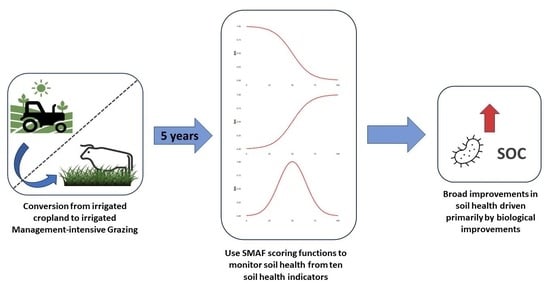
Tracking Soil Health Changes in a Management-Intensive Grazing Agroecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
- 2017: No fertilizer added;
- 2018: ~14 kg N ha−1 and ~67 kg P ha−1 as monoammonium phosphate;
- 2019: no fertilizer added;
- 2020: no fertilizer added;
- 2021: ~90 kg N ha−1, 22 kg P ha−1, and 13 kg S ha−1 as a mix of monoammonium phosphate, urea, and ammonium sulfate;
- 2022: ~56 kg N ha−1, 22 kg P ha−1, and 13 kg S ha−1 as a mix of monoammonium phosphate, urea, and ammonium sulfate.
2.2. Soil Sampling and Processing
2.3. Soil Health Analyses
2.3.1. Soil Physical Health Indicators
2.3.2. Soil Biological Health Indicators
2.3.3. Soil Chemical Health Indicators
2.3.4. Soil Nutrient Content
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil Physical Indicators
| Soil Indicator | 2021 | 2022 | 2021 | 2022 | ANOVA | ANOVA | ANOVA | Transformation |
|---|---|---|---|---|---|---|---|---|
| (0–5 cm Depth) | (5–15 cm Depth) | (Year) | (Depth) | (Year × Depth) | ||||
| Physical | ||||||||
| Bd (g cm−3) | 1.42 ± 0.04 | 1.56 ± 0.04 | 1.41 ± 0.05 | 1.70 ± 0.03 | ** | |||
| WSA (%) | 59.2 ± 4.5 | 64.3 ± 3.3 | 61.3 ± 3.3 | 63.5 ± 3.4 | ||||
| Biological | ||||||||
| BG (mg pnp kg−1 soil hr−1) | 490 ± 34 | 839 ± 34 | 229 ± 21 | 300 ± 19 | ** at 0–5 cm | ** for both years | ** | |
| MBC (mg g−1) | 219 ± 15 | 438 ± 16 | 137 ± 13 | 203 ± 10 | ** at both depths | ** for both years | ** | |
| SOC (%) | 2.10 ± 0.10 | 2.38 ± 0.17 | 2.06 ± 0.09 | 1.86 ± 0.12 | * | |||
| PMN (mg kg−1) | 15.5 ± 2.8 | 50.7 ± 4.2 | 16.1 ± 1.0 | 42.2 ± 3.4 | ** | |||
| Chemical | ||||||||
| pH, 1:1 | 7.87 ± 0.02 | 8.07 ± 0.04 | 7.99 ± 0.03 | 8.05 ± 0.03 | ** at 0–5 cm | * | ||
| EC, 1:1 (dS m−1) | 1.27 ± 0.17 | 1.04 ± 0.16 | 1.59 ± 0.23 | 2.19 ± 0.22 | ** for both years | ** | Aligned-Rank | |
| Nutrient | ||||||||
| P (mg kg−1) | 29.7 ± 3.8 | 27.0 ± 2.9 | 14.8 ± 2.0 | 9.9 ± 1.2 | ** | Logarithmic | ||
| K (mg kg−1) | 487 ± 50 | 523 ± 63 | 281 ± 29 | 410 ± 59 | ** | |||
| Soil Indicator | 2021 | 2022 | 2021 | 2022 | ANOVA | ANOVA | ANOVA | Transformation |
|---|---|---|---|---|---|---|---|---|
| (0–5 cm Depth) | (5–15 cm Depth) | (Year) | (Depth) | (Year × Depth) | ||||
| Physical | ||||||||
| Bd | 0.47 ± 0.06 | 0.31 ± 0.02 | 0.47 ± 0.07 | 0.24 ± 0.01 | ** | Aligned-Rank | ||
| WSA | 0.94 ± 0.03 | 0.99 ± 0.01 | 0.96 ± 0.02 | 0.98 ± 0.02 | Aligned-Rank | |||
| Biological | ||||||||
