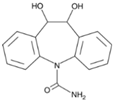
Abstract
The use of reclaimed wastewater for irrigation could result in the release of pharmaceutically active compounds (PhACs) and their metabolites into the agroecosystem. In this study, we investigated the fate of carbamazepine (CBZ) and its metabolites, with the aim of clarifying their behavior in a soil–plant system in a greenhouse experiment. The research was carried out using irrigation water especially fortified with high doses of CBZ (200 or 600 ppb) in order to evaluate the dynamics of CBZ and its metabolites in the soil and basil organs. The results of the study showed that CBZ is easily absorbed by the aerial part of the basil plant. The soil contained two metabolites of CBZ, namely acridine and carbamazepine-10,11-epoxide, as revealed by high-resolution mass spectrometry analyses. In addition, acridine was found in the aerial parts of basil plants. Furthermore, the greater presence of CBZ and its metabolites in bulk soil indicated a positive role of the basil rhizosphere in the degradation of such compounds or a positive role of the plant in the removal of the contaminant by uptake. Considering the observed morphological parameters and the mean CBZ content in wastewater, significantly lower than that used in the experiment, basil can be considered resistant to the application of irrigation water contaminated with CBZ.
1. Introduction
The global use of pharmaceutically active compounds (PhACs) has increased dramatically, by more than 2.5 times, in the past decade [1], also considering the COVID-19 pandemic [2]. PhACs and/or their metabolites reach wastewater treatment plants (WWTPs) through urine and feces, but it is known that conventional WWTPs do not completely remove PhACs; thus, they can be released into the environment [3]. In fact, according to some chemical-physical properties of soils (e.g., pH, texture, and organic matter) and PhACs (water solubility, pKa, molecular size, etc.), many of these compounds enter the soil–plant system through irrigation with wastewater [4] or by the application of sewage sludges on agricultural land [5]. The accumulation of these anthropogenic contaminants and/or their metabolites in soils and plants has raised concerns due to their potential risks to human health [6,7].
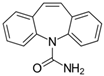
Carbamazepine (CBZ) belongs to the category of anticonvulsants and is used in chronic drug therapies for epileptic patients. However, its extensive use and the high dosages administered to patients (800–1200 mg/day) have raised concerns about its presence in the environment, particularly in wastewater [8]. Approximately 30% of the CBZ administered to patients has been estimated to be excreted in the unmodified form, and the remaining is metabolized by humans [9,10]. For this reason, it is one of the active ingredients detected most frequently in untreated wastewater at a relatively high concentration [11]. Li et al. [12] showed that CBZ may also be present in reclaimed wastewater effluents at concentrations of up to 6.3 μg L−1 or in biosolids at concentrations of up to 258 μg kg−1. CBZ can be included among environmentally recalcitrant molecules due to its limited removal (≤10%) during conventional wastewater treatment processes, and therefore, it can easily enter the food chain [13]. In fact, as reported by Walters et al. [14], CBZ has a long half-life in both WWTPs and soil and can enter and accumulate in plant species, including radish, lettuce, spinach, artichokes, cucumbers, and peppers [4,15,16], reaching concentrations in the range of 2.9 to 67 ng g−1 in edible parts.
Until now, more than 30 metabolites of CBZ have been identified [17]. The degradation pathways of CBZ in soils are complex, and many intermediates with different levels of stability can be formed [18]. In this regard, Li et al. [12] identified common intermediates in soils: CBZ is oxidized to carbamazepine-10,11-epoxide [19], and this compound rapidly undergoes further transformations. Among these, the cleavage of the epoxy ring can occur [12], with the final formation of acridine. More attention should be paid to the fate of such metabolites, since recent studies have shown that they can also be taken up by plants. Paltiel et al. [9] detected in human urine CBZ and its metabolites, not derived from the consumption of drugs but derived from the consumption of fresh vegetables irrigated with reclaimed wastewater.
Evaluation of the fate of PhACs and their metabolites in the plant–soil system is important because in drought-prone areas, such as the Mediterranean basin, plants can increasingly be irrigated with reclaimed wastewater. This practice could represent a sustainable alternative, especially for growing plants that require large amounts of irrigation water, such as basil [20]. It has a large leaf area, resulting in a water consumption per unit area of up to 849 mm [21]. Sweet basil (Ocimum basilicum L.), cultivated primarily in the Mediterranean regions of Europe, as well as in Asia and Africa, is an annual herbaceous plant of the Lamiaceae family [22]. The aromatic plants most used as food and in cosmetics belong to the genus Ocimum, and it is estimated that up to 160 species of basil exist [23]. Unfortunately, aromatic plants, such as sweet basil, can absorb and accumulate contaminants and their metabolites released into the soil, as reported by Kowalska [24].
To the best of our knowledge, only a few works in the recent literature have reported the behavior of CBZ metabolites in soils and plants [12,25,26]. For this reason, in this study, we aimed to investigate the environmental fate of CBZ and its metabolites to clarify their uptake and transformation in soils and basil plants irrigated with artificially enriched CBZ water.
2. Materials and Methods
2.1. Chemicals and Materials
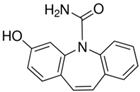
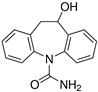
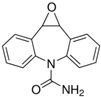
Acetonitrile (ACN), LC-MS-grade water, anhydrous magnesium sulfate (MgSO4), sodium citrate (Na citrate), and primary secondary amine (PSA) were selected from the Sigma-Aldrich catalog (Darmstadt, Germany), and QuE-Lab® tubes by Lab Instruments (Castellana Grotte, Italy) were used for extractions. Preliminary qualitative analyses of experimental samples (soil and plant organs), using the pre-scan function of the high-resolution mass spectrometer, identified CBZ and five metabolites, i.e., 3-hydroxycarbamazepine, 10,11-dihydro-10-hydroxycarbamazepine (>97%), acridine (>99%), carbamazepine 10,11-epoxide (>97%), and 10,11-dihydro-10,11-dihydroxy carbamazepine (>98%). For this reason, they were provided by Lab Instruments and used for subsequent quantification.
The chemical structures and properties of CBZ and its metabolites are given in Table 1.

Table 1.
Main properties of CBZ and its metabolites (Kow: octanol/water partition coefficient; pKa: acid ionization constant; non-aq: not soluble in water).
2.2. Experimental Design
The trial was carried out in 1 L pots and in a greenhouse located at the University of Bari under controlled conditions (24 °C, 60% humidity). All pots were prepared with silty clay loam soil (clay: 37.2%; sand: 7.5%; silt: 55.3%) whose main chemical properties, determined according to the analytical methods of Swift et al. [29], were as follows: pHH2O, 7.3; pHKCl, 6.5; electrical conductivity, 603 µS cm−1; organic carbon, 18.9 g kg−1; organic matter, 3.3%; total nitrogen, 3.1 g kg−1; C/N ratio, 6; available phosphorus (P2O5), 71.1 mg kg−1; and total carbonates, 3.3 g kg−1.
The experimental design was randomized, with 3 treatments and 3 replicates. Twenty-seven pots were prepared without plants (Figure 1), and the treatments were (i) soil without contamination (control), (ii) soil irrigated with water spiked with 200 ppb of CBZ, and (iii) soil irrigated with water spiked with 600 ppb of CBZ. A second set of pots with the same treatments was prepared with basil plants (Ocimum basilicum L.) aged 30 days (1 pot = 1 basil plant; Figure 1). Comparing pots with and without plants can help to investigate the contribution of the rhizosphere in the degradation or otherwise of CBZ and its metabolites. The second set was also used to determine some morphological parameters of basil plants. The experiment lasted 30 days to simulate the time needed to complete the growth of the potted basil seedlings.

Figure 1.
Experimental pot design and photo of the trial.
The contaminated pots were obtained by preparing two CBZ solutions (200 or 600 ppb) and irrigating the pots with the solutions up to the soil field capacity. High concentrations of CBZ were used to make any stresses on the plants and CBZ metabolites more evident. The control pots were irrigated considering the soil field capacity as well but using tap water. Soil moisture was detected using sensors (X-Farm) placed on the top and in the middle of each pot. Irrigation started each time the soil moisture reached 25% of the available water. Soil and plant analyses were conducted after 10, 20, and 30 days.
2.3. Extraction and Quantification of Carbamazepine and Its Metabolites
The extraction of CBZ and its metabolites from the soil was performed according to the modified QuEChERS method reported by De Mastro et al. [30], while the corresponding extraction from plant organs was performed by slightly modifying the QuEChERS method proposed by Brunetti et al. [31]. Before CBZ extraction, roots were first washed with tap water, then rinsed with deionized water, and finally dried with a paper towel. Roots, leaves, and stems were chopped and placed in a 15 mL centrifuge tube in the dark at −20 °C until subsequent analyses. The quantification of CBZ and its metabolites in different matrices was performed using an Ultimate 3000 System (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a degasser, a high-pressure-gradient pump, a WPS autosampler and a column oven, and a Q Exactive mass spectrometer. Details of the quantification procedure are reported elsewhere [30].
2.4. Enzymatic Activities
The activities of many soil enzymes are indicative of the state of the soil’s microbial community. Indeed, these enzymes participate in the biogeochemical cycles of different elements, and their activity is sensitive to changes occurring in soils.
Β-Glucosidase activity was assessed using the method of Eivazi and Tabatabai [32]. Briefly, 1 g of soil was added to 0.25 mL of toluene, 4 mL of modified universal buffer (MUB, pH 6.0), and 1 mL of 0.025 M p-nitrofenil-β-D-glucopiranoside solution in a 50 mL Erlenmeyer flask. The flask was incubated at 37 °C for 1 h, and successively, 1 mL of 0.5 M CaCl2 solution and 4 mL of 0.1 M TRIS (pH 12) were added. The soil suspension was filtered through Whatman No. 2 filter paper, and the absorbance at 400 nm was measured using a PerkinElmer model Lambda15 UV–VIS spectrophotometer (Shelton, CT, USA).
The phosphatase activities In the soils were determined according to the method of Eivazi and Tabatabai [33] with the determination of p-nitrophenol released by 1 g of soil to which 0.2 mL of toluene, 4 mL of MUB (pH 6.5 for acid phosphatase and pH 11 for alkaline phosphatase), and 1 mL of 0.025 M sodium p-nitrophenyl phosphate were added and the resulting mixture incubated at 37 °C for 1 h. Successively, the p-nitrophenol released by the mixture was determined colorimetrically after extraction with 1 mL of 0.5 M CaCl2 and 4 mL of 0.5 M NaOH and filtration through Whatman No. 2 filter paper. Controls were performed, as described for the assay procedure, with sodium p-nitrophenyl phosphate added immediately before filtration.
The fluorescein diacetate hydrolysis (FDA) assay was performed according to Green et al. [34]. Briefly, 1 g of air-dried soil was placed in a 125 mL Erlenmeyer flask, and 50 mL of 60 mM sodium phosphate buffer (pH 7.6) and 0.50 mL of 4.9 mM FDA lipase substrate solution (20 mg of FDA lipase substrate in 10 mL of acetone) were added. Regarding the control, 0.50 mL of acetone was added instead of FDA solution. The contents of the flask were mixed and incubated for 3 h at 37 °C. After that, to stop the reaction, 2 mL of acetone was added. Next, about 30 mL of the soil suspension was transferred to a 50 mL centrifuge tube and centrifuged at 8000 rpm min−1 for 5 min. The supernatant was filtered through a Whatman No. 2 filter paper, and the absorbance at 490 nm was measured by a PerkinElmer Lambda15 UV–VIS spectrophotometer.
