Residue Addition Can Mitigate Soil Health Challenges with Climate Change in Drylands: Insights from a Field Warming Experiment in Semi-Arid Texas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characteristics
2.2. Experimental Design
2.3. Measurement of Environmental Variables
2.4. Soil Sample Collection and Laboratory Analysis
2.5. Soil Respiration Measurement
2.6. Harvesting and Biomass Measurements
2.7. Statistical Analysis
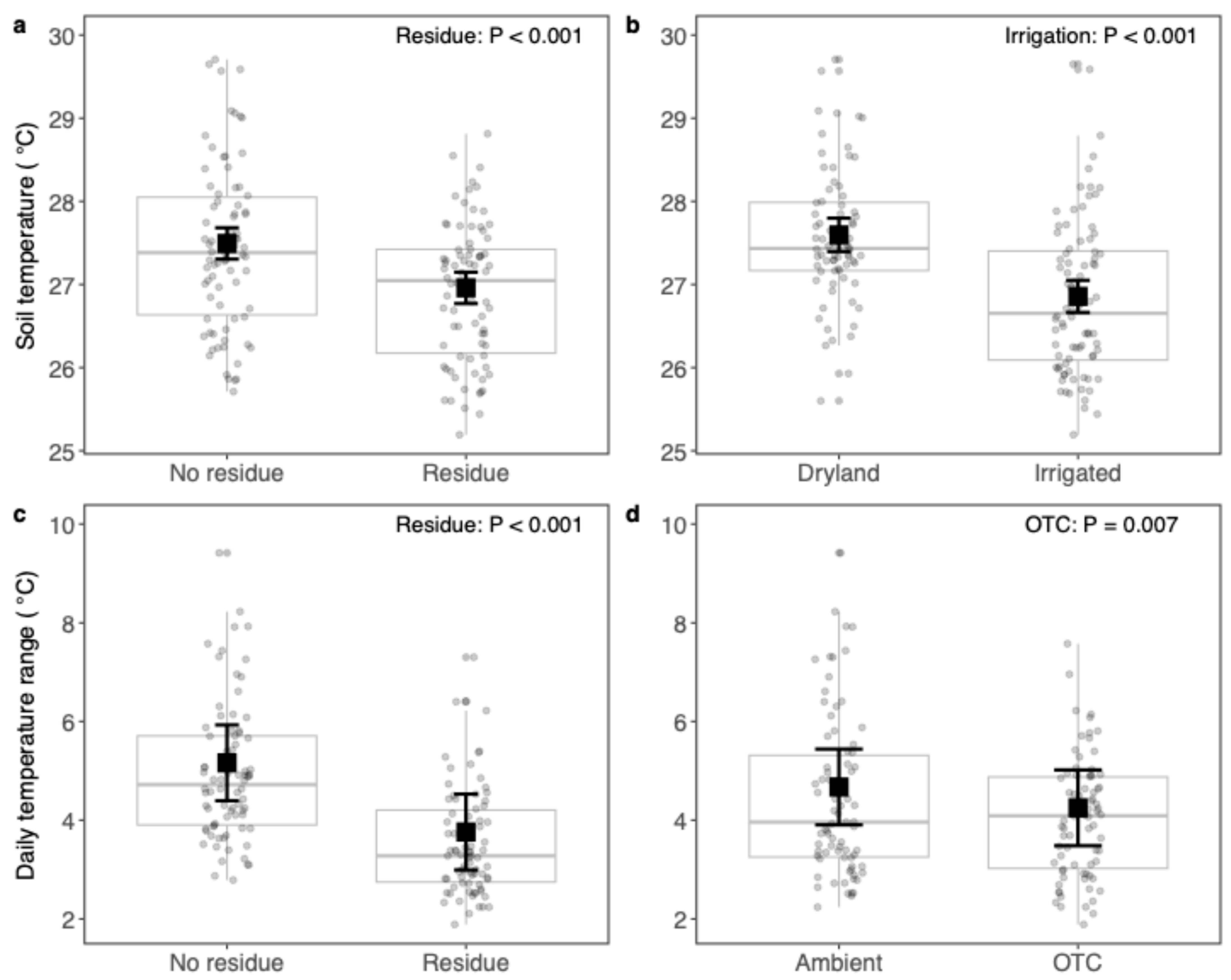
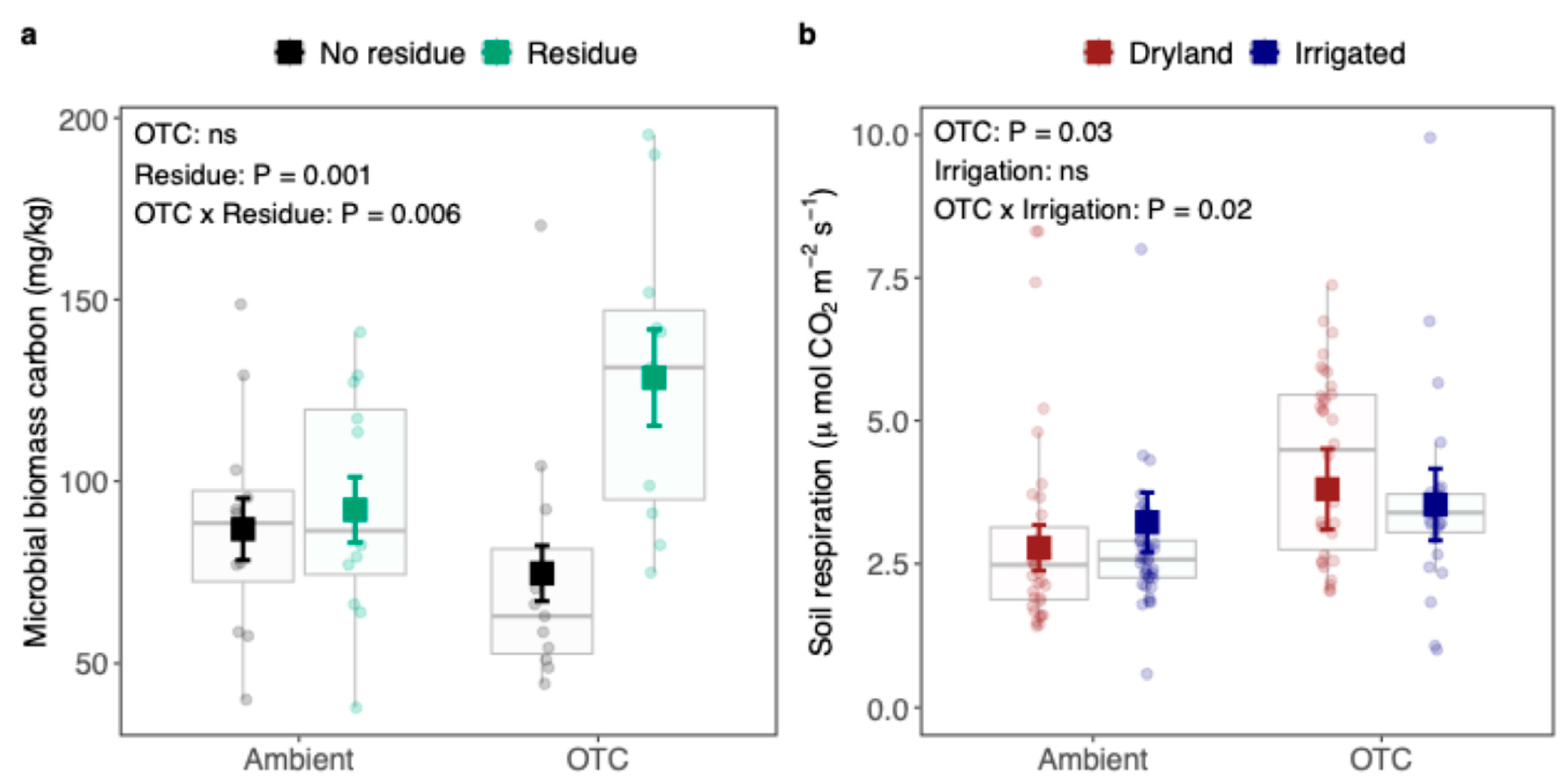
3. Results
4. Discussion
4.1. Residue Addition Stabilized the Soil Environment
4.2. Residue Addition Increased Microbial Biomass and Soil Respiration
4.3. Irrigation Was a Stronger Predictor than Warming on Plant Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acosta-Martinez, V.; Cotton, J.; Slaughter, L.C.; Ghimire, R.; Roper, W. Soil Health Assessment to Evaluate Conservation Practices in SemiArid Cotton Systems at Producer Site Scale. Soil Syst. 2023, 7, 72. [Google Scholar] [CrossRef]
- Eekhout, J.P.; De Vente, J. Global impact of climate change on soil erosion and potential for adaptation through soil conservation. Earth Sci. Rev. 2022, 226, 103921. [Google Scholar] [CrossRef]
- Lauer, S.; Sanderson, M.R. Producer Attitudes Toward Groundwater Conservation in the U.S. Ogallala-High Plains. Groundwater 2020, 58, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Dorminey, B. Dryland Farmers Work Wonders without Water in U.S. West. Available online: https://www.scientificamerican.com/article/dryland-farmers-work-wonders-without-water-us-west/ (accessed on 24 July 2024).