| BG | 0.80 ± 0.06 | 0.98 ± 0.01 | 0.34 ± 0.08 | 0.48 ± 0.08 | * | ** | ||
| MBC | 0.38 ± 0.07 | 0.85 ± 0.03 | 0.18 ± 0.04 | 0.30 ± 0.05 | ** | ** | Logarithmic | |
| SOC | 0.43 ± 0.06 | 0.53 ± 0.07 | 0.42 ± 0.06 | 0.37 ± 0.07 | Logarithmic | |||
| PMN | 0.69 ± 0.10 | 1.00 ± 0.00 | 0.89 ± 0.05 | 1.00 ± 0.00 | ** at 5–15 cm | * for 2022 | * | Aligned-Rank |
| Chemical | ||||||||
| pH | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | ** at 0–5 cm | ** for 2021 | * | Logarithmic |
| EC | 0.86 ± 0.07 | 0.93 ± 0.06 | 0.74 ± 0.09 | 0.53 ± 0.08 | ** for both years | ** | Aligned-Rank | |
| Nutrient | ||||||||
| P | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.94 ± 0.03 | 0.81 ± 0.06 | a | ** for both years | ** | Aligned-Rank |
| K | 1.00 ± 0.00 | 0.99 ± 0.01 | 0.98 ± 0.01 | 0.95 ± 0.04 | Aligned-Rank | |||
| Soil Indicator | 2021 | 2022 | 2021 | 2022 | ANOVA | ANOVA | ANOVA | Transformation |
|---|---|---|---|---|---|---|---|---|
| (0–5 cm Depth) | (5–15 cm Depth) | (Year) | (Depth) | (Year × Depth) | ||||
| Physical | 0.70 ± 0.04 | 0.65 ± 0.01 | 0.71 ± 0.04 | 0.61 ± 0.01 | * | Logarithmic | ||
| Biological | 0.58 ± 0.05 | 0.84 ± 0.02 | 0.46 ± 0.04 | 0.54 ± 0.04 | ** at both depths | ** for both years | ** | Aligned-Rank |
| Chemical | 0.45 ± 0.03 | 0.47 ± 0.03 | 0.38 ± 0.04 | 0.27 ± 0.04 | ** for both years | ** | Aligned-Rank | |
| Nutrient | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.96 ± 0.02 | 0.88 ± 0.05 | a | ** for both years | * | Aligned-Rank |
| Overall | 0.66 ± 0.02 | 0.76 ± 0.01 | 0.59 ± 0.03 | 0.57 ± 0.02 | ** at 0–5 cm | ** for both years | ** | Aligned-Rank |
3.2. Soil Biological Indicators
3.3. Soil Chemical Indicators
3.4. Soil Nutrient Indicators
3.5. Overall Soil Health
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Soil Health | 2017 | 2018 | 2017 | 2018 |
|---|---|---|---|---|
| Indicators | (0–5 cm Depth) | (5–15 cm Depth) | ||
| Physical | ||||
| Bd (g cm−3) | 1.15 ± 0.05 | 1.52 ± 0.05 | 1.29 ± 0.04 | 1.59 ± 0.04 |
| WSA (%) | 40.1 ± 3.9 | 44.3 ± 6.1 | 54.3 ± 3.8 | 55.6 ± 5.6 |
| Biological | ||||
| BG (mg pnp kg−1 soil hr−1) | 65.3 ± 2.8 | 84.9 ± 4.2 | 66.9 ± 3.3 | 70.2 ± 5.3 |
| MBC (mg g−1) | 122 ± 6 | 355 ± 25 | 136 ± 9 | 271 ± 22 |
| SOC (%) | 1.24 ± 0.06 | 1.31 ± 0.08 | 1.21 ± 0.09 | 1.33 ± 0.06 |
| PMN (mg kg−1) | 11.8 ± 1.9 | 17.3 ± 1.0 | 11.2 ± 1.7 | 14.9 ± 1.0 |
| Chemical | ||||
| pH, 1:1 | 8.00 ± 0.02 | 8.17 ± 0.03 | 7.90 ± 0.02 | 8.05 ± 0.02 |