2.5. Plant Characterization
To verify the effects of CBZ and its metabolites on plants during the trial, indirect measurements of the chlorophyll content were carried out using a SPAD-502 chlorophyll meter (Konica Minolta, Tokyo, Japan). Furthermore, plant samples were taken after 10, 20, and 30 days for growth measurement. The height of the plant, the number of leaves, and fresh and dry weights were determined to assess the influence of CBZ on the morphological parameters of basil plants [35].
2.6. Statistical Analysis
The results obtained were tested against the normal distribution of variables (Shapiro–Wilk’s test) and the homogeneity of variance (Bartlett’s test) using R software (version 3.2.3). Since the variables were normally distributed and showed homogeneity of variance, they were subjected to an analysis of variance (ANOVA), followed by the Tukey test. Statistical significance was determined at p ≤ 0.05.
3. Results and Discussion
3.1. Soil
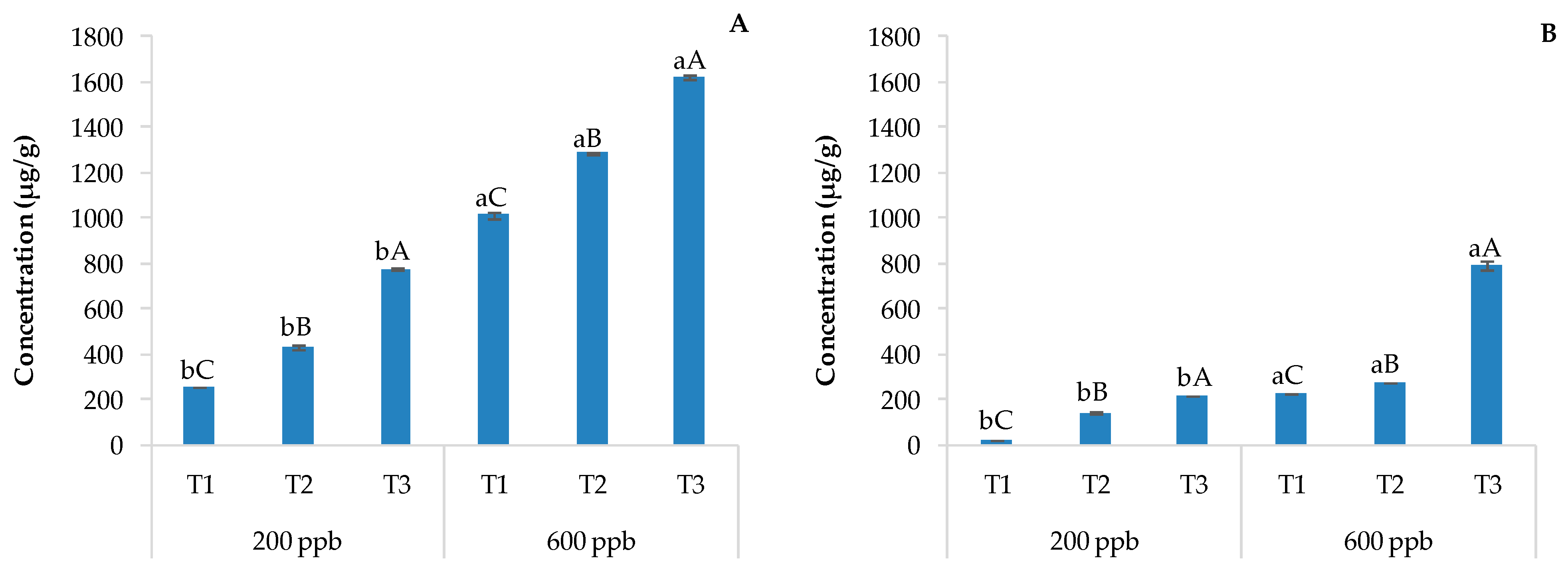
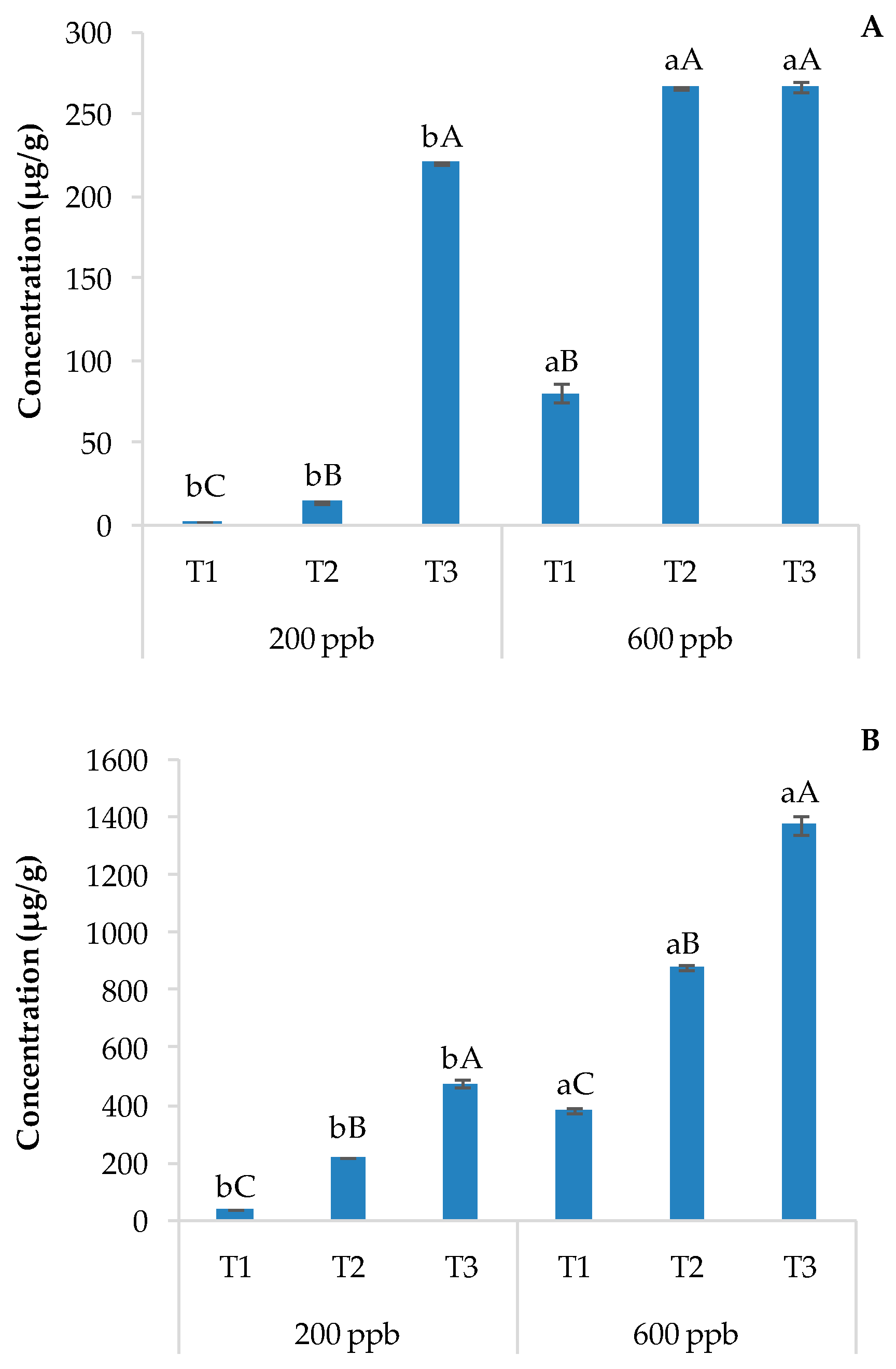
The evaluation of pharmaceutical drugs and their metabolites is usually performed via chromatographic techniques coupled with mass spectrometry after typical processing and extraction/purification techniques [11]. The LC-MS output is reported in Figure 2 as histograms showing the content of CBZ in soils without plants (A) and with plants (B) irrigated with the 200 or 600 ppb CBZ solution and sampled after 10, 20, and 30 days of trial.
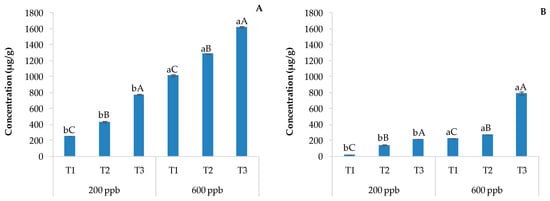
Figure 2.
Average CBZ concentration detected in soils without plants (A) and with plants (B) and the corresponding standard deviation. The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences between concentrations (200 and 600 ppb) at the same time. Uppercase letters represent differences among times (T1, T2, and T3) at the same concentration.
Not surprisingly, the amount of CBZ detected in the soil was directly and significantly related to the dose of the contaminant applied and the time. The presence of sensitive and metabolically active plants strongly decreases the CBZ concentration in the soil due to its possible uptake by roots and/or the rhizosphere, which could potentially allow the related degradation of CBZ (Figure 2). The bioaccumulation of pharmaceutical drugs by the rhizosphere and the populating vegetable species is often followed by active degradation through the activation of specific microorganisms [36]. The combination of these two processes reduces leaching to the surface and into groundwater. In fact, the rhizosphere is an environment rich in microorganisms with respect to the bulk soil [37] due to root exudates [38].
Regarding the specific degradation, carbamazepine (5H-dibenz[b,f]azepine-5-carboxamide) is highly stable, considered persistent because its macrocycle structure remains unchanged even after the conventional activated mass treatment process. De facto, the most effective processes leading degradation include the advanced oxidation process (AOP) alone [39] or after biotreatments [40]. UV and visible light energies, gamma radiation, or the presence of metal catalysts induces the organic radical conversion of CBZ, which can then become the perfect substrate for mono-hydroxylation (MH), bis-hydroxylation (BH), and epoxidation (EP) reactions due to oxidative biochemical processes. These three processes can occur directly on the chemical core of CBZ or indirectly after the action of an amidase biochemical pathway, leading to iminostilbene derivatives [41]. Moreover, the degradation metabolites can be already found in environmental matrices deriving from enzyme liver metabolism via cytochrome p450 oxidase and delivery from human bodies [42]. Regarding the five metabolites shown in Table 1, quantitative analysis highlighted that 3-hydroxycarbamazepine, 10,11-dihydro-10-hydroxycarbamazepine, and 10,11-dihydro-10,11-dihydroxy carbamazepine were below the limit of quantification. Only the carbamazepine-10,11-epoxide derivative could be detected and compared in soil samples. In detail, Figure 3 shows the content of carbamazepine-10,11-epoxide in soils without plants (A) and with plants (B) irrigated with the 200 or 600 ppb solution of CBZ and sampled after 10, 20, and 30 days of trial.
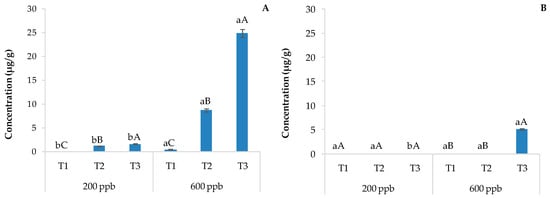
Figure 3.
Average concentration of carbamazepine-10,11-epoxide detected in experimental soils without plants (A) and with plants (B). The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences between concentrations (200 and 600 ppb) at the same time. Uppercase letters represent differences among times (T1, T2, and T3) at the same concentration.
This epoxide derivative is produced via alkene epoxidation. Alkenes are more reactive than aromatic π-bonds, richer in electrons, and allowed to metabolically epoxidize in the liver and other human tissues. The soil with plants showed no presence of carbamazepine-10,11-epoxide, apart from the highest dose and at the end of the experiment (Figure 3). Therefore, it is evident that there is a strong contribution of the basil rhizosphere to CBZ degradation and possible consequent uptake of carbamazepine-10,11-epoxide by plants. As a second hypothesis, unnoticeable degradation of CBZ could have occurred in the soil with plants (Figure 3).
