Glutamic-N,N-Diacetic Acid as an Innovative Chelating Agent in Microfertilizer Development: Biodegradability, Lettuce Growth Promotion, and Impact on Endospheric Bacterial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Microfertilizer
2.2. Degradability Analysis of GLDA-Based Chelates
2.3. Vegetation Experiment
2.3.1. Overall Experimental Design
2.3.2. Hydroponic Cultivation
2.3.3. Soil Cultivation
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results
3.1. Biodegradability of the Chelating Agents
3.2. Influence of GLDA-Based Chelates on Lettuce Growth and Microelement Uptake
3.3. Influence of GLDA Chelate Fertilization on Leaf and Root Endophytic Bacterial Communities
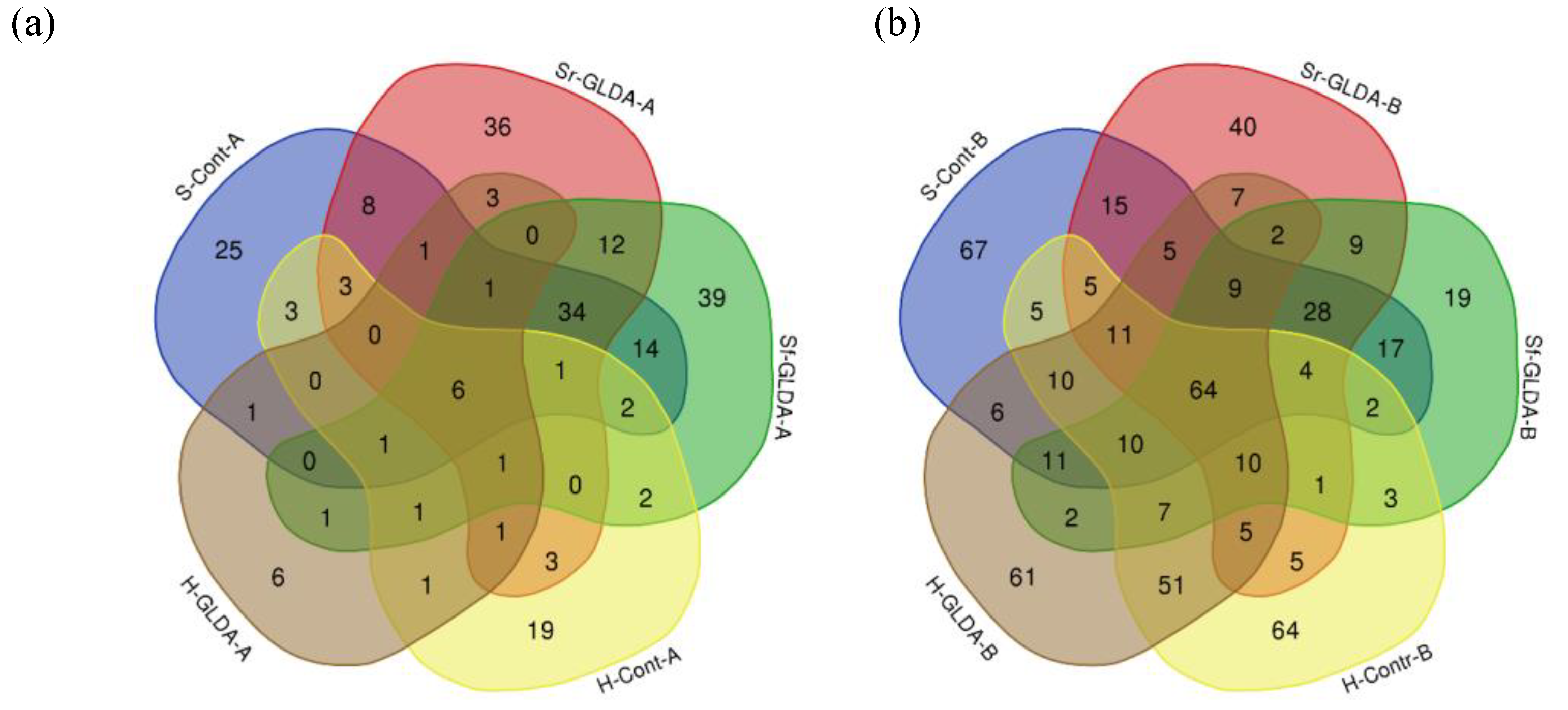
3.3.1. Influence of GLDA Chelate Fertilization on Endophytic Bacterial Communities of Plants Grown in Hydroponics
3.3.2. Influence of GLDA Chelate Fertilization on Endophytic Bacterial Communities of Plants Grown in Soil
3.3.3. Alpha and Beta Diversity of the Lettuce Endophytic Bacterial Communities
4. Discussion
4.1. Biodegradability
4.2. Influence of GLDA-Based Chelates on Lettuce Growth and Microelements Uptake
4.3. Influence of GLDA-Chelate Fertilization on Leaf and Root Endophytic Bacterial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolodynska, D. Polyacrylate anion exchangers in sorption of heavy metal ions with the biodegradable complexing agent. Chem. Eng. J. 2009, 150, 280–288. [Google Scholar] [CrossRef]
- Bucheli-Witschel, M.; Egli, T. Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol. Rev. 2001, 25, 69–106. [Google Scholar] [CrossRef]
