Cowpea (Vigna unguiculata) Water Relations, Growth, and Productivity as Affected by Salinity in Two Soils with Contrasting Mineralogies
Abstract
1. Introduction
2. Materials and Methods
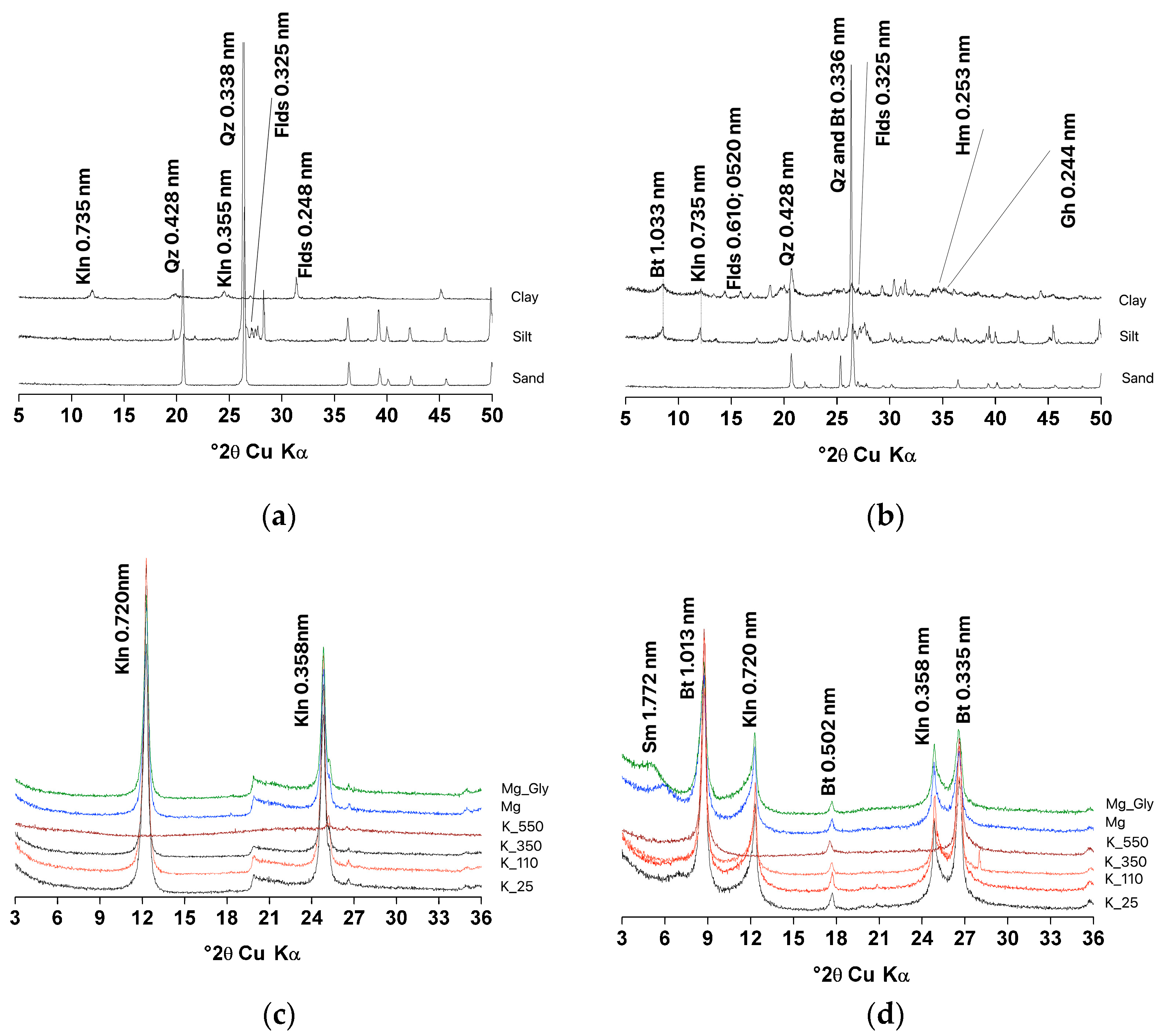
2.1. Soil Collection and Characterization
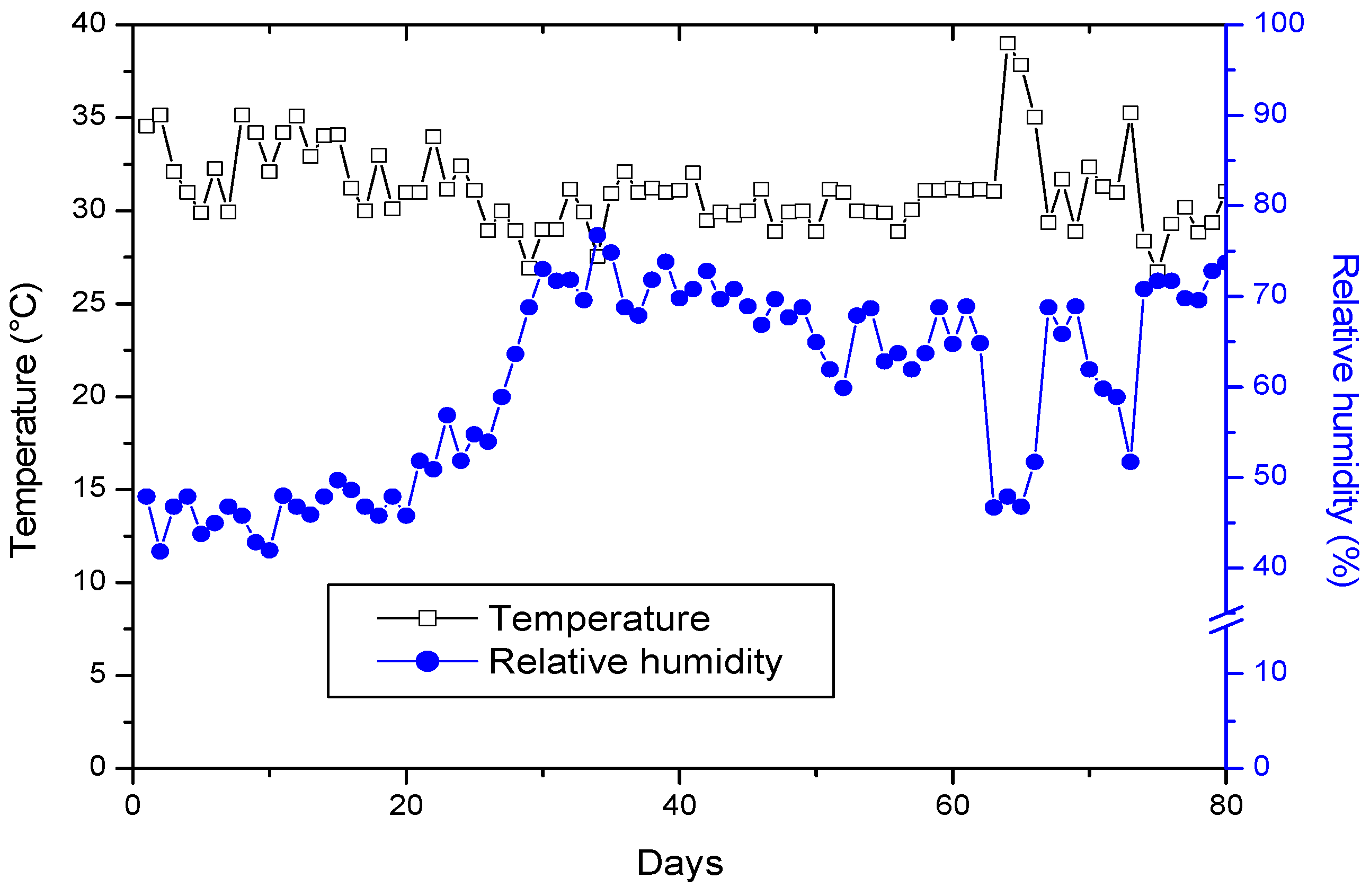
2.2. Experimental Site and Plant Material
2.3. Irrigation Management
2.4. Plant Growth and Yield
2.5. Plant Water Potential (Ψw) and Leaf Osmotic Potential (Ψs)
2.6. Osmotic Adjustment (OA)
2.7. Electrolyte Leakage (EL)
2.8. Relative Water Content (RWC)
2.9. Soil CO2 Efflux
2.10. Statistical Analyses
3. Results
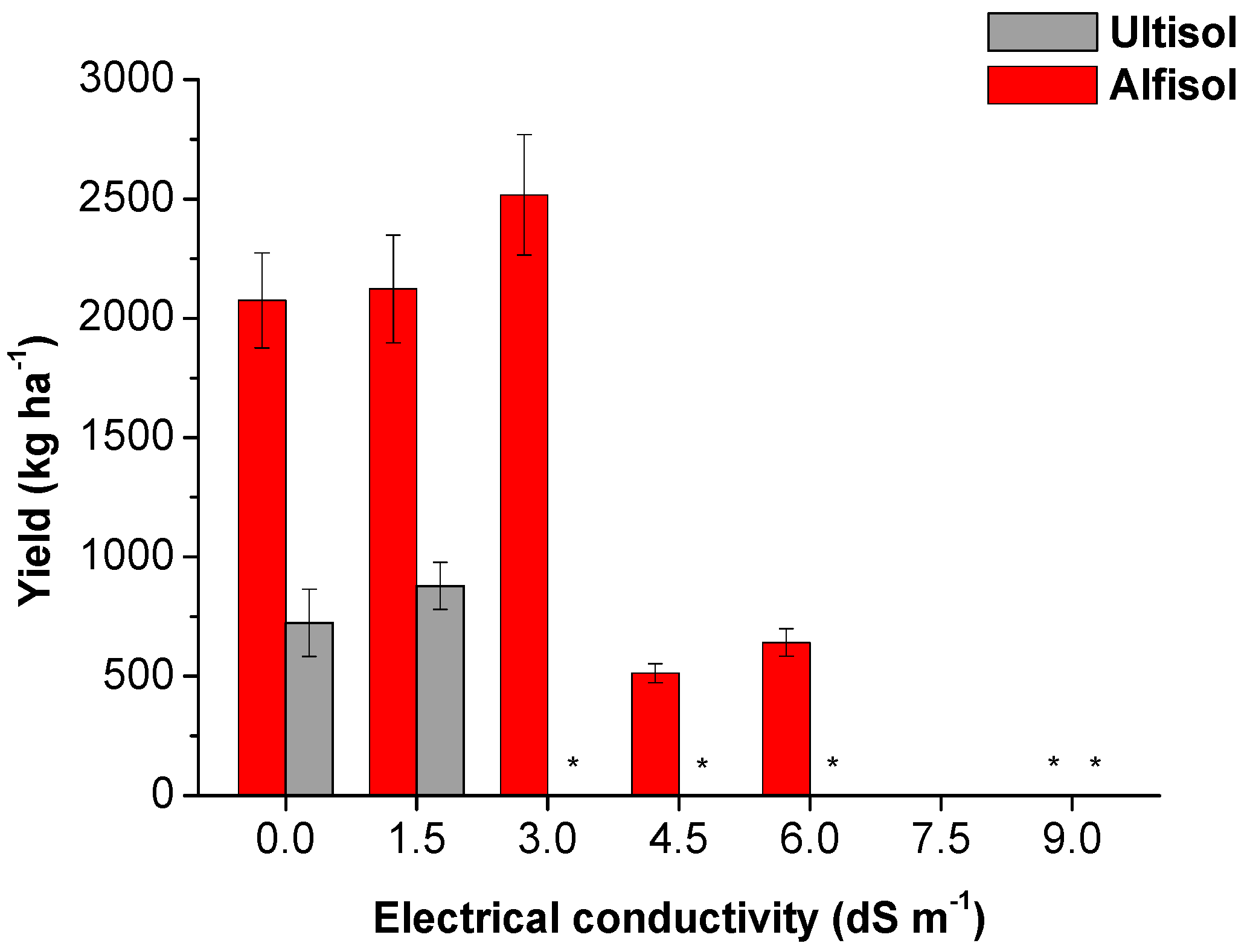
3.1. Biometrics and Produce
3.2. Physiological Variables
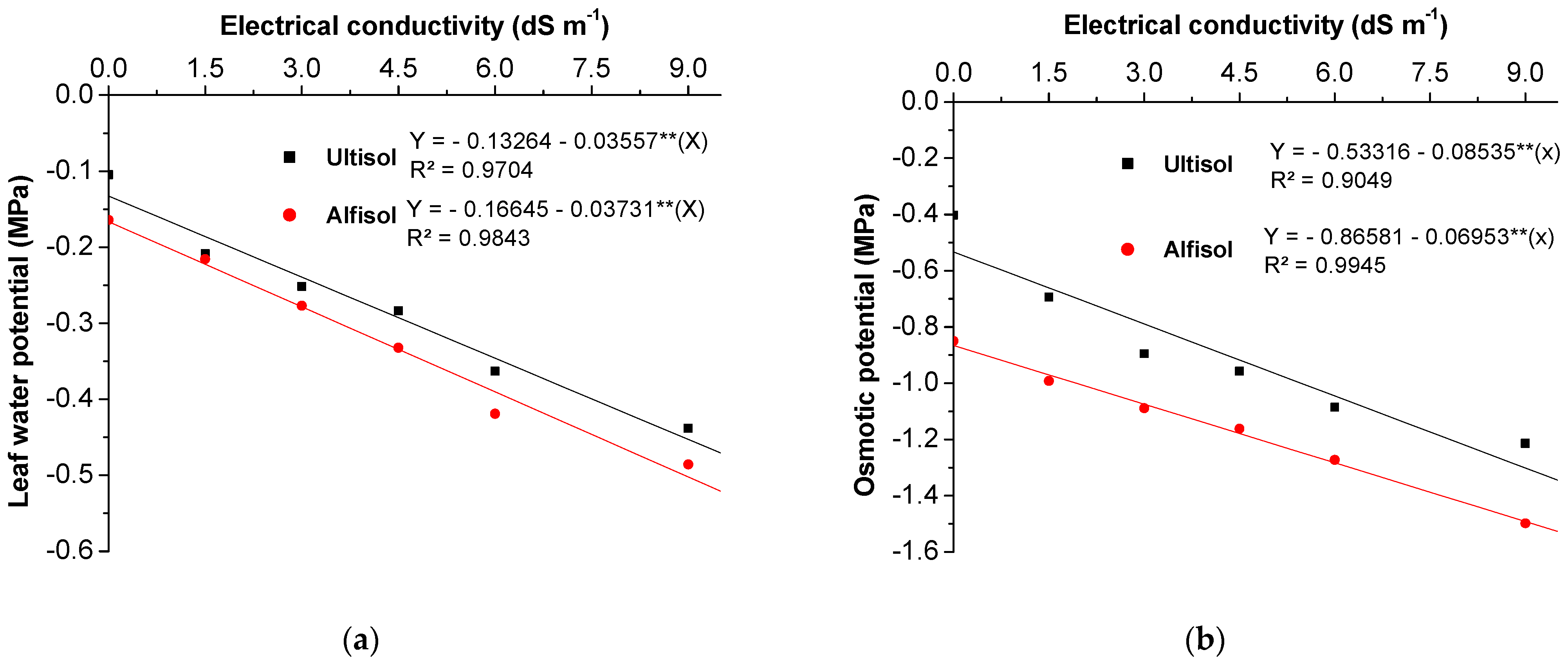
3.2.1. Water Relations
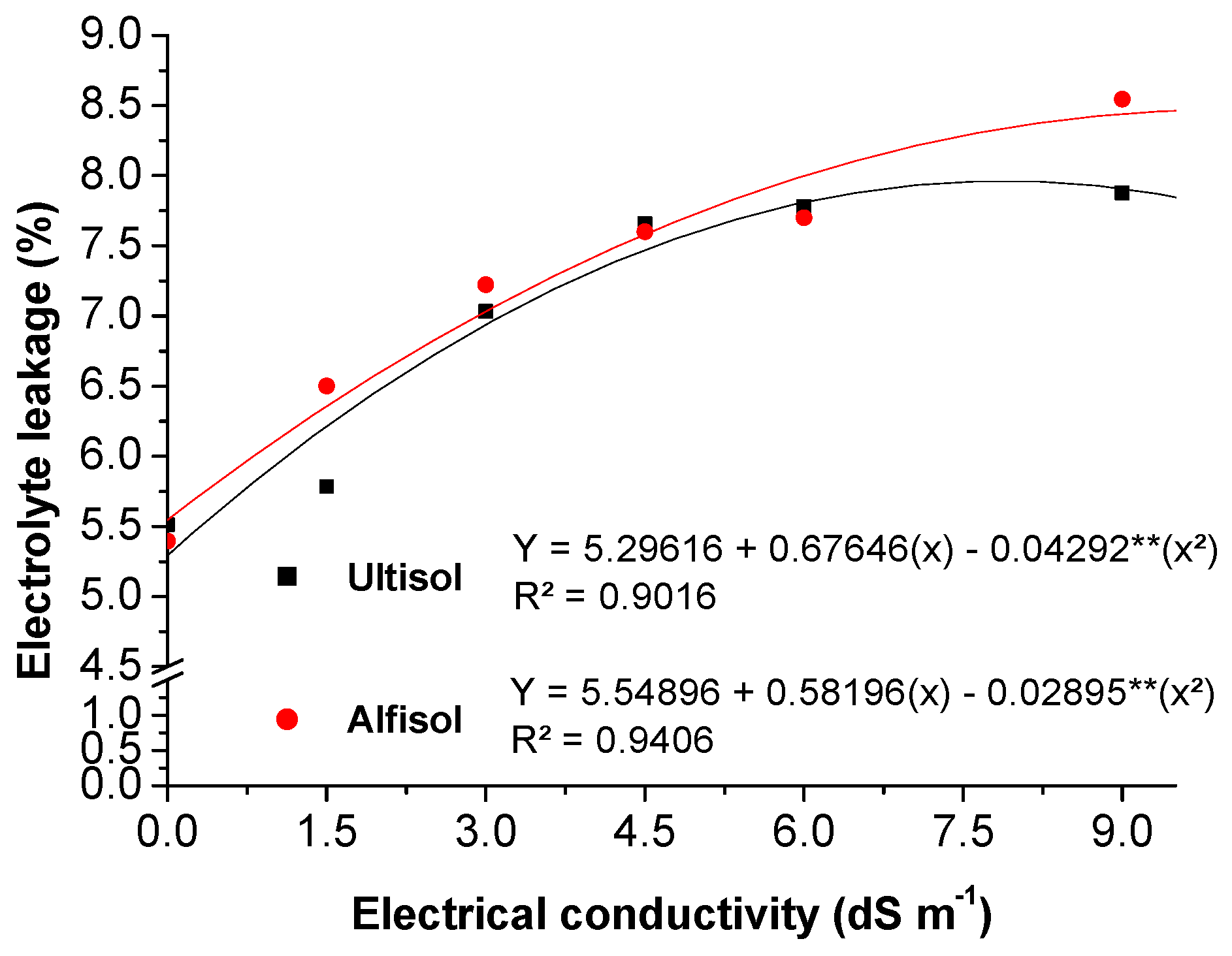
3.2.2. Electrolyte Leakage
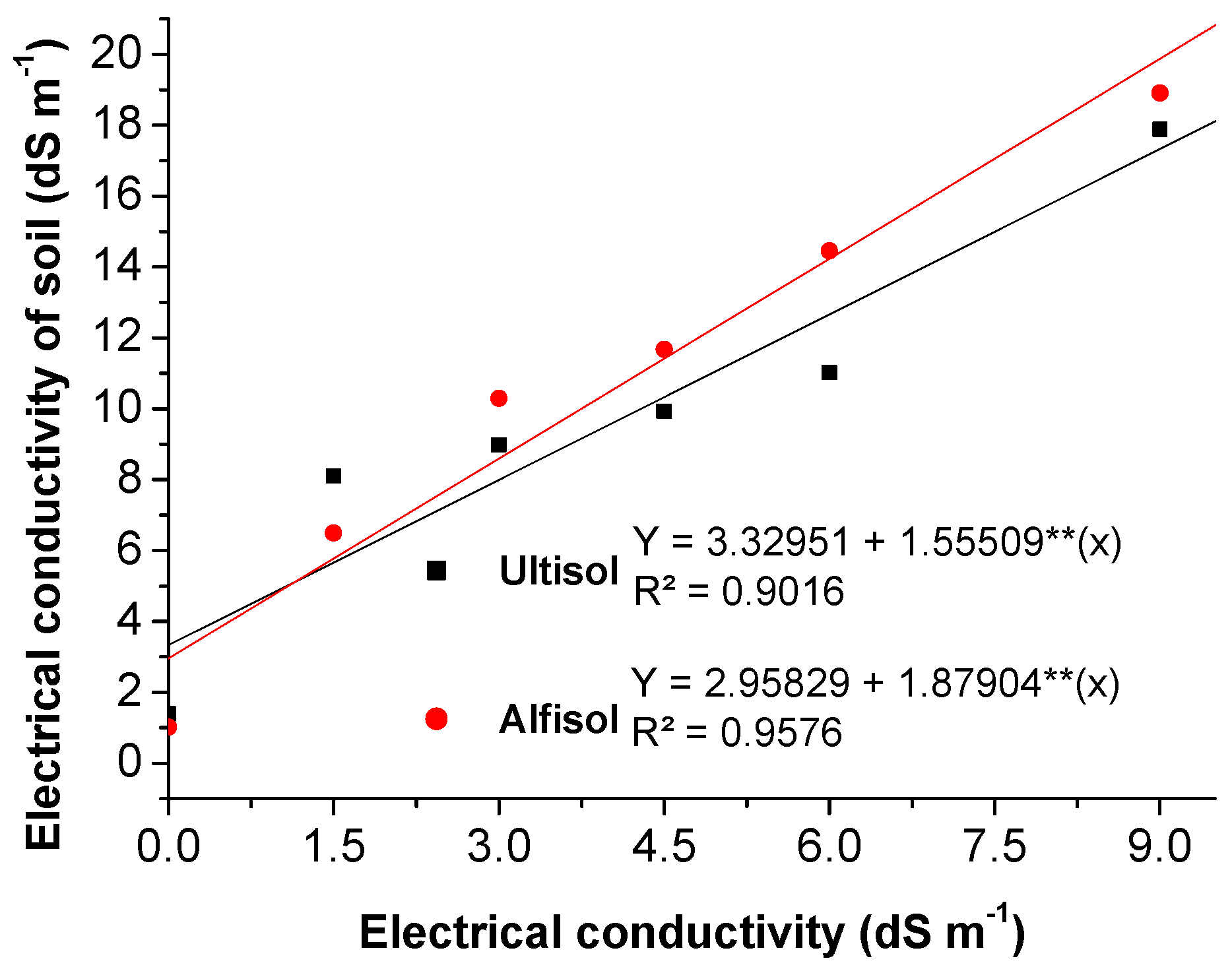
3.2.3. Final Electrical Conductivity
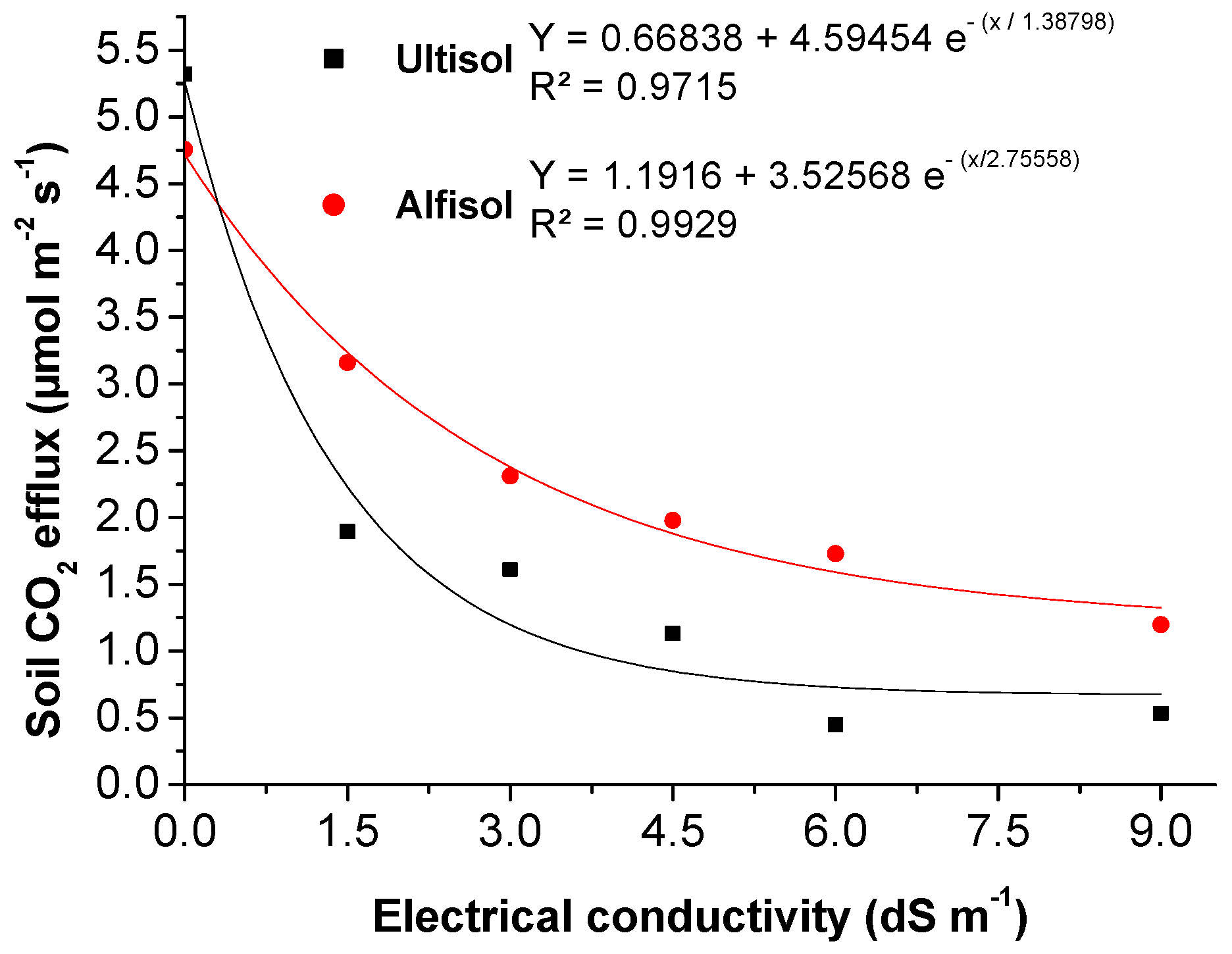