With time, the accumulation of carbamazepine-10,11-epoxide was observed in soils without plants (Figure 3A). Since carbamazepine-10,11-epoxide is considered more biologically active than the parent compound [43], its monitoring in the soil–plant system is necessary.
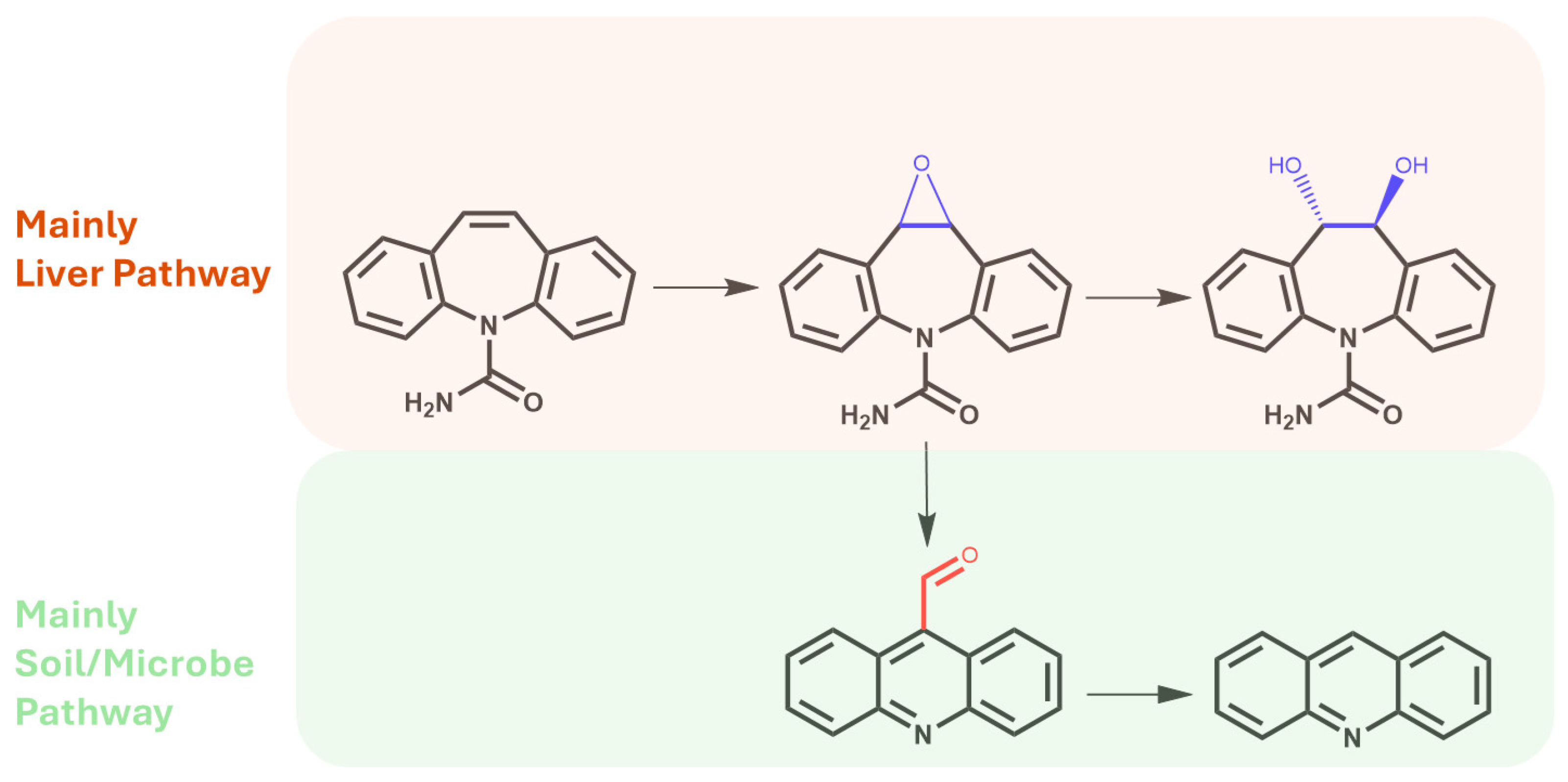
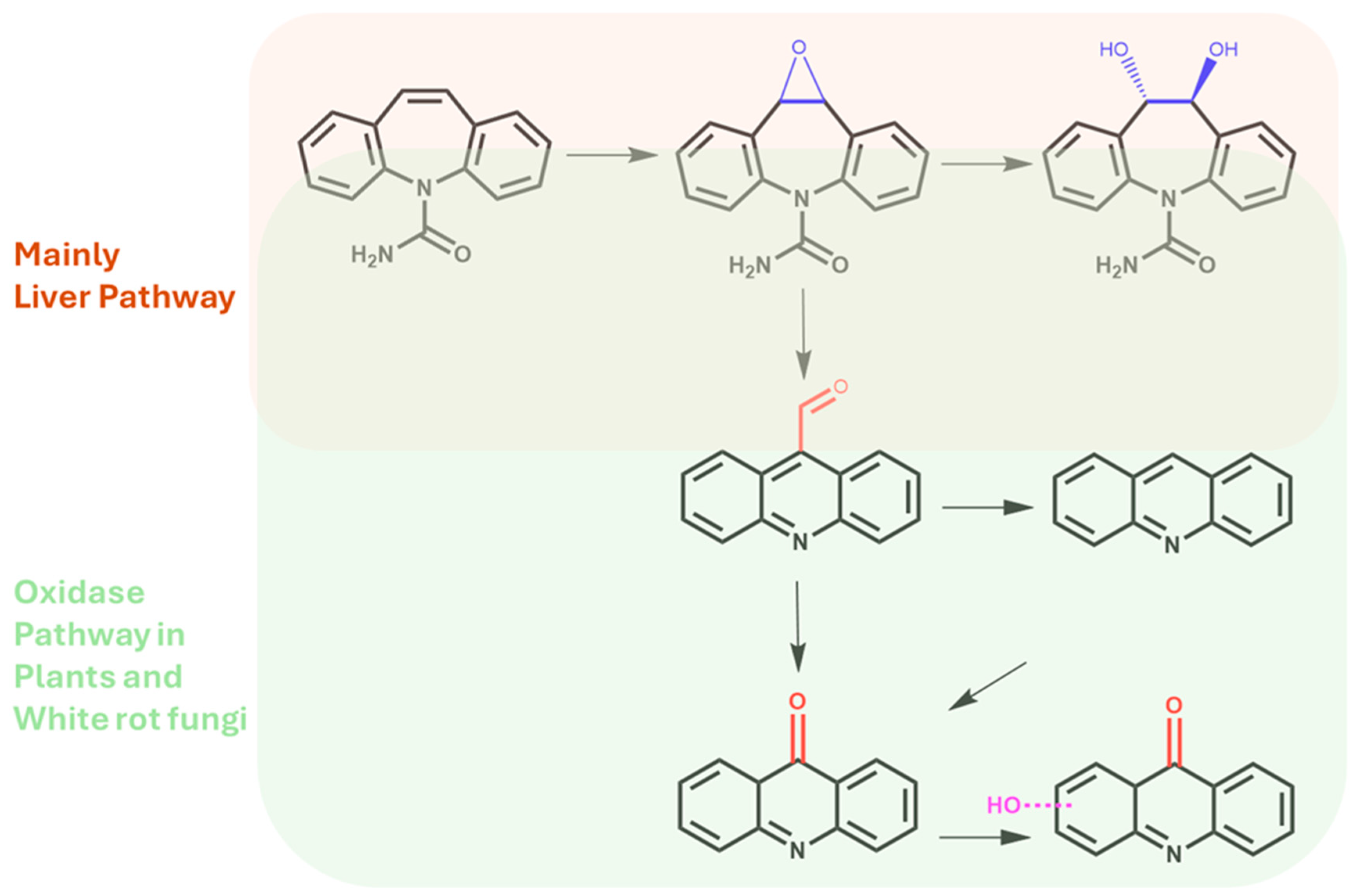
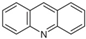
Another metabolite, acridine, is considered the rarest among CBZ derivatives. A few works have established the possibility of the production of acridine directly from CBZ epoxide via a carbonyl intermediate and after ring rearrangement (Figure 4) [44].
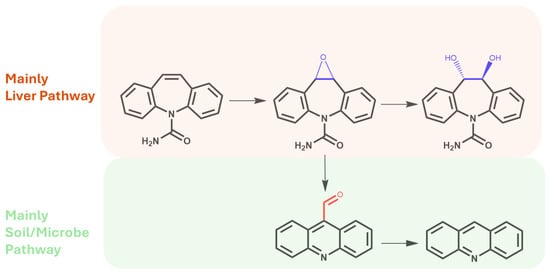
Figure 4.
Main metabolites produced via metabolic pathways in the human liver and after biotransformation via soil microorganisms and/or the rhizosphere.
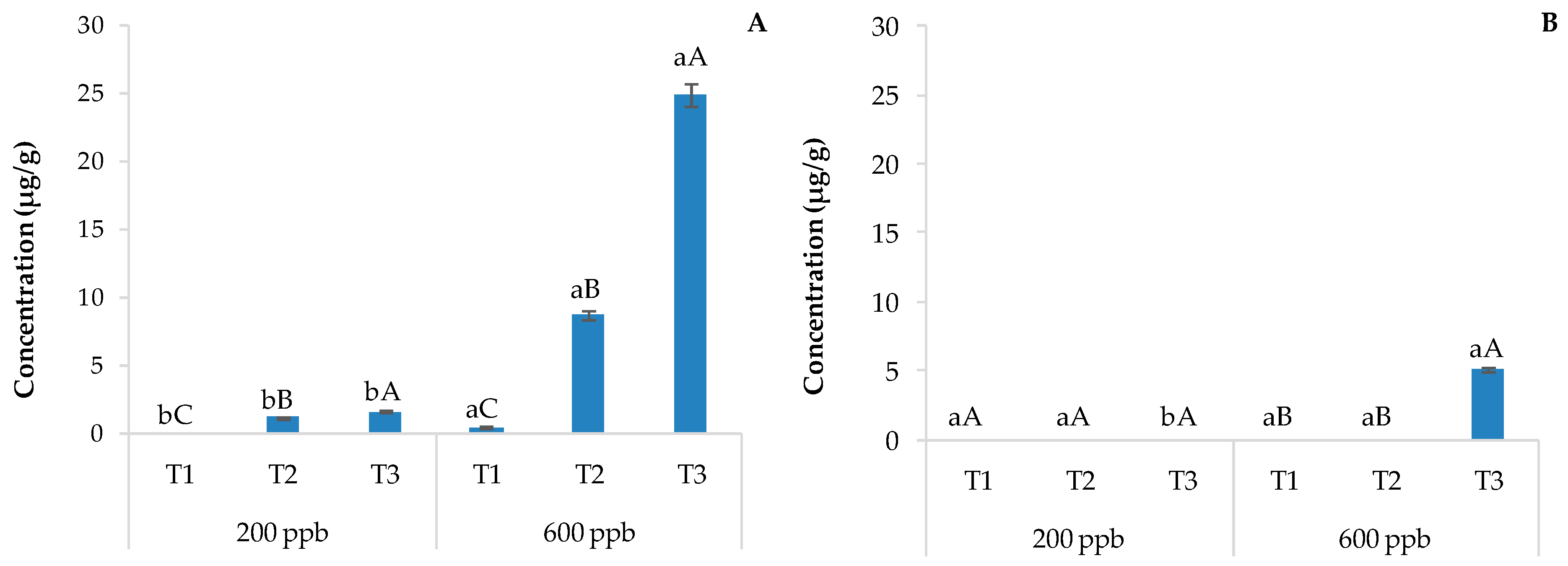
The accumulation of acridine in the soil creates concerns about its possible entry into the food chain and the toxic action exerted by this molecule, as it can inhibit DNA repair and cell growth [45]. In fact, a previous study highlighted that acridine is considerably more toxic than CBZ at multiple trophic levels [46]. Figure 5 shows the content of acridine in the soils without plants (A) and with plants (B) irrigated with the 200 or 600 ppb CBZ solution and sampled after 10, 20, and 30 days of trial.