| EC, 1:1 (dS m−1) | 1.96 ± 0.25 | 1.12 ± 0.25 | 2.94 ± 0.20 | 2.52 ± 0.23 |
| Nutrient | ||||
| P (mg kg−1) | 11.8 ± 1.1 | 6.9 ± 0.9 | 8.0 ± 1.0 | 5.7 ± 0.9 |
| K (mg kg−1) | 175 ± 9 | 351 ± 25 | 172 ± 13 | 186 ± 21 |
| Soil Health | 2017 | 2018 | 2017 | 2018 |
| Indicator Scores | (0–5 cm depth) | (5–15 cm depth) | ||
| Physical | ||||
| Bd | 0.81 ± 0.06 | 0.37 ± 0.04 | 0.61 ± 0.07 | 0.31 ± 0.05 |
| WSA | 0.77 ± 0.05 | 0.76 ± 0.08 | 0.93 ± 0.02 | 0.88 ± 0.06 |
| Biological | ||||
| BG | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| MBC | 0.17 ± 0.05 | 0.67 ± 0.06 | 0.17 ± 0.03 | 0.49 ± 0.08 |
| SOC | 0.19 ± 0.04 | 0.20 ± 0.05 | 0.20 ± 0.06 | 0.21 ± 0.04 |
| PMN | 0.64 ± 0.10 | 0.97 ± 0.01 | 0.56 ± 0.09 | 0.90 ± 0.04 |
| Chemical | ||||
| pH | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| EC | 0.62 ± 0.09 | 0.87 ± 0.09 | 0.25 ± 0.06 | 0.43 ± 0.07 |
| Nutrient | ||||
| P | 0.92 ± 0.03 | 0.62 ± 0.07 | 0.71 ± 0.08 | 0.47 ± 0.08 |
| K | 0.92 ± 0.03 | 0.99 ± 0.00 | 0.93 ± 0.02 | 0.91 ± 0.04 |
| Soil Health | 2017 | 2018 | 2017 | 2018 |
| Indices | (0–5 cm depth) | (5–15 cm depth) | ||
| Physical | 0.79 ± 0.04 | 0.56 ± 0.05 | 0.77 ± 0.04 | 0.59 ± 0.04 |
| Biological | 0.26 ± 0.04 | 0.48 ± 0.03 | 0.25 ± 0.03 | 0.42 ± 0.03 |
| Chemical | 0.32 ± 0.04 | 0.44 ± 0.04 | 0.14 ± 0.03 | 0.22 ± 0.04 |
| Nutrient | 0.94 ± 0.02 | 0.81 ± 0.03 | 0.82 ± 0.04 | 0.69 ± 0.05 |
| Overall | 0.51 ± 0.03 | 0.55 ± 0.02 | 0.45 ± 0.02 | 0.47 ± 0.02 |
References
- Shawver, C.J.; Ippolito, J.A.; Brummer, J.E.; Ahola, J.K.; Rhoades, R.D. Soil health changes following transition from an annual cropping to perennial management-intensive grazing agroecosystem. Agrosystems Geosci. Environ. 2021, 4, e20181. [Google Scholar] [CrossRef]
- Wang, T.; Jin, H.; Kreuter, U.; Teague, R. Expanding grass-based agriculture on marginal land in the U.S. Great Plains: The role of management intensive grazing. Land Use Policy 2021, 104, 105155. [Google Scholar] [CrossRef]
- Martz, F.A.; Gerrish, J.; Belyea, R.; Tate, V. Nutrient Content, Dry Matter Yield, and Species Composition of Cool-Season Pasture with Management-Intensive Grazing. J. Dairy Sci. 1999, 82, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Hancock, D.; Andrae, J. What Is Management-Intensive Grazing (MiG) and What Can It Do for My Farm? 2009. Available online: http://www.caes.uga.edu/commodities/fieldcrops/forages/questions/023FAQ-grazmethods.pdf (accessed on 5 June 2023).