3.2.4. Soil CO2 Efflux
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Bd | Bulk density |
| CS | Coarse sand |
| DCW | Dispersed clay in water |
| DD | Dispersion degree |
| DMAP | Dry mass of the aerial portion of the plant |
| DMR | Dry mass of the roots |
| CEC | Effective cation exchange capacity |
| EC | Electrical conductivity |
| EL | Electrolyte leakage |
| FS | Fine sand |
| DF | Flocculation degree |
| FC | Free conductivity |
| H | Plant height |
| IRGA | Infrared gas analyzer |
| OA | Osmotic adjustment |
| Pd | Particle density |
| ROS | Reactive oxygen species |
| RWC | Relative water content |
| SD | Stem diameter |
| TC | Total conductivity |
References
- Zyada, H.G.; Ibraheim, S.K.A.; El-Saadony, F.M.; Mohaseb, M.I. Response of growth and productivity of cowpea plants to nano mixture of micronutrients under different potassium sulphate fertilizer rates. J. Bio-Agric. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). Mapping of Salt-Affected Soils-Technical Specifications and Country Guidelines; FAO: Roma, Italy, 2020. [Google Scholar]
- Ribeiro, M.R.; Ribeiro Filho, M.R.; Jacomine, P.K.T. Origem e classificação dos solos afetados por sais. In Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados, 2nd ed.; Ghey, H.R., Dias, N.S., Lacerda, C.F., Gomes Filho, E., Eds.; INCTSal: Fortaleza, Brazil, 2016; pp. 9–15. [Google Scholar]
- Castro, F.C.; Santos, A.M.D. Salinidade do solo e risco de desertificação na região semiárida. Mercator 2020, 19, e19002. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Souza, P.J.D.O.P.D.; Ferreira, D.P.; Sousa, D.D.P.; Nunes, H.G.G.C.; Barbosa, A.V.C. Trocas gasosas do feijão-caupi cultivado no Nordeste Paraense em resposta à deficiência hídrica forçada durante a fase reprodutiva. Rev. Bras. Meteorol. 2020, 35, 13–22. [Google Scholar] [CrossRef]