- Jones, P.W.; Williams, D.R. Chemical speciation used to assess [S,S′]-ethylenediaminedisuccinic acid (EDDS) as a readily-biodegradable replacement for EDTA in radiochemical decontamination formulations. Appl. Radiat. Isot. 2001, 54, 587–593. [Google Scholar] [CrossRef]
- Henneken, L.; Nörtemann, B.; Hempel, D.C. Influence of physiological conditions on EDTA degradation. Appl. Microbiol. Biotechnol. 1995, 44, 190–197. [Google Scholar]
- Beltyukova, M.; Kuryntseva, P.; Galitskaya, P.; Selivanovskaya, S.; Brusko, V.; Dimiev, A. Biodegradation rate of EDTA and IDS and their metal complexes. Horticulturae 2023, 9, 623. [Google Scholar] [CrossRef]
- Metsärinne, S.; Ronkainen, E.; Tuhkanen, T.; Aksela, R.; Sillanpää, M. Biodegradation of novel amino acid derivatives suitable for complexing agents in pulp bleaching applications. Sci. Total Environ. 2007, 377, 45–51. [Google Scholar] [CrossRef]
- Thomas, R.A.P.; Lawlor, K.; Bailey, M.; Macaskie, L.E. Biodegradation of metal-EDTA complexes by an enriched microbial population. Appl. Environ. Microbiol. 1998, 64, 1319. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Ramanathan, R.; Nasr-El-Din, H.A. From initial treatment design to final disposal of chelating agents: A review of corrosion and degradation mechanisms. RSC Adv. 2022, 12, 1813. [Google Scholar] [CrossRef]
- Begum, Z.A.; Rahman, I.M.M.; Sawai, H.; Tate, Y.; Maki, T.; Hasegawa, H. Stability constants of Fe(III) and Cr(III) complexes with dl-2-(2-carboxymethyl)nitrilotriacetic acid (GLDA) and 3-hydroxy-2,2’- iminodisuccinic acid (HIDS) in aqueous solution. J. Chem. Eng. Data 2012, 57, 2723–2732. [Google Scholar] [CrossRef]
- Discover Our Dissolvine® Product Range. Available online: https://www.nouryon.com/products/chelates/dissolvine/ (accessed on 9 January 2024).