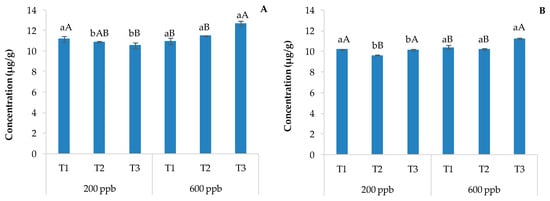
Figure 5.
Average concentration of acridine detected in soils without plants (A) and with plants (B) and the corresponding standard deviations. The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences between concentrations (200 and 600 ppb) at the same time. Uppercase letters represent differences among times (T1, T2, and T3) at the same concentration.
The amount of acridine in the soils was between 9.6 and 12.7 μg g−1, and unlike the results of carbamazepine-10,11-epoxide, its concentration was about the same in the soils with and without plants regardless of the time of sampling and the dose applied. This metabolite appears not to degrade as quickly as carbamazepine-10,11-epoxide. In fact, since carbamazepine-10,11-epoxide is a precursor of acridine, its greater presence in soils without plants suggests a slower degradation of the parent compound in such soils and a contribution of the plants in the degradation process or/and the absorption of CBZ and its degradation products. The second hypothesis about the lack of degradation of CBZ in soils with plants seems to fall short because there was also acridine in this soil. However, photodegradation on soil surfaces can also be considered a way of degradation to obtain acridine from CBZ [47]. In this regard, humic substances in wastewater and soil can absorb light, contributing to the degradation of CBZ to acridine [48]. However, acridine can also be derived from other pathways [49].
Table 2 summarizes the content of CBZ and its metabolites in soils.

Table 2.
Analysis of variance and mean values of CBZ, carbamazepine-10,11-epoxide, and acridine found in soils without plants (A) and with plants (B). The values in each column followed by a different letter are significantly different. ** Significant at p ≤ 0.01. *** Significant at p ≤ 0.001. n.s.: not significant.
3.2. Plants
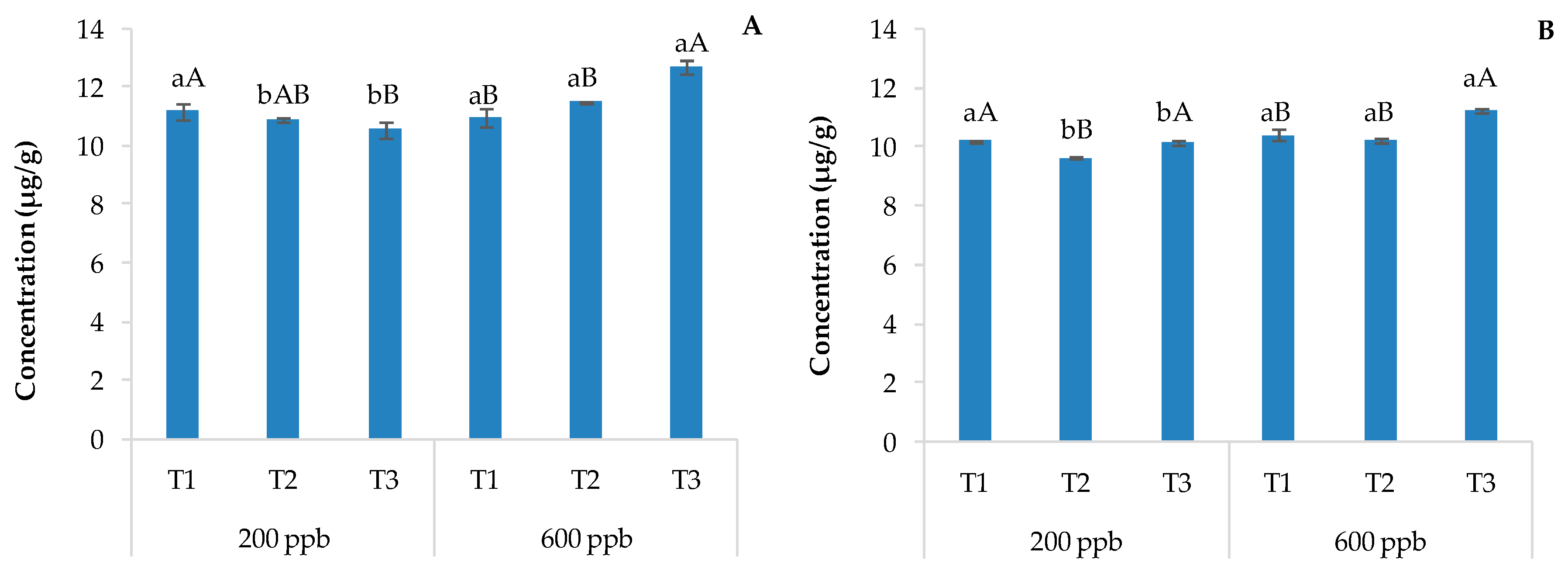
Figure 6 shows the results related to the content of CBZ in the roots, leaves, and stems of basil plants irrigated with the 200 or 600 ppb solution of CBZ and sampled 10, 20, and 30 days after transplantation.
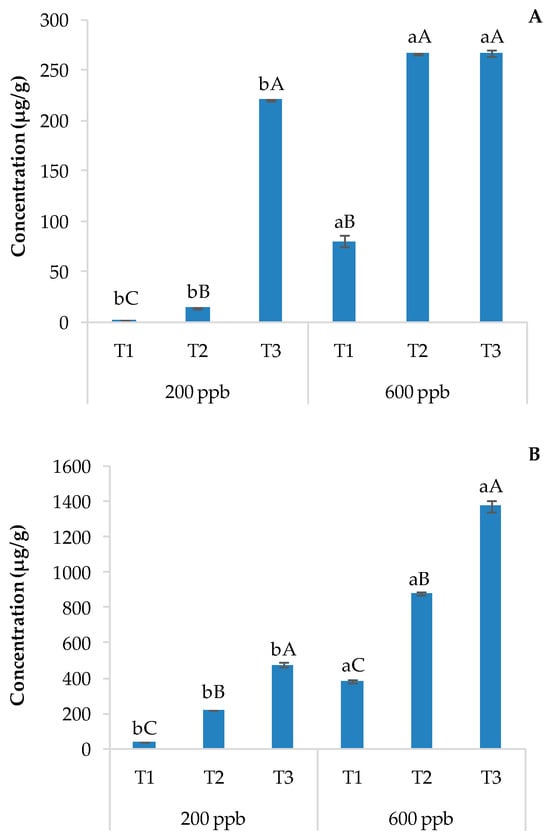
Figure 6.
Average CBZ concentration detected in the roots (A) and leaves and stems (B) of basil plants cultivated in soils contaminated with two different concentrations of CBZ. The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences between concentrations (200 and 600 ppb) at the same time. Uppercase letters represent differences among times (T1, T2, and T3) at the same concentration.
The amount of CBZ detected in plant organs was directly related to the time and dose of the applied contaminant, as observed for soils. In general, the amount of CBZ found in the roots was equal to or less than that found in the corresponding soil but much lower than that found in the aerial parts of the plant, suggesting high mobility of the contaminant through the plant organs. These results agree with several studies reporting the uptake of CBZ by different plants [26,43,50,51,52] and its higher accumulation in plant leaves [53,54,55], possibly due to its chemical properties. A previous study [38] hypothesized that the uptake and translocation of CBZ is due to diffusion into the vascular system, especially due to 0 < log Kow < 4 values; consequently, CBZ tends to move easily in the xylem, reaching the aerial parts of plants. Even Trapp et al. [56] reported that neutral compounds with −1 < log Kow < 5 move to aerial tissues through the xylem.
Regarding carbamazepine metabolites, previous studies have reported that the two CBZ metabolites that are commonly found in leaves and fruits are carbamazepine-10,11-epoxide and 10,11-dihydro-10,11-dihydroxy-carbamazepine [53,55,57]. This latter metabolite derives from a dehydrogenative pathway directly from epoxide CBZ in the human oxidative enzymatic set (Figure 4) [17].
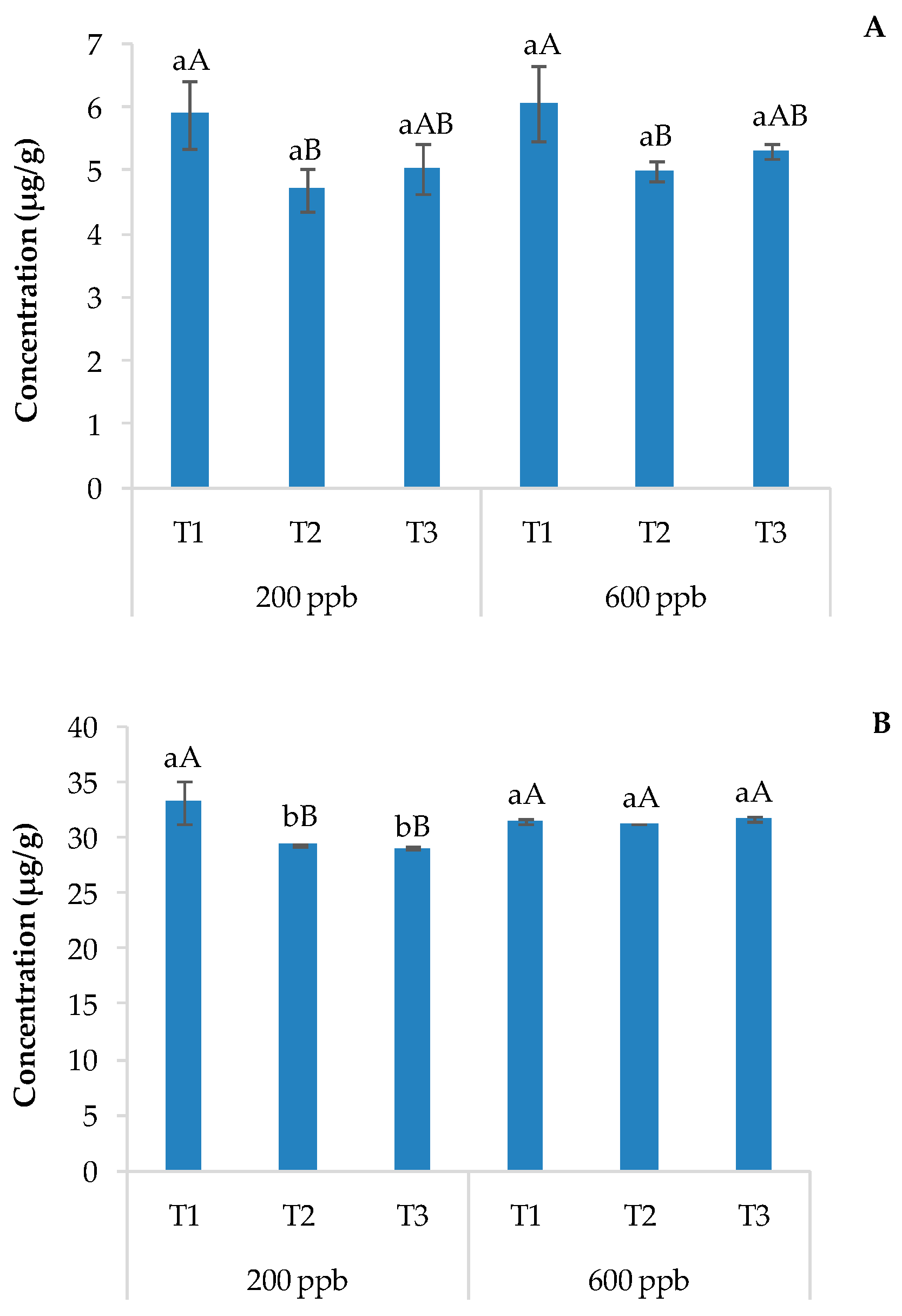
In this study, only acridine was found in plant organs, suggesting rapid degradation of CBZ to carbamazepine-10,11-epoxide and then to acridine. These results are shown in Figure 7A (roots) and Figure 7B (leaves and stems).
