A method for estimating coefficients of soil organic matter dynamics based on long-term experiments. Soil Tillage Res. 2006, 91, 217–226. [Google Scholar] [CrossRef]
- Guimarães, C.C.; Schumacher, M.V.; Witshoreck, R.; Souza, H.P.; Santo, J.C. BIomass and nutrients of Eucalyptus dunnii Maiden stand in pampa gaúcho. Rev. Árvore 2015, 39, 873–882. [Google Scholar] [CrossRef]
- Dieckow, J.; Bayer, C.; Conceição, P.C.; Zanatta, J.A.; Martin-Neto, L.; Milori, D.B.M.; Salton, J.C.; Macedo, M.M.; Mielniczuk, J.; Hernani, L.C. Land use, tillage, texture and organic matter stock and composition in tropical and subtropical Brazilian soils. Eur. J. Soil Sci. 2009, 60, 240–249. [Google Scholar] [CrossRef]
- Cao, J.B.; He, X.X.; Chen, Y.Q.; Chen, Y.P.; Zhang, Y.J.; Yu, S.Q.; Zhou, L.X.; Liu, Z.F.; Zhang, C.L.; Fu, S.L. Leaf litter contributes more to soil organic carbon than fine roots in two 10-year-old subtropical plantations. Sci. Total Environ. 2020, 704, 135–341. [Google Scholar] [CrossRef] [PubMed]
- Lajtha, K.; Jones, J. Forest harvest legacies control dissolved organic carbon export in small watersheds, western Oregon. Biogeochemistry 2018, 140, 299–315. [Google Scholar] [CrossRef]
- Tanner, E.V.J.; Sheldrake, M.W.A.; Turner, B.L. Changes in soil carbon and nutrients following 6 years of litter removal and addition in a tropical semi-evergreen rain forest. Biogeosciences 2016, 13, 6183–6190. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall manipulation experiments. Biogeosciences 2013, 10, 7423–7433. [Google Scholar] [CrossRef]
- Sena, K.N.; Maltoni, K.L.; Troleis, M.J.B.; Faria, G.A. Forest harvest management systems and residual phytomass affecting physical properties of a sandy soil. Rev. Bras. Cienc. 2021, 45, e0200190. [Google Scholar] [CrossRef]
- Laganiére, J.; Angers, D.A.; Paré, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Pegoraro, R.F.; Silva, I.R.; Souza, I.F.; Novais, R.F.; Barros, N.F.; Fonseca, S. Carbon accumulation and partitioning above and belowground under coppiced and replanted eucalypt plantations. For. Sci. 2022, 68, 162–171. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Silva, I.R.; Neves, J.C.; Novais, R.F.; Barros, N.F.; Mendonça, E.S.; Leite, F.P. Soil organic carbon dynamics following afforestation of degraded pastures with eucalyptus in southeastern Brazil. For. Ecol. Manag. 2006, 235, 219–231. [Google Scholar] [CrossRef]
- Cook, R.L.; Binkley, D.; Mendes, J.C.T.; Stape, J.L. Soil carbon stocks and forest biomass following conversionof pasture to broadleaf and conifer plantations in southeastern Brazil. For. Ecol. Manag. 2014, 324, 37–45. [Google Scholar] [CrossRef]
- Hernández, J.; del Pino, A.; Vance, E.D.; Califra, Á.; Del Giorgio, F.; Martínez, L.; González-Barrios, P. Eucalyptus and Pinus stand density effects on soil carbon sequestration. For. Ecol. Manag. 2016, 368, 28–38. [Google Scholar] [CrossRef]
- Kätterer, T.; Bolinder, M.A.; Andrén, O.; Kirchmann, H.; Menichetti, L. Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric. Ecosyst. Environ. 2011, 141, 184–192. [Google Scholar] [CrossRef]
- Oliveira, F.C.C.; Silva, I.R.; Ferreira, G.W.D.; Soares, E.M.B.; Silva, S.R.; Silva, E.F. Contribution of eucalyptus harvest residues and nitrogen fertilization to carbon stabilization in Ultisols of southern Bahia. Rev. Bras. Cienc. 2018, 42, e0160340. [Google Scholar] [CrossRef]
- Oliveira Filho, J.D.S.; Pereira, M.G.; Aquino, B.F. Organic matter labile fractions and carbon stocks in a typic quartzipsamment cultivated with sugarcane harvested without burning. Rev. Caatinga 2017, 30, 24–31. [Google Scholar] [CrossRef]
- De Bona, F.D.; Bayer, C.; Dieckow, J.; Bergamaschi, H. Soil quality assessed by carbon management index in a subtropical Acrisol subjected to tillage systems and irrigation. Aust. J. Soil Res. 2008, 46, 469–475. [Google Scholar] [CrossRef]