- Li, N.; Hu, Y.; Xiong, G.; Liu, P.; Xiong, Y.; Luo, Z.; Zhang, Q.; Li, Y.; Zhu, S.; Feng, W.; et al. Research and application of eco-friendly chelating agents in plugging removal systems: A review. Geoenergy Sci. Eng. 2023, 229, 212135. [Google Scholar] [CrossRef]
- Morelli, M.; Bahar, O.; Papadopoulou, K.K.; Hopkins, D.L.; Obradović, A. Editorial: Role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 2020, 11, 577603. [Google Scholar]
- Tiwari, P.; Bae, H. Trends in harnessing plant endophytic microbiome for heavy metal mitigation in plants: A perspective. Plants 2023, 12, 1515. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Cao, C.; Han, S.; Zhao, W.; Li, Q.; Liu, X.; Kong, L. Effects of fertilizer reduction coupled with straw returning on soil fertility, wheat root endophytic bacteria, and the occurrence of wheat crown rot. Front. Microbiol. 2023, 14, 1143480. [Google Scholar]
- Wang, Y.Z.; Zhou, S.Y.D.; Zhou, X.Y.; An, X.L.; Su, J.Q. Manure and biochar have limited effect on lettuce leaf endophyte resistome. Sci. Total Environ. 2023, 860, 160515. [Google Scholar] [CrossRef]
- Danilova, N.; Galieva, G.; Kuryntseva, P.; Selivanovskaya, S.; Galitskaya, P. Influence of the antibiotic oxytetracycline on the morphometric characteristics and endophytic bacterial community of lettuce (Lactuca sativa L.). Microorganisms 2023, 11, 2828. [Google Scholar]
- Smith, R.M.; Martell, A.; Motekaitis, R.; Smith, R.; Motekaitis, R. NIST Critically Selected Stability Constants of Metal Complexes Database; NIST: Gaithersburg, MD, USA, 2013; Volume 46. [Google Scholar]
- Begum, Z.A.; Rahman, I.M.M.; Tate, Y.; Egawa, Y.; Maki, T.; Hasegawa, H. Formation and Stability of Binary Complexes of Divalent Ecotoxic Ions (Ni, Cu, Zn, Cd, Pb) with Biodegradable Aminopolycarboxylate Chelants (Dl-2-(2-Carboxymethyl)Nitrilotriacetic Acid, GLDA, and 3-Hydroxy-2,2′-Iminodisuccinic Acid, HIDS) in Aqueous Solutions. J. Solut. Chem. 2012, 41, 1713–1728. [Google Scholar] [CrossRef]
- OECD Guideline for Testing of Chemicals Test No. 301: Ready Biodegradability; OECD Guidelines for the Testing of Chemicals, Section 3; OECD: Paris, France, 1992. [CrossRef]
- Puccinelli, M.; Landi, M.; Maggini, R.; Pardossi, A.; Incrocci, L. Iodine biofortification of sweet basil and lettuce grown in two hydroponic systems. Sci. Hortic. 2021, 276, 109783. [Google Scholar] [CrossRef]
- Brusko, V.; Garifullin, B.; Geniyatullina, G.; Kuryntseva, P.; Galieva, G.; Galitskaya, P.; Selivanovskaya, S.; Dimiev, A.M. Novel biodegradable chelating agents for micronutrient fertilization. J. Agric. Food Chem. 2023, 71, 14979–14988. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251. [Google Scholar] [CrossRef]
- Zhang, Q.; Acuña, J.J.; Inostroza, N.G.; Mora, M.L.; Radic, S.; Sadowsky, M.J.; Jorquera, M.A. Endophytic bacterial communities associated with roots and leaves of plants growing in chilean extreme environments. Sci. Rep. 2019, 9, 4950. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nature methods. 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Bioinformatics & Evolutionary Genomics. Available online: https://bioinformatics.psb.ugent.be/webtools/Venn/ (accessed on 9 January 2024).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Technol. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Beisel, J.N.; Moreteau, J.C. A simple formula for calculating the lower limit of Shannon’s diversity index. Ecol. Model. 1997, 99, 289–292. [Google Scholar] [CrossRef]