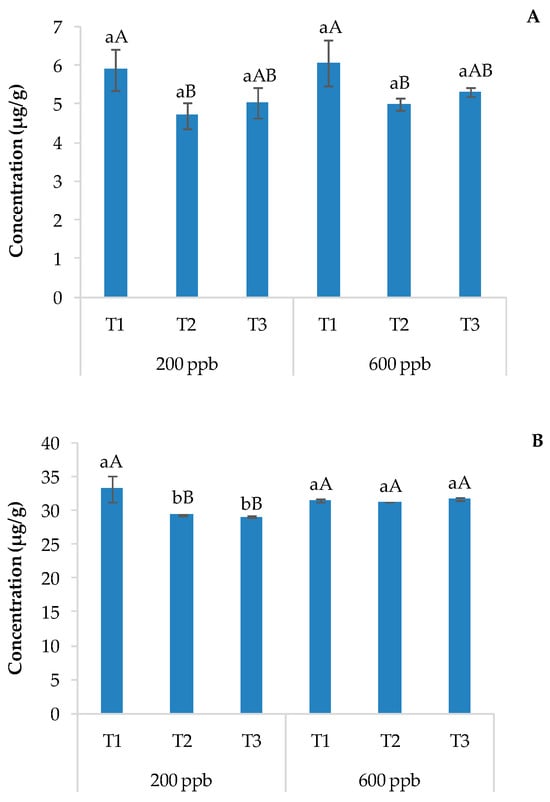
Figure 7.
Average concentration of acridine detected in roots (A) and leaves and stems (B). The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences between concentrations (200 and 600 ppb) at the same time. Uppercase letters represent differences among times (T1, T2, and T3) at the same concentration.
The acridine root concentration did not show significant differences between the two doses applied regardless of sampling times, while a significant decrease in acridine was observed with time. The aerial parts of basil showed 5 to 6 times the concentration of acridine found in the roots. The application of a 200 ppb solution of CBZ resulted in a higher acridine content in T1 compared to the other sampling times, while no differences were observed with the highest dose of CBZ (Figure 7B).
A possible explanation related to the findings of oxidized metabolites of CBZ in such parts of the plants is the presence of specific metal-dependent oxidases and polyphenoloxidase expressed and translocated along the xylematic tissues and roots of the plants. These enzymes, also activated with low amounts of water and at different and huge pH ranges, directly catalyze the formation of -O- ether bridges and the insertion of mono-hydroxyl functions on the benzapine core of CBZ, as reported for laccase, peroxidase, and ligninolitic enzymes in white-rot fungi (Figure 8) [58]. This occurs even if CBZ exhibits a strong electron-withdrawing functional group (N-amide), which generates an electron deficiency, forcing a push-pull monodirectional electron transfer, leading to low susceptibility to laccase-like oxidase. In plants, and in the specific case of basil plants, the huge bioaccumulation of highly concentrated substrates and the simultaneous expression of oxidative enzymes and polyphenol oxidase at different growth stages could spark the oxidative biotransformation of CBZ over time [59].
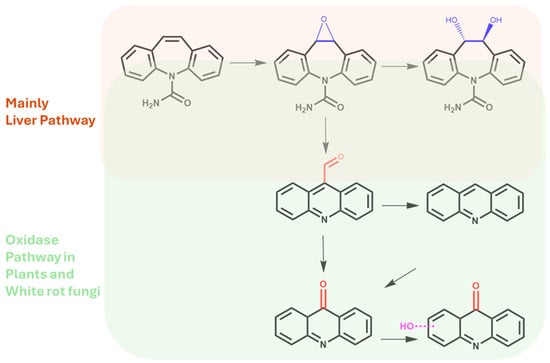
Figure 8.
Oxidative pathway via oxidase in plants and white-rot fungi.
3.3. Enzyme Activities
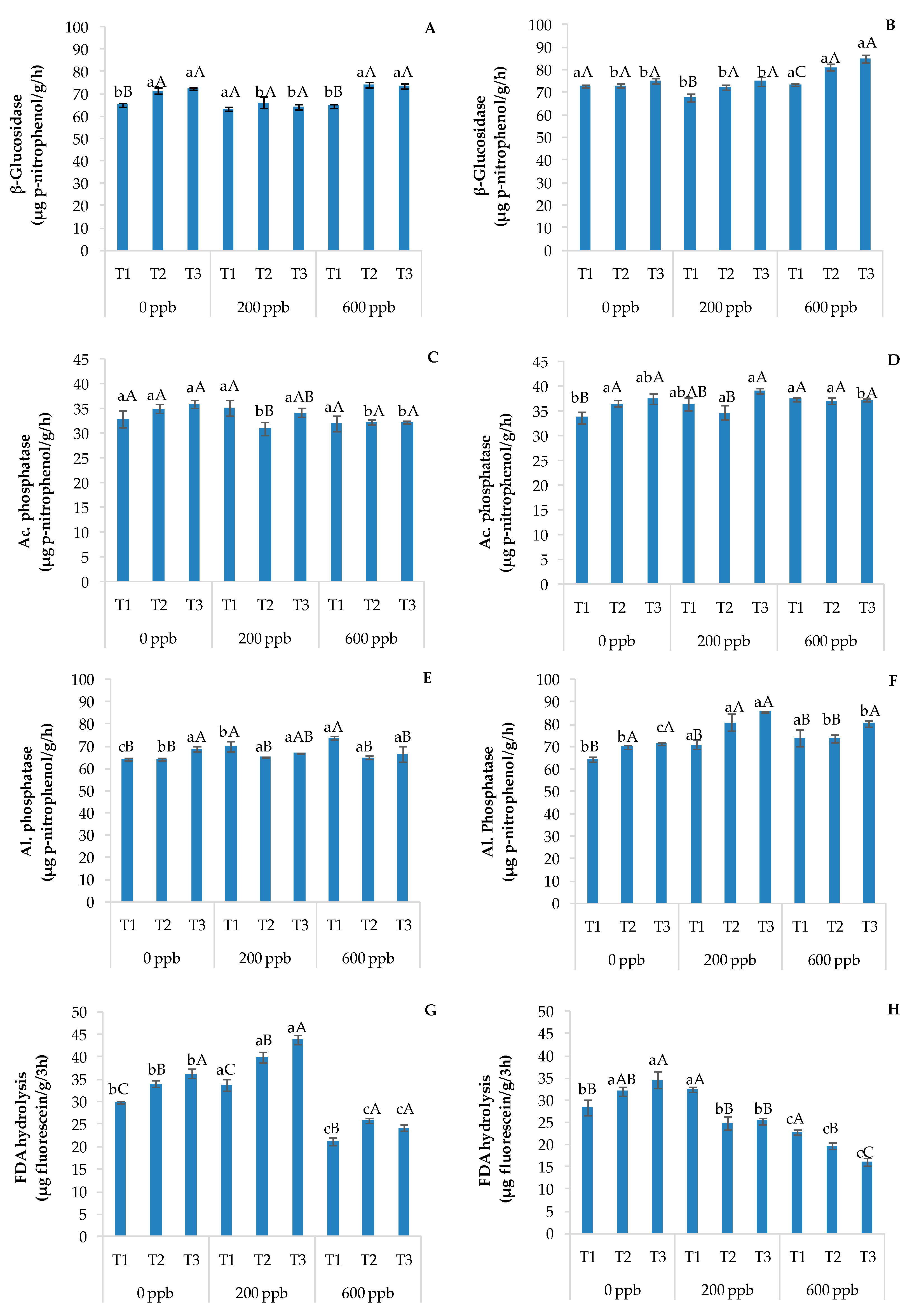
Figure 9 shows the trend of enzymes in soils without and with plants in relation to the time or the concentration of CBZ added.
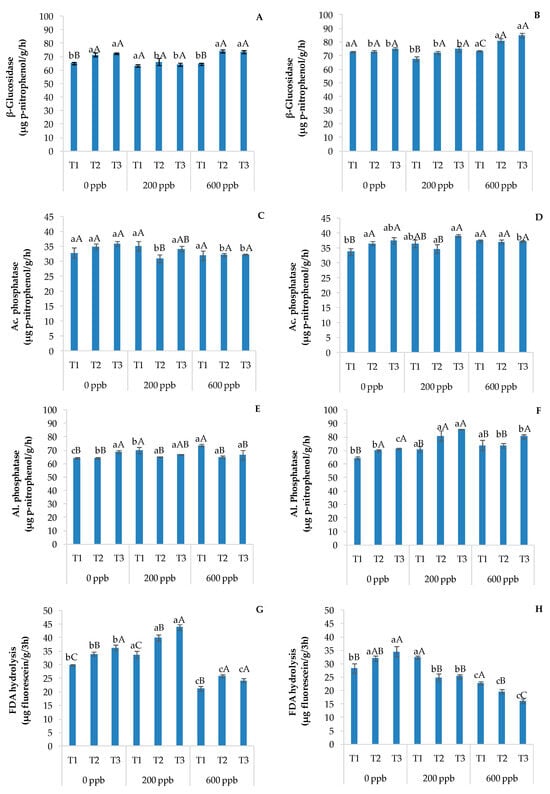
Figure 9.
Enzymatic activities of β-glucosidase in soils without plants (A) and with plants (B), acid phosphatase in soils without plants (C) and with plants (D), alkaline phosphatase in soils without plants (E) and with plants (F), and FDA hydrolysis in soils without plants (G) and with plants (H). The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences between concentrations (0, 200, and 600 ppb) at the same time. Uppercase letters represent differences among times (T1, T2, and T3) at the same concentration.
In general, β-glucosidase and alkaline phosphatase activities increased with time in soils with plants, while FDA hydrolysis increased with time in soils without plants. On the contrary, the latter enzymatic activity decreased with time at 200 and 600 ppb and in the presence of plants. These results suggest that FDA hydrolysis is sensitive to the application of CBZ. In fact, since FDA is an enzyme substrate cleaved by extracellular proteases, lipases, and esterases [60], CBZ could have negatively influenced the microbial community responsible for the release of those enzymes over time. This consideration was confirmed by the lower presence of CBZ and its metabolites in soils with plants compared to those without plants (Table 3). According to Akintoroye et al. [61], a lower concentration of PhACs in wastewater applied in fertigation results in no significant differences in FDA activity between soils with and without plants.

Table 3.
Analysis of variance and mean values of the enzymes studied in soils without plants (A) and with plants (B). n.s.: not significant. The values in each column followed by a different letter are significantly different. * Significant at p ≤ 0.05. ** Significant at p ≤ 0.01. *** Significant at p ≤ 0.001.
Table 3 summarizes the results of the enzymatic activity in soils.