- Acosta-Martínez, V.; Acosta-Mercado, D.; Sotomayor-Ramírez, D.; Cruz-Rodríguez, L. Microbial communities and enzymatic activities under different management in semiarid soils. Appl. Soil Ecol. 2008, 38, 249–260. [Google Scholar] [CrossRef]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Huang, X.; Skidmore, E.L.; Tibke, G.L. Soil quality of two Kansas soils as influenced by the Conservation Reserve Program. J. Soil Water Conserv. 2002, 57, 344–350. [Google Scholar]
- Moore, J.M.; Klose, S.; Tabatabai, M.A. Soil microbial biomass carbon and nitrogen as affected by cropping systems. Biol. Fertil. Soils 2000, 31, 200–210. [Google Scholar] [CrossRef]
- Staben, M.L.; Bezdicek, D.F.; Fauci, M.F.; Smith, J.L. Assessment of Soil Quality in Conservation Reserve Program and Wheat-Fallow Soils. Soil Sci. Soc. Am. J. 1997, 61, 124–130. [Google Scholar] [CrossRef]
- Veum, K.S.; Kremer, R.J.; Sudduth, K.A.; Kitchen, N.R.; Lerch, R.N.; Baffaut, C.; Stott, D.E.; Karlen, D.L.; Sadler, E.J. Conservation effects on soil quality indicators in the Missouri Salt River Basin. J. Soil Water Conserv. 2015, 70, 232–246. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Chae, Y.; Cui, R.; Woong Kim, S.; An, G.; Jeong, S.W.; An, Y.J. Exoenzyme activity in contaminated soils before and after soil washing: ß-glucosidase activity as a biological indicator of soil health. Ecotoxicol. Environ. Saf. 2017, 135, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.P. Soil Enzyme Activities as Indicators of Soil Quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994. [Google Scholar] [CrossRef]
- Beniston, J.W.; DuPont, S.T.; Glover, J.D.; Lal, R.; Dungait, J.A.J. Soil organic carbon dynamics 75 years after land-use change in perennial grassland and annual wheat agricultural systems. Biogeochemistry 2014, 120, 37–49. [Google Scholar] [CrossRef]
- Ledo, A.; Smith, P.; Zerihun, A.; Whitaker, J.; Vicente-Vicente, J.L.; Qin, Z.; McNamara, N.P.; Zinn, Y.L.; Llorente, M.; Liebig, M.; et al. Changes in soil organic carbon under perennial crops. Glob. Chang. Biol. 2020, 26, 4158–4168. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, E.; Sun, O.J. Soil carbon change and its responses to agricultural practices in Australian agro-ecosystems: A review and synthesis. Geoderma 2010, 155, 211–223. [Google Scholar] [CrossRef]
- O’Brien, S.L.; Jastrow, J.D. Physical and chemical protection in hierarchical soil aggregates regulates soil carbon and nitrogen recovery in restored perennial grasslands. Soil Biol. Biochem. 2013, 61, 1–13. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Song, Z. Rhizosphere soil microbial activity under different vegetation types on the Loess Plateau, China. Geoderma 2011, 161, 115–125. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Holden, N.M.; Adamowski, J.F.; Biswas, A.; Zhang, X.; Feng, Q. Soil properties and microbiome of annual and perennial cultivated grasslands on the Qinghai–Tibetan Plateau. Land Degrad. Dev. 2021, 32, 5306–5321. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, S.; Struik, P.C.; Wang, H.; Jin, K.; Wu, R.; Na, R.; Mu, H.; Ta, N. Plant and soil responses to grazing intensity drive changes in the soil microbiome in a desert steppe. Plant Soil 2022, 491, 219–237. [Google Scholar] [CrossRef]