- Melo, H.F.D.; Souza, E.R.D.; Dourado, P.R.M.; Lins, C.M.T.; Santos, H.R.B.; Monteiro, D.R.; Paulino, M.K.S.S.; de Almeida, B.G.; Santos, M.A.D. Comparison of water and osmotic potentials on Vigna unguiculata stress response. Rev. Bras. Cienc. Solo 2020, 44, e0200070. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Corn and soybean yield response to salinity influenced by soil texture. Agron. J. 2018, 110, 1243–1253. [Google Scholar] [CrossRef]
- Rodrigues, R.A.S. Ciência do Solo: Morfologia e Gênese; Editora e Distribuidora Educacional S.A.: Londrina, Brazil, 2018; p. 264. [Google Scholar]
- Gomes, A.M.F.; Draper, D.; Nhantumbo, N.; Massinga, R.; Ramalho, J.C.; Marques, I.; Ribeiro-Barros, A.I. Diversity of cowpea [Vigna unguiculata (L.) walp] landraces in Mozambique: New opportunities for crop improvement and future breeding programs. Agronomy 2021, 11, 991. [Google Scholar] [CrossRef]
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.; António, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, e00586. [Google Scholar] [CrossRef]
- Dourado, P.R.M.; de Souza, E.R.; Lins, C.M.T.; de Melo, H.F.; Santos, H.R.B.; Monteiro, D.R.; Paulino, M.K.S.S.; de Carvalho Leal, L.Y. Osmotic adjustment in cowpea plants: Interference of methods for estimating osmotic potential at full turgor. Plant Physiol. Biochem. 2019, 145, 114–119. [Google Scholar] [CrossRef]
- Köppen, W. Climatologia: Con un Estudio de los Climas de la Tierra; Fondo de Cultura Econômica: Ciudad de México, Mexico, 1948; p. 479. [Google Scholar]
- INMET. Instituto Nacional de Meteorologia. Clima. Available online: https://clima.inmet.gov.br/ (accessed on 14 March 2025).
- APAC. Agência Pernambucana de Águas e Clima. Precipitação. Available online: https://www.apac.pe.gov.br/ (accessed on 14 March 2025).
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embapa: Brasília, Brazil, 2017; p. 574. [Google Scholar]
- Jackson, M.L. Specific surface determinations for soil. In Soil Chemical Analysis: Advanced Course, 2nd ed.; United Book Prints: New Delhi, India, 2014; pp. 305–360. [Google Scholar]
- Brown, G.; Brindley, G.W. X-ray Diffraction Procedures for Clay Mineral Identification. In Crystal Structures of Clay Minerals and their X-Ray Identification: The Mineralogical Society of Great Britain and Ireland; Brindley, G.W., Brown, G., Eds.; Mineralogical Society: London, UK, 1980; Volume 5, pp. 305–360. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-Ray Diffraction and Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989; p. 352. [Google Scholar]
- Ribeiro, F.E.; Del Peloso, M.J.; Barbosa, F.R.; Gonzaga, A.D.O.; De Oliveira, L.F.C. Recomendações Técnicas Para o Cultivo do Feijoeiro-Comum (Phaseolus vulgaris L.) nas Regiões Norte e Nordeste do Brasil. Circular Técnico 89. EMBRAPA, 2011. 61p. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/916406/1/CIRCTECNICA89.pdf (accessed on 14 March 2025).