The activities of soil enzymes can be considered indicators of microbial metabolism and the degree of soil stress and pollution [62,63], due to their rapid response to soil changes [64]. However, the interpretation of enzymatic activities is not easy in the presence of organic contaminants, due to their direct and indirect effects on the microbial community [65,66]. Among the indirect effects, the important ones are (i) the growth of resistant microorganisms that can use the organic pollutant or the debris of killed sensitive microorganisms as energy sources and (ii) the changes in the microbial synthesis of the target enzymes [64,66].
3.4. Morphological Parameters
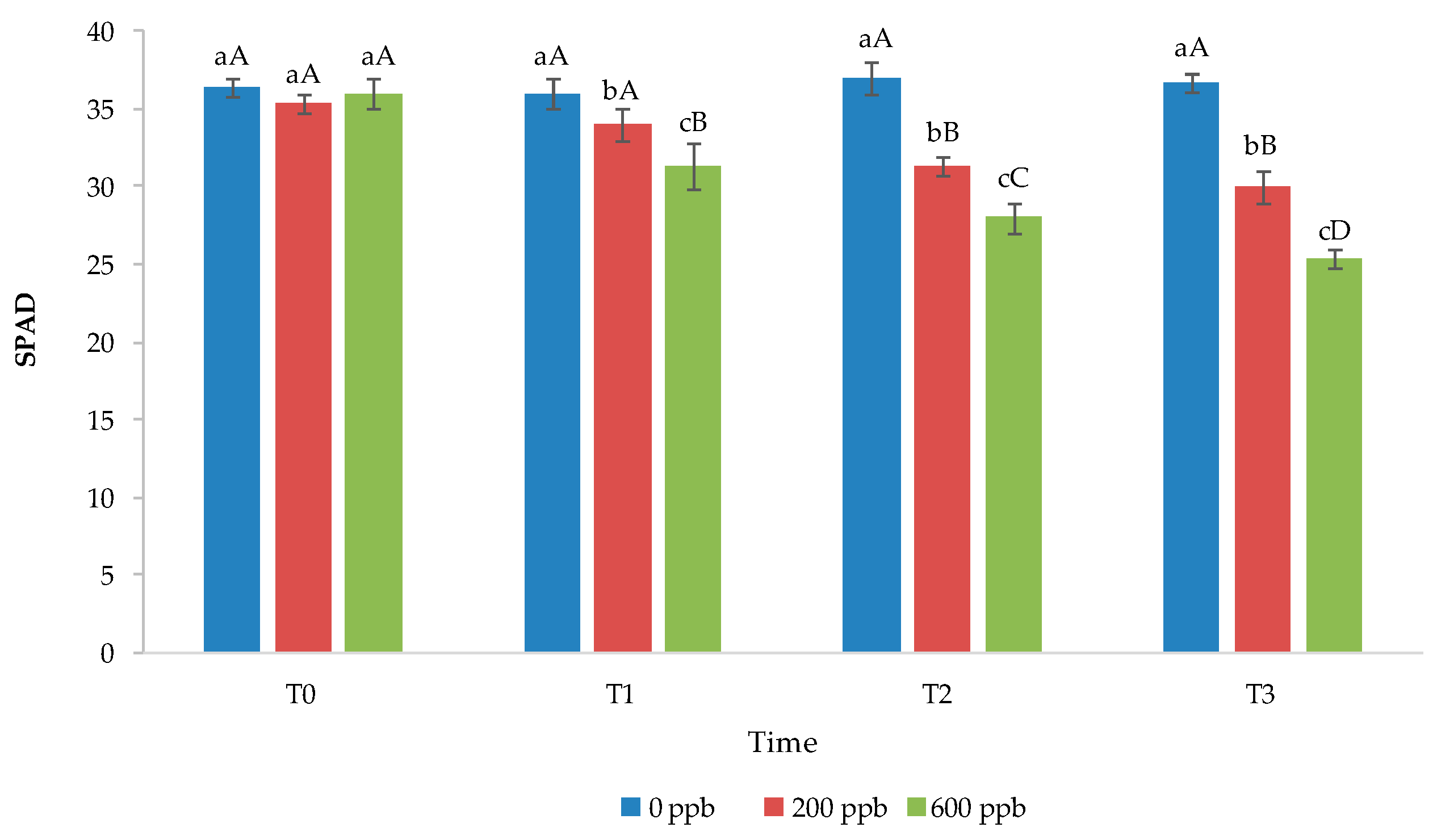
Figure 10 shows the chlorophyll content of basil plants irrigated with the 200 or 600 ppb CBZ solution and sampled after 10, 20, and 30 days of trial.
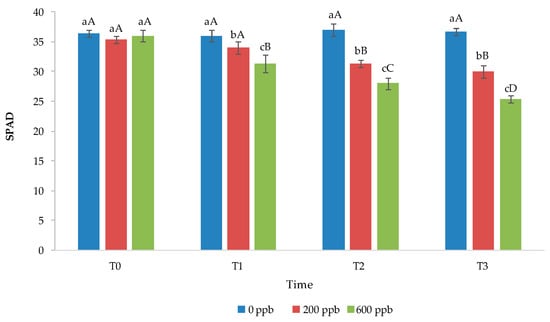
Figure 10.
Soil–plant analysis development (SPAD) of basil leaves grown under different treatments. The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences among concentrations (0, 200, and 600 ppb) at the same time. Uppercase letters represent differences among times (T1, T2, and T3) at the same concentration.
In general, the chlorophyll content decreased with time in plants irrigated with water contaminated with CBZ at 200 and, especially, 600 ppb, while it remained constant in control plants (Figure 8). In addition, corresponding to the same time, the chlorophyll content was definitely lower in plants grown in contaminated soil compared to those grown in uncontaminated soil. In general, a reduction in chlorophyll suggests a typical stress response due to metabolic disorders or due to the accumulation of reactive oxygen species in chloroplasts [67]. Recently, De Mastro et al. [68] found that the chlorophyll A and B content decreases in basil seedlings with the addition of different PhACs at different doses. Opriș et al. [69] found that the chlorophyll content decreases in green leafy vegetables with the application of non-steroidal anti-inflammatory drugs as a function of the dose applied.
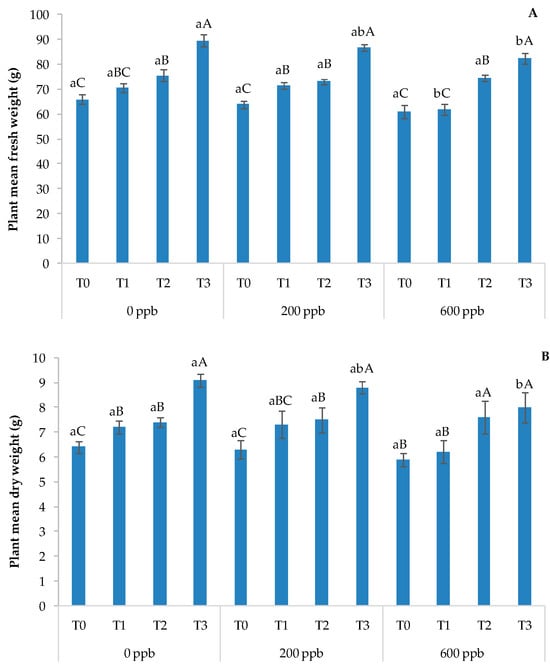
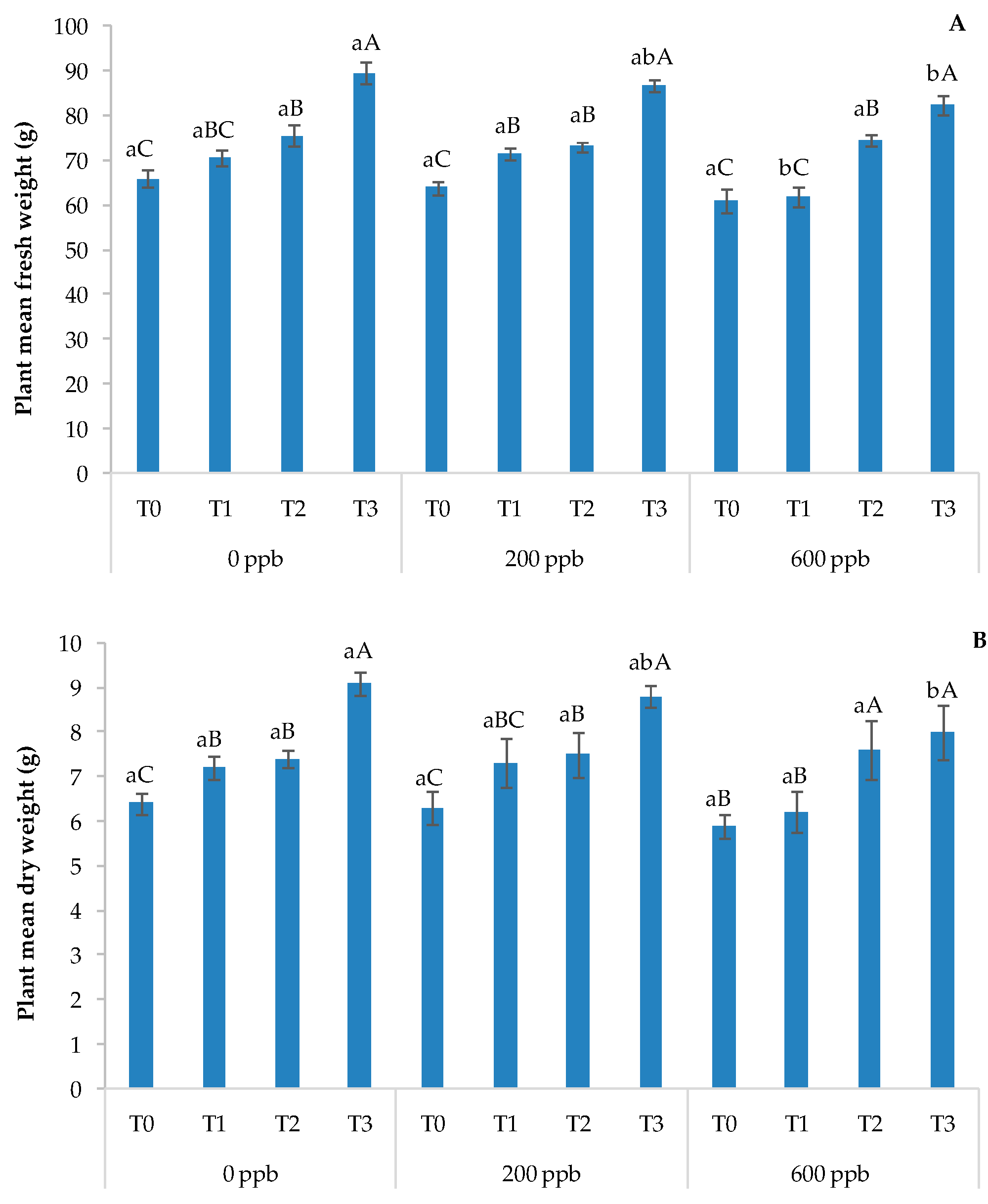
Figure 11.
Fresh weight (A) and dry weight (B) of the basil plant. The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences between concentrations (0, 200, and 600 ppb) at the same time. Uppercase letters represent differences between times (T1, T2, and T3) at the same concentration.