- Aarons, S.R.; O’Connor, C.R.; Hosseini, H.M.; Gourley, C.J.P. Dung pads increase pasture production, soil nutrients and microbial biomass carbon in grazed dairy systems. Nutr. Cycl. Agroecosyst. 2009, 84, 81–92. [Google Scholar] [CrossRef]
- Whiting, D.; Card, A.; Wilson, C. Understanding Fertilizers; CMC Garden: Fort Collins, CO, USA, 2022; pp. 1–7. [Google Scholar]
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A Global Meta-Analysis of Grazing Impacts on Soil Health Indicators. J. Environ. Qual. 2018, 47, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil compaction effects on soil health and crop productivity: An overview. Environ. Sci. Pollut. Res. 2017, 24, 10056–10067. [Google Scholar] [CrossRef] [PubMed]
- USDA NRCS. Soil Health-Guides for Educators: Soil Bulk Density/Moisture/Aeration. Soil Quality Kit-Guides for Educators, May, 1–11. 2019. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detailfull/soils/health/assessment/?cid=nrcs142p2_053870 (accessed on 15 June 2023).
- Warren, S.D.; Nevill, M.B.; Blackburn, W.H.; Garza, N.E. Soil Response to Trampling Under Intensive Rotation Grazing. Soil Sci. Soc. Am. J. 1986, 50, 1336–1341. [Google Scholar] [CrossRef]
- Colorado Climate Center-Station Normals for Nunn, CO. Colorado Climate Center-Station Normals. 2023. Available online: https://climate.colostate.edu/station_normal.html?USW00094074 (accessed on 1 July 2023).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: https://websoilsurvey.nrcs.usda.gov/app/ (accessed on 12 July 2022).
- da Luz, F.B.; da Silva, V.R.; Kochem Mallmann, F.J.; Bonini Pires, C.A.; Debiasi, H.; Franchini, J.C.; Cherubin, M.R. Monitoring soil quality changes in diversified agricultural cropping systems by the Soil Management Assessment Framework (SMAF) in southern Brazil. Agric. Ecosyst. Environ. 2019, 281, 100–110. [Google Scholar] [CrossRef]
- Keshavarz, R.; Banet, T.; Li, L.; Ippolito, J.A. Furrow-irrigated corn residue management and tillage strategies for improved soil health. Soil Tillage Res. 2022, 216, 105238. [Google Scholar]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis, Part 1—Physical and Mineralogical Methods Agronomy Monograph 9, 2nd ed.; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Green, V.; Stott, D.; Cruz, J.; Curi, N. Tillage impacts on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil Tillage Res. 2007, 92, 114–121. [Google Scholar] [CrossRef]
- Hobbie, S.E. Chloroform Fumigation Direct Extraction (CFDE) Protocol for Microbial Biomass Carbon and Nitrogen. 1998. Available online: https://web.stanford.edu/group/Vitousek/chlorofume.html (accessed on 6 July 2023).
- Nelson, D.W.; Sommers, L.E. Total C, organic C, and organic matter. In Methods of Soil Analysis, Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 975–977. [Google Scholar]
- Sherrod, L.A.; Dunn, G.; Peterson, G.; Kolberg, R. Inorganic C analysis by modified pressure-calcimeter method. Soil Sci. Soc. Am. J. 2002, 66, 299–305. [Google Scholar]
- Curtin, D.; McCallum, F.M. Biological and chemical assays to estimate nitrogen supplying power of soils with contrasting management histories. Aust. J. Soil Res. 2004, 42, 737–746. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Electrical conductivity and total dissolved solids. In Methods of Soil Analysis, Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954; p. 19.