- Oliveira, F.M.D.; Farias, S.A.; Ceballos, B.S.D.; Azevedo, C.A.D.; Gheyi, H.R.; Oliveira, A.K.D. Diagnóstico de qualidade das águas da microbacia do riacho Angico, para fins de irrigação. Rev. Bras. Eng. Agric. Amb. 2005, 9, 221–225. [Google Scholar] [CrossRef]
- Kirkham, M.B. Principles of Soil and Plant Water Relations, 1st ed.; Elsevier Academic Press Publications: Amsterdã, The Netherlands, 2004; p. 520. [Google Scholar] [CrossRef]
- Hillel, D. Soil in the Environment: Crucible of Terrestrial Life, 1st ed.; Elsevier Academic Press Publications: Burlington, VT, USA, 2007; p. 320. [Google Scholar] [CrossRef]
- Souza, E.R.; Freire, M.B.G.S.; Cunha, K.P.V.; Nascimento, C.W.A.; Ruiz, H.A.; Lins, C.T. Biomass, anatomical changes and osmotic potential in Atriplex nummularia Lindl. cultivated in sodic saline soil under water stress. Environ. Exp. Bot. 2012, 82, 20–27. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci. 1989, 29, 230–233. [Google Scholar] [CrossRef]
- Vasquez-Tello, A.; Zuily-Fodil, Y.; Thi, A.P.; da Silva, J.V. Electrolyte and Pi leakages and soluble sugar content as physiological tests for screening resistance to water stress in Phaseolus and Vigna species. J. Exp. Bot. 1990, 41, 827–832. [Google Scholar] [CrossRef]
- Szogi, A.A.; Padilla, J.T.; Shumaker, P.D. Effect of soil pH and mineralogy on the sorption and desorption of phosphite and phosphate in Ultisol of the Southeastern Coastal Plain. Soil Sci. Soc. Am. J. 2024, 88, 1248–1258. [Google Scholar] [CrossRef]
- Doaa, M.; Ashmawi, A.E. Effect of Feldspar, compost and biochar on cultivating Cowpea (Vigna unguiculata ssp. unguiculata) plant and soil sandy clay loam properties. Asian Soil Res. J. 2022, 6, 42–57. [Google Scholar] [CrossRef]
- USDA—Soil Survey Staff. Soil Taxononmy—A Basic System of Soil Classification for Making and Interpreting Soil Survey, 2nd ed.; USDA: Washington, DC, USA, 1999; p. 871.
- Bekele, D.; Birhan, M. The characteristics, distribution and management of Alfisols. Int. J. Res. Stud. Agric. Sci. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Xie, R.; Han, P.; Wang, X.; Li, B.; He, B.; Ma, F.; Bai, X. Effects of Pore Fluids and Sand Particles on Electrochemical Characteristics of Sandy Soil Containing Soluble Sodium Salt. Int. J. Electrochem. Sci. 2020, 15, 3543–3562. [Google Scholar] [CrossRef]
- Sheta, M.H.; Abd El-Wahed, A.H.; Elshaer, M.A.; Bayomy, H.M.; Ozaybi, N.A.; Abd-Elraheem, M.A.; El-Sheshtawy, A.-N.A.; El-Serafy, R.S.; Moustafa, M.M.I. Green synthesis of zinc and iron nanoparticles using Psidium guajava leaf extract stimulates cowpea growth, yield, and tolerance to saline water irrigation. Horticulturae 2024, 10, 915. [Google Scholar] [CrossRef]
- Sá, F.V.D.S.; Silva, I.E.D.; Ferreira, M.; Lima, Y.B.D.; Paiva, E.P.D.; Gheyi, H.R. Phosphorus doses alter the ionic homeostasis of cowpea irrigated with saline water. Rev. Bras. Eng. Agric. Amb. 2021, 25, 372–379. [Google Scholar] [CrossRef]
- Le, L.T.T.; Kotula, L.; Siddique, K.H.; Colmer, T.D. Na+ and/or Cl− toxicities determine salt sensitivity in soybean (Glycine max (L.) Merr.), mungbean (Vigna radiata (L.) R. Wilczek), cowpea (Vigna unguiculata (L.) Walp.), and common Bean (Phaseolus vulgaris L.). Int. J. Mol. Sci. 2021, 22, 1909. [Google Scholar] [CrossRef]
- Wests, D.W.; Francois, L.E. Effects of salinity on germination, growth and yield of cowpea. Irrig. Sci. 1982, 3, 169–175. [Google Scholar] [CrossRef]
- Coelho, J.B.M.; Barros, M.F.C.; Bezerra Neto, E.; Correa, M.M. Hydric behaviour and growth of cowpea cultivated in salinized soils. Rev. Bras. Eng. Agric. Amb. 2013, 17, 379–385. [Google Scholar] [CrossRef]
- Aryal, A.; Devkota, A.K.; Aryal, K.; Mahato, M. Effect of different levels of phosphorus on growth and yield of cowpea varieties in Dang, Nepal. J. Agric. Nat. Resour. 2021, 4, 62–78. [Google Scholar] [CrossRef]
- Faye, A.; Obour, A.K.; Akplo, T.M.; Stewart, Z.P.; Min, D.; Prasad, P.V.; Assefa, Y. Dual-purpose cowpea grain and fodder yield response to variety, nitrogen-phosphorus-potassium fertilizer, and environment. Agrosyst. Geosci. Environ. 2024, 7, e20459. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Akintola, O.A.; Tetteh, F.M.; Babalola, O.O. Combined application of inoculant, phosphorus and potassium enhances cowpea yield in savanna soils. Agronomy 2020, 11, 15. [Google Scholar] [CrossRef]
- Oliveira, W.J.D.; Souza, E.R.D.; Cunha, J.C.; Silva, Ê.F.D.F.E.; Veloso, V.D.L. Leaf gas exchange in cowpea and CO2 efflux in soil irrigated with saline water. Rev. Bras. Eng. Agric. Amb. 2017, 21, 32–37. [Google Scholar] [CrossRef]
- Behera, P.; Mahapatra, S.; Mohapatra, M.; Kim, J.Y.; Adhya, T.K.; Raina, V.; Suar, M.; Pattnaik, A.K.; Rastogi, G. Salinity and macrophyte drive the biogeography of the sedimentary bacterial communities in a brackish water tropical coastal lagoon. Sci. Total Environ. 2017, 595, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Marschner, P. Response of soil respiration and microbial biomass to changing EC in saline soils. Soil Biol. Biochem. 2013, 65, 322–328. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, R.; Petropoulos, E.; Yu, B.; Zhang, J.; Lin, X.; Gao, M.; Feng, Y. Interactive effects of salinity and SOM on the ecoenzymatic activities across coastal soils subjected to a saline gradient. Geoderma 2022, 406, e115519. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline Alkali Soils, 1st ed.; US Department of Agriculture, United States of America: Washington, DC, USA, 1954; p. 166.