- Hailu, T.; Devkota, P.; Osoko, T.O.; Singh, R.K.; Zak, J.C.; van Gestel, N. No-till and crop rotation are promising practices to enhance soil health in cotton-producing semiarid regions: Insights from on-farm research. Soil Syst. under Review.
- Zhou, M.; Liu, C.; Wang, J.; Meng, Q.; Yuan, Y.; Ma, X.; Liu, X.; Zhu, Y.; Ding, G.; Zhang, J.; et al. Soil Aggregates Stability and Storage of Soil Organic Carbon Respond to Cropping Systems on Black Soils of Northeast China. Sci. Rep. 2020, 10, 265. [Google Scholar] [CrossRef]
- Rawls, W.J.; Pachepsky, Y.A.; Ritchie, J.C.; Sobecki, T.M.; Bloodworth, H. Effect of Soil Organic Carbon on Soil Water Retention. Geoderma 2003, 116, 61–76. [Google Scholar] [CrossRef]
- Benbi, D.K.; Sharma, S.; Toor, A.S.; Brar, K.; Sodhi, G.P.S.; Garg, A.K. Differences in Soil Organic Carbon Pools and Biological Activity between Organic and Conventionally Managed Rice-Wheat Fields. Org. Agric. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon Sequestration in Soil. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar] [CrossRef]
- Godde, C.M.; Thorburn, P.J.; Biggs, J.S.; Meier, E.A. Understanding the Impacts of Soil, Climate, and Farming Practices on Soil Organic Carbon Sequestration: A Simulation Study in Australia. Front. Plant Sci. 2016, 7, 661. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Sun, O.J. Soil Carbon Change and Its Responses to Agricultural Practices in Australian Agro-Ecosystems: A Review and Synthesis. Geoderma 2010, 155, 211–223. [Google Scholar] [CrossRef]
- Mandal, A.; Patra, A.K.; Singh, D.; Swarup, A.; Ebhin Masto, R. Effect of Long-Term Application of Manure and Fertilizer on Biological and Biochemical Activities in Soil during Crop Development Stages. Bioresour. Technol. 2007, 98, 3585–3592. [Google Scholar] [CrossRef]
- Page, K.L.; Dang, Y.P.; Dalal, R.C. The Ability of Conservation Agriculture to Conserve Soil Organic Carbon and the Subsequent Impact on Soil Physical, Chemical, and Biological Properties and Yield. Front. Sustain. Food Syst. 2020, 4, 31. [Google Scholar] [CrossRef]
- Iqbal, R.; Raza, M.A.S.; Valipour, M.; Saleem, M.F.; Zaheer, M.S.; Ahmad, S.; Toleikiene, M.; Haider, I.; Aslam, M.U.; Nazar, M.A. Potential Agricultural and Environmental Benefits of Mulches—A Review. Bull. Natl. Res. Cent. 2020, 44, 75. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Basit, A.; Mohamed, H.I.; Ali, I.; Ullah, S.; Kamel, E.A.R.; Shalaby, T.A.; Ramadan, K.M.A.; Alkhateeb, A.A.; Ghazzawy, H.S. Mulching as a Sustainable Water and Soil Saving Practice in Agriculture: A Review. Agronomy 2022, 12, 1881. [Google Scholar] [CrossRef]
- Russel, J.C. The Effect of Surface Cover on Soil Moisture Losses by Evaporation. Soil. Sci. Soc. Am. J. 1940, 4, 65–70. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Viscarra Rossel, R.A. Can the Sequestered Carbon in Agricultural Soil Be Maintained with Changes in Management, Temperature and Rainfall? A Sensitivity Assessment. Geoderma 2016, 268, 22–28. [Google Scholar] [CrossRef]
- Potter, K.N.; Velazquez-Garcia, J.; Scopel, E.; Torbert, H.A. Residue Removal and Climatic Effects on Soil Carbon Content of No-till Soils. J. Soil. Water Conserv. 2007, 62, 110–114. [Google Scholar]