- Ham, B.; Choi, B.Y.; Chae, G.T.; Kirk, M.F.; Kwon, M.J. Geochemical influence on microbial communities at CO2-leakage analog sites. Front. Microbiol. 2017, 8, 264914. [Google Scholar]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hoque, M.N.; Puspo, J.A.; Islam, M.R.; Das, N.; Siddique, M.A.; Hossain, M.A.; Sultana, M. Microbiome signature and diversity regulates the level of energy production under anaerobic condition. Sci. Rep. 2021, 11, 19777. [Google Scholar] [CrossRef]
- Ali, M.; Ali, Q.; Sohail, M.A.; Ashraf, M.F.; Saleem, M.H.; Hussain, S.; Zhou, L. Diversity and taxonomic distribution of endophytic bacterial community in the rice plant and its prospective. Int. J. Mol. Sci. 2021, 22, 10165. [Google Scholar]
- Lopez-Echartea, E.; Strejcek, M.; Mukherjee, S.; Uhlik, O.; Yrjälä, K. Bacterial succession in oil-contaminated soil under phytoremediation with poplars. Chemosphere 2020, 243, 125242. [Google Scholar] [CrossRef]
- Ma, B.; Lv, X.; Warren, A.; Gong, J. Shifts in diversity and community structure of endophytic bacteria and archaea across root, stem and leaf tissues in the common reed, Phragmites australis, along a salinity gradient in a marine tidal wetland of northern China. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2013, 104, 759–768. [Google Scholar] [CrossRef]
- Kolodynska, D. Application of a new generation of complexing agents in removal of heavy metal ions from different wastes. Environ. Sci. Pollut. Res. Int. 2013, 20, 5939. [Google Scholar] [CrossRef]
- Ekinci, M.; Kul, R.; Turan, M.; Yildirim, E.; Bilgisi, Y. Effects of organic fertilizers on plant growth, yield and mineral content of lettuce (Lactuca sativa L.). J. Erciyes Agric. Anim. Sci. 2020, 1, 1319–1324. [Google Scholar]
- Liu, W.; Zha, L.; Zhang, Y. Growth and nutrient element content of hydroponic lettuce are modified by LED continuous lighting of sifferent intensities and spectral qualities. Agronomy 2020, 10, 1678. [Google Scholar] [CrossRef]
- Cardarelli, M.; El Chami, A.; Iovieno, P.; Rouphael, Y.; Bonini, P.; Colla, G. Organicfertilizer sources distinctively modulate productivity, quality, mineral composition, and soil enzyme activity of greenhouse lettuce grown in degraded soil. Agronomy 2023, 13, 194. [Google Scholar] [CrossRef]
- Liphadzi, M.S.; Kirkham, M.B. Availability and plant uptake of heavy metals in EDTA-assisted phytoremediation of soil and composted biosolids. S. Afr. J. Bot. 2006, 72, 391–397. [Google Scholar] [CrossRef]
- Fonseca-García, C.; Coleman-Derr, D.; Garrido, E.; Visel, A.; Tringe, S.G.; Partida-Martínez, L.P. The Cacti Microbiome: Interplay between habitat-filtering and host-specificity. Front. Microbiol. 2016, 7, 150. [Google Scholar]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front. Environ. Sci. 2016, 4, 217521. [Google Scholar]