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for nonparametric factorial analyses using only ANOVA procedures. In Proceedings of the Conference on Human Factors in Computing Systems–Proceedings, Vancouver, BC, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar] [CrossRef]
- Stavi, I.; Ungar, E.D.; Lavee, H.; Sarah, P. Grazing-induced spatial variability of soil bulk density and content of moisture, organic carbon and calcium carbonate in a semi-arid rangeland. Catena 2008, 75, 288–296. [Google Scholar] [CrossRef]
- Bruand, A.; Gilkes, J.R. Subsoil bulk density and organic carbon stock in relation to land use for a Western Australian Sodosol. Aust. J. Soil Res. 2002, 40, 431–459. [Google Scholar] [CrossRef]
- Devine, S.; Markewitz, D.; Hendrix, P.; Coleman, D. Soil aggregates and associated organic matter under conventional tillage, no-tillage, and forest succession after three decades. PLoS ONE 2014, 9, e84988. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Zheng, J.; Luo, Y.; Li, R.; Wang, H.; Qi, H. Effect of long-term tillage on soil aggregates and aggregate-associated carbon in black soil of northeast China. PLoS ONE 2018, 13, e0199523. [Google Scholar] [CrossRef]
- Shawver, C.; Brummer, J.; Ippolito, J.; Ahola, J.; Rhoades, R. Management-Intensive Grazing and Soil Health, Fact Sheet 0.570; Colorado State University Extension: Fort Collins, CO, USA, 2020. [Google Scholar]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Sun, C.X.; Chen, Z.H.; Zhang, G.N.; Chen, L.J.; Wu, Z.J. Stoichiometric analyses of soil nutrients and enzymes in a Cambisol soil treated with inorganic fertilizers or manures for 26 years. Geoderma 2019, 353, 382–390. [Google Scholar] [CrossRef]
- Gómez, E.J.; Delgado, J.A.; González, J.M. Persistence of microbial extracellular enzymes in soils under different temperatures and water availabilities. Ecol. Evol. 2020, 10, 10167–10176. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Dukes, J.S.; Wallenstein, M.D. Modeling the effects of temperature and moisture on soil enzyme activity: Linking laboratory assays to continuous field data. Soil Biol. Biochem. 2012, 55, 85–92. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Finzi, A.C. Seasonal variation in the temperature sensitivity of proteolytic enzyme activity in temperate forest soils. J. Geophys. Res. Biogeosciences 2012, 117. [Google Scholar] [CrossRef]
- Colorado State University. CoAgMet Homepage. CoAgMET. Available online: https://coagmet.colostate.edu/ (accessed on 1 July 2023).
- Paudel, B.R.; Udawatta, R.P.; Anderson, S.H. Agroforestry and grass buffer effects on soil health parameters for grazed pasture and row-crop systems. Appl. Soil Ecol. 2011, 48, 125–132. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Xu, M.; Zhu, J.; Wimberly, M.C.; Yu, G.; Niu, S.; Xi, Y.; Zhang, X.; Wang, J. Light-intensity grazing improves alpine meadow productivity and adaption to climate change on the Tibetan Plateau. Sci. Rep. 2015, 5, 15949. [Google Scholar] [CrossRef]
- Mahal, N.K.; Castellano, M.J.; Miguez, F.E. Conservation Agriculture Practices Increase Potentially Mineralizable Nitrogen: A Meta-Analysis. Soil Sci. Soc. Am. J. 2018, 82, 1270–1278. [Google Scholar] [CrossRef]
- Moebius-Clune, B.N.; Moebius-Clune, D.J.; Gugino, B.K.; Idowu, O.J.; Schindelbeck, R.R.; Ristow, A.J.; van Es, H.M.; Thies, J.E.; Shayler, H.A.; McBride, M.B.; et al. Comprehensive Assessment of Soil Health—The Cornell Framework Manual, Edition 3.1; Cornell University: Geneva, Switzerland; Ithaca, NY, USA, 2016. [Google Scholar]