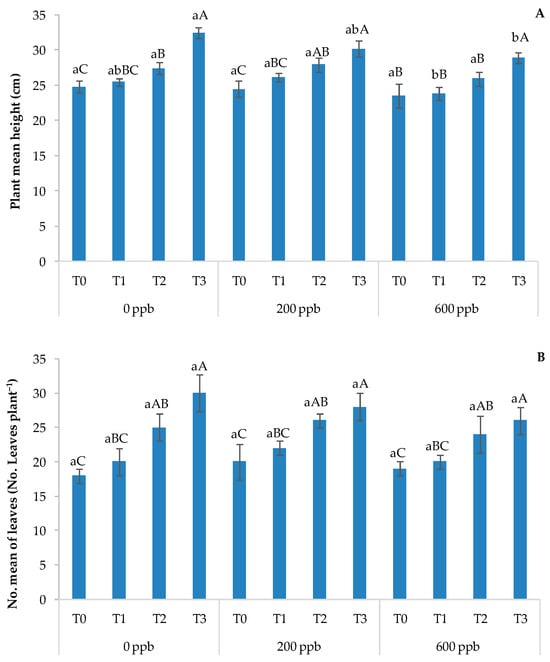
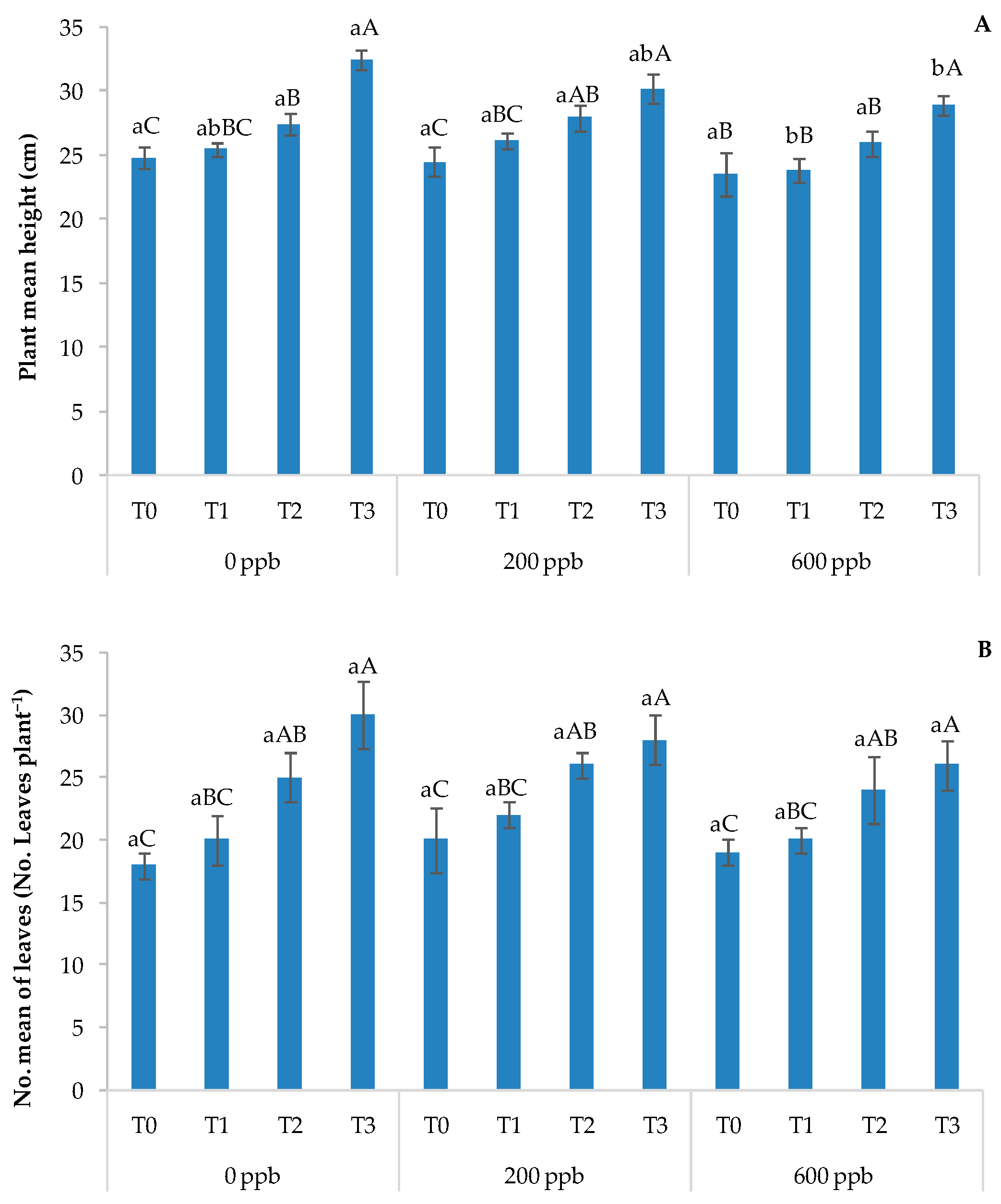
Figure 12.
Height of the basil plant (A) and number of leaves (B). The bars marked with different letters are significantly different (p < 0.05). Lowercase letters represent differences between concentrations (0, 200, and 600 ppb) at the same time. Uppercase letters represent differences between times (T1, T2, and T3) at the same concentration.
After 30 days (T3), the highest concentration of the contaminant negatively affected the fresh and dry weights of the plants compared to the intermediate dose and the control. With respect to time, the results showed a constant growth in all treatments, as expected. These results suggest a slight toxic effect of CBZ on the aforementioned plant parameters.
Regarding height and the number of leaves (Figure 12A,B), two of the most significant indicators of plant growth and development, significant differences were found among the different treatments, especially with respect to time.
The number of leaves and the height of the plant, although numerically lower at the highest dose of CBZ applied, did not appear to be significantly influenced by the addition of the contaminant. The results of the statistical analysis confirmed that the highest dose of CBZ did not negatively affect the vigor of the basil plants, even after the accumulation of the contaminant due to the different types of irrigation.
These parameters can increase the plant-to-land cover ratio, which in turn can improve photosynthesis and plant uptake. Our results are in contrast to those of Carter et al. [70] and Mascellani et al. [71], who found that the effects of CBZ on plant growth depend on its concentration.
4. Conclusions
This study proved that CBZ is a mobile contaminant that can easily accumulate in basil leaves, making it a contaminant of major interest according to various authors [15,43,57]. In addition, CBZ undergoes several degradation pathways, releasing metabolites that can be more toxic than the parent compound. Our results suggest that the identification and quantification of CBZ metabolites are essential to estimate the overall uptake by basil plants and to make a correct risk assessment. In general, basil plants reduce the contaminant in the soil through direct uptake from the root systems or indirectly through the rhizosphere, which stimulates the activity of microorganisms.
The activity of soil enzymes is influenced differently by the presence of CBZ and does not show a particular trend, while, according to the results of the morphological parameters, basil can be considered a tolerant plant. All these considerations highlight that the fate of CBZ in the soil–plant system is not easily predictable. To better identify its dynamics and long-term effects, more studies are needed in other edible plants and other types of soil.
Author Contributions
Conceptualization, F.D.M. and G.B.; methodology, F.D.M., G.B. and C.C. (Claudio Cocozza); software, F.D.M.; validation, F.D.M. and G.B.; formal analysis, F.D.M. and C.C. (Claudio Cacace); investigation, F.D.M., G.B., A.T., D.V. and C.C. (Claudio Cocozza); resources, G.B.; data curation, F.D.M. and A.T.; writing—original draft preparation, F.D.M. and A.T.; writing—review and editing, F.D.M., G.B., C.C. (Claudio Cocozza), D.V., M.R.P. and F.S.; supervision, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministero dell’Istruzione dell’Università e della Ricerca, Italy (grant number P2022PY45N).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vinayagam, V.; Murugan, S.; Kumaresan, R.; Narayanan, M.; Sillanpää, M.; Viet, N.V.; Kushwaha, O.S.; Jenis, P.; Potdar, P.; Gadiya, S. Sustainable adsorbents for the removal of pharmaceuticals from wastewater: A review. Chemosphere 2022, 300, 134597. [Google Scholar] [CrossRef]
- Khan, M.T.; Shah, I.A.; Ihsanullah, I.; Naushad, M.; Ali, S.; Shah, S.H.A.; Mohammad, A.W. Hospital wastewater as a source of environmental contamination: An overview of management practices, environmental risks, and treatment processes. J. Water Process Eng. 2021, 41, 101990. [Google Scholar] [CrossRef]
- De Mastro, F.; Cacace, C.; Traversa, A.; Pallara, M.; Cocozza, C.; Mottola, F.; Brunetti, G. Influence of chemical and mineralogical soil properties on the adsorption of sulfamethoxazole and diclofenac in Mediterranean soils. Chem. Biol. Technol. Agric. 2022, 9, 34. [Google Scholar] [CrossRef]
- Carter, L.J.; Harris, E.; Williams, M.; Ryan, J.J.; Kookana, R.S.; Boxall, A.B.A. Fate and uptake of pharmaceuticals in soil–plant systems. J. Agric. Food Chem. 2014, 62, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Bolesta, W.; Głodniok, M.; Styszko, K. From Sewage Sludge to the Soil—Transfer of Pharmaceuticals: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10246. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-term wastewater irrigation of vegetables in real agricultural systems: Concentration of pharmaceuticals in soil, uptake and bioaccumulation in tomato fruits and human health risk assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, D.; Du, L.; Song, B.; Yin, L.; Chen, Y.; Gao, L.; Li, R.; Huang, H.; Zeng, G. Antibiotic resistance in soil-plant systems: A review of the source, dissemination, influence factors, and potential exposure risks. Sci. Total Environ. 2023, 869, 161855. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.-Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef]
- Paltiel, O.; Fedorova, G.; Tadmor, G.; Kleinstern, G.; Maor, Y.; Chefetz, B. Human exposure to wastewater-derived pharmaceuticals in fresh produce: A randomized controlled trial focusing on carbamazepine. Environ. Sci. Technol. 2016, 50, 4476–4482. [Google Scholar] [CrossRef]
- Fenet, H.; Mathieu, O.; Mahjoub, O.; Li, Z.; Hillaire-Buys, D.; Casellas, C.; Gomez, E. Carbamazepine, carbamazepine epoxide and dihydroxycarbamazepine sorption to soil and occurrence in a wastewater reuse site in Tunisia. Chemosphere 2012, 88, 49–54. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dodgen, L.; Ye, Q.; Gan, J. Degradation kinetics and metabolites of carbamazepine in soil. Environ. Sci. Technol. 2013, 47, 3678–3684. [Google Scholar] [CrossRef]
- Watkinson, A.; Murby, E.; Costanzo, S. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids–soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef]
- De Mastro, F.; Brunetti, G.; De Mastro, G.; Ruta, C.; Stea, D.; Murgolo, S.; De Ceglie, C.; Mascolo, G.; Sannino, F.; Cocozza, C.; et al. Uptake of different pharmaceuticals in soil and mycorrhizal artichokes from wastewater. Environ. Sci. Pollut. Res. 2023, 30, 33349–33362. [Google Scholar] [CrossRef] [PubMed]
- Bahlmann, A.; Brack, W.; Schneider, R.J.; Krauss, M. Carbamazepine and its metabolites in wastewater: Analytical pitfalls and occurrence in Germany and Portugal. Water Res. 2014, 57, 104–114. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Padovano, E.; Giancotti, V.; Baiocchi, C. Identification of the unknown transformation products derived from clarithromycin and carbamazepine using liquid chromatography/high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1687–1704. [Google Scholar] [CrossRef]
- Sauvêtre, A.; May, R.; Harpaintner, R.; Poschenrieder, C.; Schröder, P. Metabolism of carbamazepine in plant roots and endophytic rhizobacteria isolated from Phragmites australis. J. Hazard. Mater. 2018, 342, 85–95. [Google Scholar] [CrossRef]
- Kalamartzis, I.; Menexes, G.; Georgiou, P.; Dordas, C. Effect of Water Stress on the Physiological Characteristics of Five Basil (Ocimum basilicum L.) Cultivars. Agronomy 2020, 10, 1029. [Google Scholar] [CrossRef]
- Kalamartzis, I.; Dordas, C.; Georgiou, P.; Menexes, G. The Use of Appropriate Cultivar of Basil (Ocimum basilicum) Can Increase Water Use Efficiency under Water Stress. Agronomy 2020, 10, 70. [Google Scholar] [CrossRef]
- Yilmaz, A.; Karik, Ü. AMF and PGPR enhance yield and secondary metabolite profile of basil (Ocimum basilicum L.). Ind. Crop. Prod. 2021, 176, 114327. [Google Scholar] [CrossRef]
- Çamlica, M.; Yaldiz, G. Basil (Ocimum basilicum L.): Botany, Genetic Resource, Cultivation, Conservation, and Stress Factors. In Sustainable Agriculture in the Era of the OMICs Revolution; Prakash, C.S., Fiaz, S., Nadeem, M.A., Baloch, F.S., Qayyum, A., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Kowalska, G. Pesticide Residues in Some Polish Herbs. Agriculture 2020, 10, 154. [Google Scholar] [CrossRef]
- Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Approach to the Dynamic of Carbamazepine and its Main Metabolites in Soil Contamination through the Reuse of Wastewater and Sewage Sludge. Molecules 2020, 25, 5306. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Tadmor, G.; Malchi, T.; Blotevogel, J.; Borch, T.; Polubesova, T.; Chefetz, B. Fate of carbamazepine, its metabolites, and lamotrigine in soils irrigated with reclaimed wastewater: Sorption, leaching and plant uptake. Chemosphere 2016, 160, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carbamazepine (accessed on 28 April 2024).