- Zhao, G.; Bryan, B.A.; King, D.; Luo, Z.; Wang, E.; Song, X.; Yu, Q. Impact of Agricultural Management Practices on Soil Organic Carbon: Simulation of Australian Wheat Systems. Glob. Change Biol. 2013, 19, 1585–1597. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial Contributions to Climate Change through Carbon Cycle Feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing Uncertainty in Soil Carbon Feedbacks to Climate Change. Nat. Clim. Change 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-Term Sensitivity of Soil Carbon Turnover to Warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- van Gestel, N.; Shi, Z.; van Groenigen, K.J.; Osenberg, C.W.; Andresen, L.C.; Dukes, J.S.; Hovenden, M.J.; Luo, Y.; Michelsen, A.; Pendall, E.; et al. Predicting Soil Carbon Loss with Warming. Nature 2018, 554, E4–E5. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.; Taylor, J.A. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Lefèvre, R.; Barré, P.; Moyano, F.E.; Christensen, B.T.; Bardoux, G.; Eglin, T.; Girardin, C.; Houot, S.; Kätterer, T.; van Oort, F.; et al. Higher Temperature Sensitivity for Stable than for Labile Soil Organic Carbon—Evidence from Incubations of Long-Term Bare Fallow Soils. Glob. Change Biol. 2014, 20, 633–640. [Google Scholar] [CrossRef]
- Rustad, L.; Campbell, J.; Marion, G.; Norby, R.; Mitchell, M.; Hartley, A.; Cornelissen, J.; Gurevitch, J. GCTE-NEWS A Meta-Analysis of the Response of Soil Respiration, Net Nitrogen Mineralization, and Aboveground Plant Growth to Experimental Ecosystem Warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Piao, S.; Janssens, I.A.; Tang, J.; Liu, W.; Chi, Y.; Wang, J.; Xu, S. Soil Respiration under Climate Warming: Differential Response of Heterotrophic and Autotrophic Respiration. Glob. Change Biol. 2014, 20, 3229–3237. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Zhang, Q.; Cheng, X. Soil Carbon Dynamics Following Land-Use Change Varied with Temperature and Precipitation Gradients: Evidence from Stable Isotopes. Glob. Change Biol. 2015, 21, 2762–2772. [Google Scholar] [CrossRef]
- Piao, S.; Friedlingstein, P.; Ciais, P.; Zhou, L.; Chen, A. Effect of Climate and CO2 Changes on the Greening of the Northern Hemisphere over the Past Two Decades. Geophys. Res. Lett. 2006, 33, L23402. [Google Scholar] [CrossRef]
- Liu, J.; Wennberg, P.O.; Parazoo, N.C.; Yin, Y.; Frankenberg, C. Observational Constraints on the Response of High-Latitude Northern Forests to Warming. AGU Adv. 2020, 1, e2020AV000228. [Google Scholar] [CrossRef]
- Keenan, T.F.; Riley, W.J. Greening of the Land Surface in the World’s Cold Regions Consistent with Recent Warming. Nat. Clim. Change 2018, 8, 825–828. [Google Scholar] [CrossRef]
- Luo, Y. Terrestrial Carbon–Cycle Feedback to Climate Warming. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 683–712. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Decomposition and Ecosystem Carbon Budgets. In Principles of Terrestrial Ecosystem Ecology; Chapin, F.S., Matson, P.A., Vitousek, P.M., Eds.; Springer: New York, NY, USA, 2011; pp. 183–228. ISBN 978-1-4419-9504-9. [Google Scholar]
- Robertson, G.P.; Paul, E.A. Decomposition and Soil Organic Matter Dynamics. In Methods in Ecosystem Science; Sala, O.E., Jackson, R.B., Mooney, H.A., Howarth, R.W., Eds.; Springer: New York, NY, USA, 2000; pp. 104–116. ISBN 978-1-4612-1224-9. [Google Scholar]