- Martin-Lammerding, D.; Tenorio, J.L.; Albarran, M.; Zambrana, E.; Walter, I. Influence of tillage practices on soil biologically active organic matter content over a growing season under semiarid Mediterranean climate. Span. J. Agric. Res. 2013, 11, 232–243. [Google Scholar] [CrossRef]
- Needelman, B.A.; Wander, M.M.; Bollero, G.A.; Boast, C.W.; Sims, G.K.; Bullock, D.G. Interaction of Tillage and Soil Texture Biologically Active Soil Organic Matter in Illinois. Soil Sci. Soc. Am. J. 1999, 63, 1326–1334. [Google Scholar] [CrossRef]
- Wei, H.; Yang, J.; Liu, Z.; Zhang, J. Data Integration Analysis Indicates That Soil Texture and pH Greatly Influence the Acid Buffering Capacity of Global Surface Soils. Sustainability 2022, 14, 3017. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Chadwick, D.R.; Jones, D.L.; Evans, C.D.; Jones, M.B.; Rees, R.M.; Smith, P. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2018, 253, 62–81. [Google Scholar] [CrossRef]
- Dear, B.S.; Virgona, J.M.; Sandral, G.A.; Swan, A.D.; Morris, S. Changes in soil mineral nitrogen, nitrogen leached, and surface pH under annual and perennial pasture species. Crop Pasture Sci. 2009, 60, 975–986. [Google Scholar] [CrossRef]
- Hao, Y.; He, Z. Effects of grazing patterns on grassland biomass and soil environments in China: A meta-analysis. PLoS ONE 2019, 14, e0215223. [Google Scholar] [CrossRef]
- Bauder, T.A.; Waskorn, R.M.; Sutherland, P.L.; Davis, J.G. Irrigation Water Quality Criteria; Fact Sheet No. 0.506 Crop Series; Colorado State University Extension: Fort Collins, CO, USA, 2011; pp. 10–13. [Google Scholar]
- Bremer, E.; Pauly, D.; McKenzie, R.H.; Ellert, B.H.; Janzen, H.H. Twenty-four years of contrasting cropping systems on a brown chernozem in Southern Alberta: Crop yields, soil carbon, and subsoil salinity. Can. J. Soil Sci. 2023, 103, 134–142. [Google Scholar] [CrossRef]
- Waskom, R.M.; Bauder, T.; Davis, J.G.; Andales, A.A. Diagnosing Saline and Sodic Soil Problems; Fact Sheet No. 0.521 Crop Series; Colorado State University Extension: Fort Collins, CO, USA, 2012; pp. 1–2. [Google Scholar]
- Early MS, B.; Cameron, K.C.; Fraser, P.M. The fate of potassium, calcium, and magnesium in simulated urine patches on irrigated dairy pasture soil. N. Z. J. Agric. Res. 1998, 41, 117–124. [Google Scholar] [CrossRef]
- Sherman, J.F.; Young, E.O.; Coblentz, W.K.; Cavadini, J. Runoff water quality after low-disturbance manure application in an alfalfa–grass hay crop forage system. J. Environ. Qual. 2020, 49, 663–674. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimarco, T.; Brummer, J.E.; Buchanan, C.; Ippolito, J.A. Tracking Soil Health Changes in a Management-Intensive Grazing Agroecosystem. Soil Syst. 2023, 7, 94. https://doi.org/10.3390/soilsystems7040094
Trimarco T, Brummer JE, Buchanan C, Ippolito JA. Tracking Soil Health Changes in a Management-Intensive Grazing Agroecosystem. Soil Systems. 2023; 7(4):94. https://doi.org/10.3390/soilsystems7040094
Chicago/Turabian StyleTrimarco, Tad, Joe E. Brummer, Cassidy Buchanan, and James A. Ippolito. 2023. "Tracking Soil Health Changes in a Management-Intensive Grazing Agroecosystem" Soil Systems 7, no. 4: 94. https://doi.org/10.3390/soilsystems7040094
APA StyleTrimarco, T., Brummer, J. E., Buchanan, C., & Ippolito, J. A. (2023). Tracking Soil Health Changes in a Management-Intensive Grazing Agroecosystem. Soil Systems, 7(4), 94. https://doi.org/10.3390/soilsystems7040094