- Brieudes, V.; Lardy-Fontan, S.; Lalere, B.; Vaslin-Reimann, S.; Budzinski, H. Validation and uncertainties evaluation of an isotope dilution-SPE-LC–MS/MS for the quantification of drug residues in surface waters. Talanta 2016, 146, 138–147. [Google Scholar] [CrossRef]
- Swift, R.S. Method of Soil Analysis: Part 3. Chemical Methods; SSSA Book Series No. 5; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1011–1069. [Google Scholar]
- De Mastro, F.; Cocozza, C.; Traversa, A.; Cacace, C.; Mottola, F.; Mezzina, A.; Brunetti, G. Validation of a modified QuEChERS method for the extraction of multiple classes of pharmaceuticals from soils. Chem. Biol. Technol. Agric. 2022, 9, 80. [Google Scholar] [CrossRef]
- Brunetti, G.; Traversa, A.; De Mastro, F.; Dichio, B.; Mottola, F.; Mininni, A.N.; Nigro, P.; Cocozza, C. Evaluation of the QuEChERS extraction approach for the analysis of active compounds of pharmaceuticals in olive tree portions. Chem. Biol. Technol. Agric. 2023, 10, 80. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Parmar, M.R.; Bhalodiya, V.B.; Kapdi, S.S. Temperature effect on drying and phytochemicals of basil leaves. Int. J. Eng. Sci. Investig. 2018, 7, 34–44. [Google Scholar]
- Blair, P.M.; Land, M.L.; Piatek, M.J.; Jacobson, D.A.; Lu, T.Y.S.; Doktycz, M.J.; Pelletiera, D.A. Exploration of the Biosynthetic Potential of the Populus Microbiome. MSystems 2018, 3, e00045-18. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, D.M.; Fuhrmann, J.J.; Hartel, P.G.; Zuberer, D.A. Principles and Applications of Soil Microbiology, 2nd ed.; Pearson: Upper Saddle River, NJ, USA, 2005; pp. 41–51. [Google Scholar]
- Miller, E.L.; Nason, S.L.; Karthikeyan, K.G.; Pedersen, J.A. Root Uptake of Pharmaceuticals and Personal Care Product Ingredients. Environ. Sci. Technol. 2016, 50, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Rosario-Ortiz, F.L.; Snyder, S.A. Effect of ozone exposure on the oxidation of trace organic contaminants in wastewater. Water Res. 2009, 43, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, J.; Zhou, S.; Lian, M. Microbial degradation of carbamazepine by a newly isolated of Gordonia polyophrenivorans. Environ. Technol. Innov. 2023, 32, 103322. [Google Scholar] [CrossRef]
- Attia, S.M. Deleterious Effects of Reactive Metabolites. Oxidative Med. Cell. Longev. 2010, 3, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Ben Mordechay, E.; Tarchitzky, J.; Chen, Y.; Shenker, M.; Chefetz, B. Composted biosolids and treated wastewater as sources of pharmaceuticals and personal care products for plant uptake: A case study with carbamazepine. Environ. Pollut. 2018, 232, 164–172. [Google Scholar] [CrossRef]
- Tak, S.; Tiwari, A.; Vellanki, B.P. Identification of emerging contaminants and their transformation products in a moving bed biofilm reactor (MBBR)-based drinking water treatment plant around River Yamuna in India. Environ. Monit. Assess. 2020, 192, 365. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Denny, W.A. The genetic toxicology of acridines. Mutat. Res. Genet. Toxicol. 1991, 258, 123–160. [Google Scholar] [CrossRef] [PubMed]
- Donner, E.; Kosjek, T.; Qualmann, S.; Kusk, K.O.; Heath, E.; Revitt, D.M.; Ledin, A.; Andersen, H.R. Ecotoxicity of carbamazepine and its UV photolysis transformation products. Sci. Total Environ. 2013, 443, 870–876. [Google Scholar] [CrossRef]
- Chiron, S.; Minero, C.; Vione, D. Photodegradation processes of the antiepileptic drug carbamazepine, relevant to estuarine waters. Environ. Sci. Technol. 2006, 40, 5977–5983. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, M.; Mathieu, O.; Gomez, E.; Casellas, C.; Fenet, H.; Hillaire-Buys, D. Presence and Fate of Carbamazepine, Oxcarbazepine, and Seven of Their Metabolites at Wastewater Treatment Plants. Arch. Environ. Contam. Toxicol. 2009, 56, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Osborne, P.J.; Preston, M.R.; Chen, H.-Y. Azaarenes in sediments, suspended particles and aerosol associated with the River Mersey estuary. Mar. Chem. 1997, 58, 73–83. [Google Scholar] [CrossRef]
- Bartrons, M.; Peñuelas, J. Pharmaceuticals and Personal-Care Products in Plants. Trends Plant Sci. 2017, 22, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Bhalsod, G.D.; Chuang, Y.-H.; Jeon, S.; Gui, W.; Lim, H.; Ryser, E.T.; Guber, A.K.; Zhang, W. Uptake and Accumulation of Pharmaceuticals in Overhead- and Surface-Irrigated Greenhouse Lettuce. J. Agric. Food Chem. 2018, 66, 822–830. [Google Scholar] [CrossRef] [PubMed]
- García, M.G.; Fernández-López, C.; Pedrero-Salcedo, F.; Alarcón, J.J. Absorption of carbamazepine and diclofenac in hydroponically cultivated lettuces and human health risk assessment. Agric. Water Manag. 2018, 206, 42–47. [Google Scholar] [CrossRef]
- Goldstein, M.; Shenker, M.; Chefetz, B. Insights into the Uptake Processes of Wastewater-Borne Pharmaceuticals by Vegetables. Environ. Sci. Technol. 2014, 48, 5593–5600. [Google Scholar] [CrossRef]
- Hurtado, C.; Trapp, S.; Bayona, J.M. Inverse modeling of the biodegradation of emerging organic contaminants in the soil-plant system. Chemosphere 2016, 156, 236–244. [Google Scholar] [CrossRef][Green Version]
- Malchi, T.; Maor, Y.; Tadmor, G.; Shenker, M.; Chefetz, B. Irrigation of Root Vegetables with Treated Wastewater: Evaluating Uptake of Pharmaceuticals and the Associated Human Health Risks. Environ. Sci. Technol. 2014, 48, 9325–9333. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.; McFarlane, J.C. Plant Contamination: Modeling and Simulation of Organic Chemical Processes; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Malchi, T.; Maor, Y.; Chefetz, B. Comments on “Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation”. Environ. Int. 2015, 82, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Shintate, H.; Kawai, S.; Okamura, H.; Nishida, T. Elimination of carbamazepine by repeated treatment with laccase in the presence of 1-hydroxybenzotriazole. J. Hazard. Mater. 2010, 181, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Shafran, E.; Dudai, N.; Mayer, A.M. Polyphenol oxidase in Ocimum basilicum during growth, development and following cold stress. J. Food Agric. Environ. 2007, 5, 254–257. [Google Scholar]
- Serafini, C.G.; Clerici, N.J.; Della-Flora, I.K.; Dupont, G.K.; Cabrera, L.d.C.; Daroit, D.J. Effects of atrazine on soil microbial indicators and the evaluation of herbicide attenuation in microcosms. J. Soils Sediments 2022, 22, 1165–1175. [Google Scholar] [CrossRef]
- Akintoroye, M.; Newton, R.A.; Kříženecká, S.; Hejda, S.; Krystyník, P.; Ahnert, M.; Trögl, J.; Krebs, P.; Al Souki, K.S. Utilization of Biochar for Eliminating Residual Pharmaceuticals from Wastewater Used in Agricultural Irrigation: Application to Ryegrass. Agronomy 2022, 12, 2987. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Wang, Y.; Zhang, Y.; Zhang, X. Responses of soil bacterial communities, enzyme activities, and nutrients to agricultural-to-natural ecosystem conversion in the Loess Plateau, China. J. Soils Sediments 2019, 19, 1427–1440. [Google Scholar] [CrossRef]
- Nannipieri, P. The potential use of soil enzymes as indicators of productivity, sustainability and pollution. In Soil Biota: Management in Sustainable Farming Systems; Pankhurst, C.E., Double, B.M., Gupta, V.V.S.R., Grace, P.R., Eds.; CSIRO: Adelaide, Australia, 1994; pp. 238–244. [Google Scholar]
- Schaffer, A. Pesticide effects on enzyme activities in the soil ecosystems. In Soil Biochemistry; Bollag, J.M., Stotzky, G., Eds.; Marcel Dekker: New York, NY, USA, 1993; Volume 8, pp. 273–340. [Google Scholar]
- Cao, X.; Cui, X.; Xie, M.; Zhao, R.; Xu, L.; Ni, S.; Cui, Z. Amendments and bioaugmentation enhanced phytoremediation and micro-ecology for PAHs and heavy metals co-contaminated soils. J. Hazard. Mater. 2021, 426, 128096. [Google Scholar] [CrossRef]
- De Mastro, F.; Brunetti, G.; Traversa, A.; Cacace, C.; Cocozza, C. Investigation of the Effect of Twelve Pharmaceuticals on Germination and Growth Parameters of Basil (Ocimum basilicum L.). Appl. Sci. 2023, 13, 6759. [Google Scholar] [CrossRef]
- Opriș, O.; Lung, I.; Soran, M.-L.; Ciorîță, A.; Copolovici, L. Investigating the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on the composition and ultrastructure of green leafy vegetables with important nutritional values. Plant Physiol. Biochem. 2020, 151, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Williams, M.; Böttcher, C.; Kookana, R.S. Uptake of Pharmaceuticals Influences Plant Development and Affects Nutrient and Hormone Homeostases. Environ. Sci. Technol. 2015, 49, 12509–12518. [Google Scholar] [CrossRef]
- Mascellani, A.; Mercl, F.; Kurhan, S.; Pierdona, L.; Kudrna, J.; Zemanova, V.; Hnilicka, F.; Kloucek, P.; Tlustos, P.; Havlik, J. Biochemical and physiological changes in Zea mays L. after exposure to the environmental pharmaceutical pollutant carbamazepine. Chemosphere 2023, 329, 138689. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).