- Jia, Y.; Kuzyakov, Y.; Wang, G.; Tan, W.; Zhu, B.; Feng, X. Temperature Sensitivity of Decomposition of Soil Organic Matter Fractions Increases with Their Turnover Time. Land. Degrad. Dev. 2020, 31, 632–645. [Google Scholar] [CrossRef]
- Dolschak, K.; Gartner, K.; Berger, T.W. The Impact of Rising Temperatures on Water Balance and Phenology of European Beech (Fagus sylvatica L.) Stands. Model. Earth Syst. Environ. 2019, 5, 1347–1363. [Google Scholar] [CrossRef] [PubMed]
- Licht, M.A.; Al-Kaisi, M. Strip-Tillage Effect on Seedbed Soil Temperature and Other Soil Physical Properties. Soil Tillage Res. 2005, 80, 233–249. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H. Thermal Properties of Soils as Affected by Density and Water Content. Biosyst. Engin. 2003, 86, 97–102. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root Biomass and Exudates Link Plant Diversity with Soil Bacterial and Fungal Biomass. Sci. Rep. 2017, 7, 44641. [Google Scholar] [CrossRef]
- Yin, H.; Li, Y.; Xiao, J.; Xu, Z.; Cheng, X.; Liu, Q. Enhanced Root Exudation Stimulates Soil Nitrogen Transformations in a Subalpine Coniferous Forest under Experimental Warming. Glob. Change Biol. 2013, 19, 2158–2167. [Google Scholar] [CrossRef]
- Carlyle, C.N.; Fraser, L.H.; Turkington, R. Tracking Soil Temperature and Moisture in a Multi-Factor Climate Experiment in Temperate Grassland: Do Climate Manipulation Methods Produce Their Intended Effects? Ecosystems 2011, 14, 489–502. [Google Scholar] [CrossRef]
- Bell, T.H.; Klironomos, J.N.; Henry, H.A.L. Seasonal Responses of Extracellular Enzyme Activity and Microbial Biomass to Warming and Nitrogen Addition. Soil Sci. Soc. Am. J. 2010, 74, 820–828. [Google Scholar] [CrossRef]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature Effects on Soil Organic Carbon, Soil Labile Organic Carbon Fractions, and Soil Enzyme Activities under Long-Term Fertilization Regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Wang, Y.; Xu, Z.; Han, H.; Li, L.; Wan, S. Warming and Increased Precipitation Have Differential Effects on Soil Extracellular Enzyme Activities in a Temperate Grassland. Sci. Total Environ. 2013, 444, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Allison, S.D.; Xia, J.; Liu, L.; Wan, S. Precipitation Regime Drives Warming Responses of Microbial Biomass and Activity in Temperate Steppe Soils. Biol. Fertil. Soils 2016, 52, 469–477. [Google Scholar] [CrossRef]
- Yamada, H.; Miller, J.; Stockton, J. Desiccated Grass Mulch Increases Irrigation Efficiency for Cotton. Hilgardia 1963, 17, 12–13. [Google Scholar]
- Adekalu, K.O.; Olorunfemi, I.A.; Osunbitan, J.A. Grass Mulching Effect on Infiltration, Surface Runoff and Soil Loss of Three Agricultural Soils in Nigeria. Bioresour. Technol. 2007, 98, 912–917. [Google Scholar] [CrossRef]
- Lauer, S.; Sanderson, M.R.; Manning, D.T.; Suter, J.F.; Hrozencik, R.A.; Guerrero, B.; Golden, B. Values and Groundwater Management in the Ogallala Aquifer Region. J. Soil Water Conserv. 2018, 73, 593–600. [Google Scholar] [CrossRef]
- NOAA Lubbock 1991–2020 Climate Normals. Available online: https://www.weather.gov/lub/climate-klbb-norm-2020 (accessed on 25 July 2024).