- Chivenge, P.; Vanlauwe, B.; Gentile, R.; Six, J. Comparison of organic versus mineral resource effects on short-term aggregate carbon and nitrogen dynamics in a sandy soil versus a fine textured soil. Agric. Ecosyst. Environ. 2011, 140, 361–371. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Lal, R.; Resck, D.V. Changes in soil organic carbon stocks under agriculture in Brazil. Soil Tillage Res. 2005, 84, 28–40. [Google Scholar] [CrossRef]
- Puttaso, A.; Vityakon, P.; Rasche, F.; Saenjan, P.; Treloges, V.; Cadisch, G. Does organic residue quality influence carbon retention in a tropical sandy soil? Soil Sci. Soc. Am. J. 2013, 77, 1001–1011. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bandyopadhyay, K.K.; Pradhan, S.; Singh, R.; Datta, S.P. Effects of irrigation, crop residue mulch and nitrogen management in maize (Zea mays L.) on soil carbon pools in a sandy loam soil of Indo-gangetic plain region. Catena 2018, 165, 207–216. [Google Scholar] [CrossRef]


| Eucalyptus Residue Management | Litter a | Composition of Harvest Residues | ||||
|---|---|---|---|---|---|---|
| Leaves | Branches | Bark | Root | Total | ||
| Residue biomass (Mg ha−1 year−1) | ||||||
| NRs | 0.00 | 0.00 | 0.00 | 0.00 | 5.41 b | 5.41 |
| NR | 3.05 | 0.00 | 0.00 | 0.00 | 5.41 | 8.46 |
| NBr | 3.05 | 0.07 | 0.00 | 1.10 | 5.41 | 9.62 |
| NB | 3.05 | 0.07 | 2.17 | 0.00 | 5.41 | 10.70 |
| AR | 3.05 | 0.07 | 2.17 | 1.10 | 5.41 | 11.80 |
| Carbon input (Mg ha−1 year−1) | ||||||
| NRs | 0.00 | 0.00 | 0.00 | 0.00 | 2.04 c | 2.04 |
| NR | 1.56 | 0.00 | 0.00 | 0.00 | 2.04 | 3.60 |
| NBr | 1.56 | 0.03 | 0.00 | 0.45 | 2.04 | 4.08 |
| NB | 1.56 | 0.03 | 0.96 | 0.00 | 2.04 | 4.59 |
| AR | 1.56 | 0.03 | 0.96 | 0.45 | 2.04 | 5.04 |
| Biochemical composition | ||||||
| C (g kg−1) | 501.07 ± 25.97 | 474.77 ± 7.1 | 442.53 ± 2.7 | 413.35 ± 9.2 | - | - |
| N (g kg−1) | 11 ± 0.1 | 15.74 ± 0.7 | 1.99 ± 1.1 | 3.74 ± 0.2 | 2.8 d | - |
| C/N | 47.1 ± 2.0 | 30.2 ± 0.9 | 316.2 ± 255.8 | 110.6 ± 9.0 | 162.7 e | - |
| Lignin (g kg−1) | 274.4 ± 0.3 | 227.1 ± 2.8 | 142.5 ± 2.7 | 87.1 ± 0.3 | - | - |
| Lignin/N | 25.8 ± 2.0 | 14.4 ± 1.9 | 112.3 ± 111.0 | 23.2 ± 0.8 | 82.9 e | |
| Hemicellulose (g kg−1) | 104.4 ± 0.3 | 122.2 ± 0.2 | 192.4 ± 0.4 | 158.6 ± 1.0 | - | - |
| Cellulose (g kg−1) | 204.4 ± 0.9 | 143.3 ± 0.9 | 580.2 ± 2.4 | 450.1 ± 1.5 | - | - |
| Residue Management | C/N 1 | Lignin/N 1 |
|---|---|---|
| NRs | 162.7 | 82.9 |
| NR | 112.6 | 58.1 |
| NBr | 111.7 | 53.9 |
| NB | 154.6 | 69.1 |
| AR | 150.7 | 65.0 |
| Management | POC | MAC | L | LI | CSI | CMI |
|---|---|---|---|---|---|---|
| ――― g kg−1 ――― | ||||||
| NRs | 2.92 | 0.033 | 88.4 | 1.00 | 1.00 | 100 |
| NR | 3.33 | 0.032 | 104.0 | 1.17 | 1.14 | 133 |
| NBr | 4.32 | 0.036 | 120.0 | 1.35 | 1.50 | 202 |
| NB | 4.47 | 0.038 | 117.6 | 1.33 | 1.49 | 203 |
| AR | 4.42 | 0.039 | 113.3 | 1.28 | 1.46 | 186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de São José, J.F.B.; Vargas, L.K.; Lisboa, B.B.; Vieira, F.C.B.; Zanatta, J.A.; Araujo, E.F.; Bayer, C. Soil Carbon Stock and Indices in Sandy Soil Affected by Eucalyptus Harvest Residue Management in the South of Brazil. Soil Syst. 2023, 7, 93. https://doi.org/10.3390/soilsystems7040093
de São José JFB, Vargas LK, Lisboa BB, Vieira FCB, Zanatta JA, Araujo EF, Bayer C. Soil Carbon Stock and Indices in Sandy Soil Affected by Eucalyptus Harvest Residue Management in the South of Brazil. Soil Systems. 2023; 7(4):93. https://doi.org/10.3390/soilsystems7040093
Chicago/Turabian Stylede São José, Jackson Freitas Brilhante, Luciano Kayser Vargas, Bruno Britto Lisboa, Frederico Costa Beber Vieira, Josiléia Acordi Zanatta, Elias Frank Araujo, and Cimelio Bayer. 2023. "Soil Carbon Stock and Indices in Sandy Soil Affected by Eucalyptus Harvest Residue Management in the South of Brazil" Soil Systems 7, no. 4: 93. https://doi.org/10.3390/soilsystems7040093