- Januszkiewicz, R.; Kulczycki, G.; Samoraj, M. Foliar fertilization of crop plants in polish agriculture. Agriculture 2023, 13, 1715. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Galieva, G.S.; Galitskaya, P.Y.; Selivanovskaya, S.Y. Plant microbiome: Origin, composition, and functions. Uchenye Zap. Kazan. Universiteta. Seriya Estestv. Nauk. 2023, 165, 231–262. [Google Scholar] [CrossRef]
- Leach, J.E.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the phytobiome. Cell 2017, 169, 587–596. [Google Scholar] [CrossRef]
- Nadarajah, K.; Abdul Rahman, N.S.N. The microbial connection to sustainable agriculture. Plants 2023, 12, 2307. [Google Scholar] [CrossRef]
- Resendiz-Nava, C.N.; Alonso-Onofre, F.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Rivera-Pastrana, D.M.; Stasiewicz, M.J.; Nava, G.M.; Mercado-Silva, E.M. Tomato plant microbiota under conventional and organic fertilization regimes in a soilless culture system. Microorganisms 2023, 11, 1633. [Google Scholar] [CrossRef]
- Epelde, L.; Hernández-Allica, J.; Becerril, J.M.; Blanco, F.; Garbisu, C. Effects of chelates on plants and soil microbial community: Comparison of EDTA and EDDS for lead phytoextraction. Sci. Total Environ. 2008, 401, 21–28. [Google Scholar] [CrossRef]
- Gul, I.; Manzoor, M.; Hashim, N.; Shah, G.M.; Waani, S.P.T.; Shahid, M.; Antoniadis, V.; Rinklebe, J.; Arshad, M. Challenges in microbially and chelate-assisted phytoextraction of cadmium and lead—A review. Environ. Pollut. 2021, 287, 117667. [Google Scholar] [CrossRef]
- Lee, J.; Sung, K. Effects of chelates on soil microbial properties, plant growth and heavy metal accumulation in plants. Ecol. Eng. 2014, 73, 386–394. [Google Scholar] [CrossRef]
- Kaurin, A.; Gluhar, S.; Tilikj, N.; Lestan, D. Soil washing with biodegradable chelating agents and EDTA: Effect on soil properties and plant growth. Chemosphere 2020, 260, 127673. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S.L. EDTA: An antimicrobial and antibiofilm agent for use in wound care. Adv. Wound Care 2015, 4, 415. [Google Scholar] [CrossRef]
- Huang, R.; Cui, X.; Luo, X.; Mao, P.; Zhuang, P.; Li, Y.; Li, Y.; Li, Z. Effects of plant growth regulator and chelating agent on the phytoextraction of heavy metals by Pfaffia glomerata and on the soil microbial community. Environ. Pollut. 2021, 283, 117159. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]







| Treatment Type | Growing Substrate | |||||
|---|---|---|---|---|---|---|
| Hydroponics | Soil | |||||
| Above-Ground Part of Plant (Leaves) | Below-Ground Part of Plant (Roots) | Above-Ground Part of Plant (Leaves) | Below-Ground Part of Plant (Roots) | |||
| Root Treatment | Foliar Treatment | Root Treatment | Foliar Treatment | |||
| Not fertilized (control) | H-Cont-A | H-Cont-B | S-Cont-A | S-Cont-B | ||
| Fertilized (GLDA) | H-GLDA-A | H-GLDA-B | Sr-GLDA-A | Sf-GLDA-A | Sf-GLDA-B | Sf-GLDA-B |
| Substrate | Sample | Index | ||
|---|---|---|---|---|