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Nunan, N.; Morgan, M.A.; Herlihy, M. Ultraviolet Absorbance (280 Nm) of Compounds Released from Soil during Chloroform Fumigation as an Estimate of the Microbial Biomass. Soil Biol. Biochem. 1998, 30, 1599–1603. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The Fumigation-Extraction Method to Estimate Soil Microbial Biomass: Calibration of the kEN Value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- R Core Team. R: The R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; SAGE Publications: New Delhi, India, 2018; ISBN 978-1-5443-3648-0. [Google Scholar]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Hollister, R.D.; Elphinstone, C.; Henry, G.H.R.; Bjorkman, A.D.; Klanderud, K.; Björk, R.G.; Björkman, M.P.; Bokhorst, S.; Carbognani, M.; Cooper, E.J.; et al. A Review of Open Top Chamber (OTC) Performance across the ITEX Network. Arct. Sci. 2023, 9, 331–344. [Google Scholar] [CrossRef]
- Welshofer, K.B.; Zarnetske, P.L.; Lany, N.K.; Thompson, L.A.E. Open-Top Chambers for Temperature Manipulation in Taller-Stature Plant Communities. Methods Ecol. Evol. 2018, 9, 254–259. [Google Scholar] [CrossRef]
- Hollister, R.D.; Webber, P.J.; Nelson, F.E.; Tweedie, C.E. Soil Thaw and Temperature Response to Air Warming Varies by Plant Community: Results from an Open-Top Chamber Experiment in Northern Alaska. Arct. Antarct. Alp. Res. 2006, 38, 206–215. [Google Scholar] [CrossRef]
- León-Sánchez, L.; Nicolás, E.; Goberna, M.; Prieto, I.; Maestre, F.T.; Querejeta, J.I. Poor Plant Performance under Simulated Climate Change Is Linked to Mycorrhizal Responses in a Semi-Arid Shrubland. J. Ecol. 2018, 106, 960–976. [Google Scholar] [CrossRef]
- Escolar, C.; Martínez, I.; Bowker, M.A.; Maestre, F.T. Warming Reduces the Growth and Diversity of Biological Soil Crusts in a Semi-Arid Environment: Implications for Ecosystem Structure and Functioning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3087–3099. [Google Scholar] [CrossRef]
- Turmel, M.-S.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaerts, B. Crop Residue Management and Soil Health: A Systems Analysis. Agric. Syst. 2015, 134, 6–16. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Crop Residue Removal Impacts on Soil Productivity and Environmental Quality. Crit. Rev. Plant Sci. 2009, 28, 139–163. [Google Scholar] [CrossRef]
- Greiser, C.; Hederová, L.; Vico, G.; Wild, J.; Macek, M.; Kopecký, M. Higher Soil Moisture Increases Microclimate Temperature Buffering in Temperate Broadleaf Forests. Agric. For. Meteorol. 2024, 345, 109828. [Google Scholar] [CrossRef]
- Irmak, S. Impacts of Extreme Heat Stress and Increased Soil Temperature on Plant Growth and Development. CropWatch, 21 June 2016. [Google Scholar]
- Ma, L.; Shao, M.; Li, T. Characteristics of Soil Moisture and Evaporation under the Activities of Earthworms in Typical Anthrosols in China. Sustainability 2020, 12, 6603. [Google Scholar] [CrossRef]
- Qin, S.; Chen, L.; Fang, K.; Zhang, Q.; Wang, J.; Liu, F.; Yu, J.; Yang, Y. Temperature Sensitivity of SOM Decomposition Governed by Aggregate Protection and Microbial Communities. Sci. Adv. 2019, 5, eaau1218. [Google Scholar] [CrossRef]
- Li, Y.; Lv, W.; Jiang, L.; Zhang, L.; Wang, S.; Wang, Q.; Xue, K.; Li, B.; Liu, P.; Hong, H.; et al. Microbial Community Responses Reduce Soil Carbon Loss in Tibetan Alpine Grasslands under Short-Term Warming. Glob. Change Biol. 2019, 25, 3438–3449. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Thomson, A. Temperature-Associated Increases in the Global Soil Respiration Record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, Y.; Tissue, D.T.; Bruna, S.; Maier, C.; Dijkstra, F.A. Drought Impacts on Tree Root Traits Are Linked to Their Decomposability and Net Carbon Release. Front. For. Glob. Change 2022, 5, 836062. [Google Scholar] [CrossRef]
- Ma, W.; Tang, S.; Dengzeng, Z.; Zhang, D.; Zhang, T.; Ma, X. Root Exudates Contribute to Belowground Ecosystem Hotspots: A Review. Front. Microbiol. 2022, 13, 937940. [Google Scholar] [CrossRef]
- Shen, X.; Yang, F.; Xiao, C.; Zhou, Y. Increased Contribution of Root Exudates to Soil Carbon Input during Grassland Degradation. Soil. Biol. Biochem. 2020, 146, 107817. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon Flow in the Rhizosphere: Carbon Trading at the Soil–Root Interface. Plant Soil. 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Schimel, J.P. Life in Dry Soils: Effects of Drought on Soil Microbial Communities and Processes. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial Stress-Response Physiology and Its Implications for Ecosystem Function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- van Gestel, N.C.; Dhungana, N.; Tissue, D.T.; Zak, J.C. Seasonal Microbial and Nutrient Responses during a 5-Year Reduction in the Daily Temperature Range of Soil in a Chihuahuan Desert Ecosystem. Oecologia 2016, 180, 265–277. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M.; et al. Temperature and Soil Organic Matter Decomposition Rates—Synthesis of Current Knowledge and a Way Forward. Glob. Change Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Chen, J.; Elsgaard, L.; van Groenigen, K.J.; Olesen, J.E.; Liang, Z.; Jiang, Y.; Lærke, P.E.; Zhang, Y.; Luo, Y.; Hungate, B.A.; et al. Soil Carbon Loss with Warming: New Evidence from Carbon-Degrading Enzymes. Glob. Change Biol. 2020, 26, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-Carbon Response to Warming Dependent on Microbial Physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Tajik, S.; Ayoubi, S.; Zeraatpisheh, M. Digital Mapping of Soil Organic Carbon Using Ensemble Learning Model in Mollisols of Hyrcanian Forests, Northern Iran. Geoderma Regional. 2020, 20, e00256. [Google Scholar] [CrossRef]
- Schnecker, J.; Borken, W.; Schindlbacher, A.; Wanek, W. Little Effects on Soil Organic Matter Chemistry of Density Fractions after Seven Years of Forest Soil Warming. Soil. Biol. Biochem. 2016, 103, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Hamido, S.; Guertal, E.; Wood, W. Seasonal Variation of Carbon and Nitrogen Emissions from Turfgrass. Am. J. Clim. Chang. 2016, 5, 448–463. [Google Scholar] [CrossRef]
- Winsome, T.; Silva, L.C.R.; Scow, K.M.; Doane, T.A.; Powers, R.F.; Horwath, W.R. Plant-Microbe Interactions Regulate Carbon and Nitrogen Accumulation in Forest Soils. For. Ecol. Manag. 2017, 384, 415–423. [Google Scholar] [CrossRef]
- Córdova, S.C.; Olk, D.C.; Dietzel, R.N.; Mueller, K.E.; Archontouilis, S.V.; Castellano, M.J. Plant Litter Quality Affects the Accumulation Rate, Composition, and Stability of Mineral-Associated Soil Organic Matter. Soil. Biol. Biochem. 2018, 125, 115–124. [Google Scholar] [CrossRef]