| Shannon | Simpson | |||
| Leaves | Hydroponic | H-Cont-A | 2.83 | 0.91 |
| H-GLDA-A | 2.00 | 0.82 | ||
| Soil | S-Cont-A | 2.77 | 0.87 | |
| Sr-GLDA-A | 3.17 | 0.91 | ||
| Sf-GLDA-A | 3.24 | 0.92 | ||
| Roots | Hydroponic | H-Cont-B | 4.03 | 0.96 |
| H-GLDA-B | 3.45 | 0.92 | ||
| Soil | S-Cont-B | 3.71 | 0.93 | |
| Sr-GLDA-B | 3.39 | 0.92 | ||
| Sf-GLDA-B | 3.46 | 0.93 | ||
| Bacterial OTUs | Samples | |||||
|---|---|---|---|---|---|---|
| Cont | GLDA | S Cont | S GLDA | H Cont | H GLDA | |
| g_Acinetobacter; s_ | 0.869 *** | 0 | 0.783 ** | 0 | 0.974 ** | 0 |
| g_Ralstonia; s_ | 0.788 * | 0 | 0 | 0 | 0.952 * | 0 |
| g_Staphylococcus; s_ | 0.717 * | 0 | 0 | 0 | 0 | 0 |
| g_Helicobacter; s_ | 0.698 * | 0 | 0 | 0 | 0.996 * | 0 |
| g_Romboutsia; s_ | 0.694 * | 0 | 0 | 0 | 0.995 * | 0 |
| g_Staphylococcus; s_ | 0.666 * | 0 | 0 | 0 | 0 | 0 |
| g_Flavisolibacter; s_ | 0.548 * | 0 | 0 | 0 | 0 | 0 |
| f_Burkholderiaceae; g; s_ | 0.548 * | 0 | 0 | 0 | 0 | 0 |
| f_Chitinophagaceae; g; s_ | 0.542 * | 0 | 0.707 * | 0 | 0 | 0 |
| f_Sandaracinaceae; g; s_ | 0.536 * | 0 | 0.693 * | 0 | 0 | 0 |
| g_Rhodococcus; s_ | 0 | 0 | 0.814 * | 0 | 0 | 0 |
| c_Acidimicrobiia; o; f; g; s_ | 0 | 0 | 0.799 * | 0 | 0 | 0 |
| g_Pseudarthrobacter; s_ | 0 | 0 | 0.798 * | 0 | 0 | 0 |
| g_Massilia; s_ | 0 | 0 | 0.680 * | 0 | 0 | 0 |
| p_WS2; c; o; f; g; s_ | 0 | 0 | 0.672 * | 0 | 0 | 0 |
| f_JG30-KF-CM45; g; s_ | 0 | 0 | 0 | 0.814 * | 0 | 0 |
| f_Lachnospiraceae; g; s_ | 0 | 0 | 0 | 0 | 0.864 * | 0 |
| o_Obscuribacterales; f; g; s_ | 0 | 0 | 0 | 0 | 0 | 0.959 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galieva, G.; Kuryntseva, P.; Selivanovskaya, S.; Brusko, V.; Garifullin, B.; Dimiev, A.; Galitskaya, P. Glutamic-N,N-Diacetic Acid as an Innovative Chelating Agent in Microfertilizer Development: Biodegradability, Lettuce Growth Promotion, and Impact on Endospheric Bacterial Communities. Soil Syst. 2024, 8, 67. https://doi.org/10.3390/soilsystems8020067
Galieva G, Kuryntseva P, Selivanovskaya S, Brusko V, Garifullin B, Dimiev A, Galitskaya P. Glutamic-N,N-Diacetic Acid as an Innovative Chelating Agent in Microfertilizer Development: Biodegradability, Lettuce Growth Promotion, and Impact on Endospheric Bacterial Communities. Soil Systems. 2024; 8(2):67. https://doi.org/10.3390/soilsystems8020067
Chicago/Turabian StyleGalieva, Gulnaz, Polina Kuryntseva, Svetlana Selivanovskaya, Vasiliy Brusko, Bulat Garifullin, Ayrat Dimiev, and Polina Galitskaya. 2024. "Glutamic-N,N-Diacetic Acid as an Innovative Chelating Agent in Microfertilizer Development: Biodegradability, Lettuce Growth Promotion, and Impact on Endospheric Bacterial Communities" Soil Systems 8, no. 2: 67. https://doi.org/10.3390/soilsystems8020067
APA StyleGalieva, G., Kuryntseva, P., Selivanovskaya, S., Brusko, V., Garifullin, B., Dimiev, A., & Galitskaya, P. (2024). Glutamic-N,N-Diacetic Acid as an Innovative Chelating Agent in Microfertilizer Development: Biodegradability, Lettuce Growth Promotion, and Impact on Endospheric Bacterial Communities. Soil Systems, 8(2), 67. https://doi.org/10.3390/soilsystems8020067