- Ågren, G.I.; Bosatta, N. Theoretical Analysis of the Long-Term Dynamics of Carbon and Nitrogen in Soils. Ecology 1987, 68, 1181–1189. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon Use Efficiency of Microbial Communities: Stoichiometry, Methodology and Modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef]
- Li, N.; Lin, H.; Wang, T.; Li, Y.; Liu, Y.; Chen, X.; Hu, X. Impact of Climate Change on Cotton Growth and Yields in Xinjiang, China. Field Crops Res. 2020, 247, 107590. [Google Scholar] [CrossRef]
- Pettigrew, W.T. The Effect of Higher Temperatures on Cotton Lint Yield Production and Fiber Quality. Crop Sci. 2008, 48, 278–285. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature Extremes: Effect on Plant Growth and Development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Arshad, A.; Raza, M.A.; Zhang, Y.; Zhang, L.; Wang, X.; Ahmed, M.; Habib-ur-Rehman, M. Impact of Climate Warming on Cotton Growth and Yields in China and Pakistan: A Regional Perspective. Agriculture 2021, 11, 97. [Google Scholar] [CrossRef]
- Reddy, K.R.; Davidonis, G.H.; Johnson, A.S.; Vinyard, B.T. Temperature Regime and Carbon Dioxide Enrichment Alter Cotton Boll Development and Fiber Properties. Agron. J. 1999, 91, 851–858. [Google Scholar] [CrossRef]
- Bibi, A.; Oosterhuis, D.; Gonias, E. Molecular Biology and Physiology Photosynthesis, Quantum Yield of Photosystem Ii and Membrane Leakage as Affected by High Temperatures in Cotton Genotypes. J. Cotton Sci. 2008, 12, 150–159. [Google Scholar]
- DeLaune, P.B.; Mubvumba, P.; Ale, S.; Kimura, E. Impact of No-till, Cover Crop, and Irrigation on Cotton Yield. Agric. Water Manag. 2020, 232, 106038. [Google Scholar] [CrossRef]
- Ale, S.; Himanshu, S.K.; Mauget, S.A.; Hudson, D.; Goebel, T.S.; Liu, B.; Baumhardt, R.L.; Bordovsky, J.P.; Brauer, D.K.; Lascano, R.J.; et al. Simulated Dryland Cotton Yield Response to Selected Scenario Factors Associated With Soil Health. Front. Sustain. Food Syst. 2021, 4, 617509. [Google Scholar] [CrossRef]
- Broughton, K.J.; Bange, M.P.; Baker, J.T.; Yates, C.; Tan, D.K.Y.; Tissue, D.T.; Payton, P. Effects of Elevated CO2 and Warmer Temperature on Early Season Field-Grown Cotton in High-Input Systems. Crop Sci. 2021, 61, 657–671. [Google Scholar] [CrossRef]
- Wang, J.; Du, G.; Tian, J.; Jiang, C.; Zhang, Y.; Zhang, W. Mulched Drip Irrigation Increases Cotton Yield and Water Use Efficiency via Improving Fine Root Plasticity. Agric. Water Manag. 2021, 255, 106992. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. Ratios of Microbial Biomass Carbon to Total Organic Carbon in Arable Soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devkota, P.; Singh, R.K.; Smith, N.G.; Slaughter, L.C.; van Gestel, N. Residue Addition Can Mitigate Soil Health Challenges with Climate Change in Drylands: Insights from a Field Warming Experiment in Semi-Arid Texas. Soil Syst. 2024, 8, 102. https://doi.org/10.3390/soilsystems8040102
Devkota P, Singh RK, Smith NG, Slaughter LC, van Gestel N. Residue Addition Can Mitigate Soil Health Challenges with Climate Change in Drylands: Insights from a Field Warming Experiment in Semi-Arid Texas. Soil Systems. 2024; 8(4):102. https://doi.org/10.3390/soilsystems8040102
Chicago/Turabian StyleDevkota, Pawan, Rakesh K. Singh, Nicholas G. Smith, Lindsey C. Slaughter, and Natasja van Gestel. 2024. "Residue Addition Can Mitigate Soil Health Challenges with Climate Change in Drylands: Insights from a Field Warming Experiment in Semi-Arid Texas" Soil Systems 8, no. 4: 102. https://doi.org/10.3390/soilsystems8040102