- Leal, L.Y.C.; de Souza, E.R.; Júnior, J.A.S.; Dos Santos, M.A. Comparison of soil and hydroponic cultivation systems for spinach irrigated with brackish water. Sci. Hortic. 2020, 274, e109616. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture, 1st ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance—Current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Maas, E.V.; Poss, J.A. Salt sensitivity of cowpea at various growth stages. Irrig. Sci. 1989, 10, 313–320. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, Y.; Wang, T.; Han, Q. Salinity Effects on Soil Structure and Hydraulic Properties: Implications for Pedotransfer Functions in Coastal Areas. Land 2024, 13, 2077. [Google Scholar] [CrossRef]
- Dos Santos, O.D.O.; Mendes, K.; Martins, S.V.C.; Batista-Silva, W.; Dos Santos, M.A.; De Figueirôa, J.M.; De Souza, E.R.; Pompelli, M.F. Physiological parameters and plasticity as key factors to understand pioneer and late successional species in the Atlantic Rainforest. Acta Physiol. Plant. 2019, 41, 1–18. [Google Scholar] [CrossRef]
- Melo, H.F.; de Souza, E.R.; de Almeida, B.G.; Mulas, M. Water potential in soil and Atriplex nummularia (phytoremediator halophyte) under drought and salt stresses. Int. J. Phytore Mediat. 2018, 20, 249–255. [Google Scholar] [CrossRef]
- Manaf, H.H.; Zayed, M.S. Productivity of cowpea as affected by salt stress in presence of endomycorrhizae and Pseudomonas fluorescens. Ann. Agric. Sci. 2015, 60, 219–226. [Google Scholar] [CrossRef]
- Coelho, J.B.M.; Barros, M.F.C.; Bezerra Neto, E.; Souza, E.R. Physiological permanent wilting point and osmotic potential of cowpea grown in saline soils. Rev. Bras. Eng. Agric. Amb. 2014, 18, 708–713. [Google Scholar] [CrossRef]
- Iwuagwu, M.O.; Ogbonnaya, C.I.; Onyike, N.B. Physiological response of cowpea Vigna unguiculata to drought: The osmotic adjustment resistance strategy. Acad. J. Sci. 2017, 7, 329–344. [Google Scholar]


| Soils | Municipality | Localization (GPS) | Altitude (m) | Precipitation (mm) | Temperature (°C) | Climate (Köppen–Geiger) |
|---|---|---|---|---|---|---|
| Ultisol | Goiana | 7.64° S, 34.96° W | 13 | 938 | 23 a 28 | Aw |
| Alfisol | Belém do São Francisco | 8.74° S, 38.85° W | 305 | 432 | 22 a 31 | BSh |
| Attributes | Ultisol | Alfisol |
|---|---|---|
| pH (H2O 1:2.5) | 4.50 | 6.70 |
| EC (dS m−1) | 0.4 | 0.8 |
| H + Al (cmolc dm−3) | 3.52 | 0.66 |
| Ca (cmolc dm−3) | 0.80 | 5.0 |
| Mg (cmolc dm−3) | 0.60 | 2.30 |
| Al (cmolc dm−3) | 0.43 | 0.00 |
| Na (cmolc dm−3) | 0.05 | 0.21 |
| K (cmolc dm−3) | 0.03 | 0.35 |
| P (mg dm−3) | 0.00 | 6.00 |
| CEC (cmolc dm−3 pH 7) | 5.00 | 8.52 |
| Bd (g cm−3) | 1.24 | 1.87 |
| Pd (g cm−3) | 2.55 | 2.56 |
| CS (g Kg−1) | 428 | 220 |
| FS (g Kg−1) | 236 | 170 |
| Silt (g Kg−1) | 156 | 250 |
| Clay (g Kg−1) | 180 | 360 |
| DCW (%) | 45 | 64 |
| FD (%) | 75 | 73 |
| DD (%) | 25 | 26 |
| EC (dS m−1) | DMAP (g) | DMR (g) | H (cm) | SD (cm) | |
|---|---|---|---|---|---|
| 0 | 4.00 Ab ± 0.87 | 1.21 Ab ± 0.15 | 101.65 Ab ± 8.23 | 5.05 Aa ± 0.34 | |
| 1.5 | 1.91 Bb ± 0.32 | 1.23 Ab ± 0.20 | 85.50 Bb ± 16.66 | 5.66 Aa ± 1.33 | |
| Ultisol | 3.0 | 1.04 Cb ± 0.34 | 0.51 Bb ± 0.10 | 75.32 Bb ± 20.54 | 4.60 Ab ± 0.87 |
| 4.5 | 0.14 Db ± 0.04 | 0.42 Bb ± 0.09 | 51.75 Cb ± 16.90 | 3.86 Bb ± 0.14 | |
| 6.0 | 0.14 Db ± 0.07 | 0.29 Bb ± 0.03 | 35.00 Db ± 2.16 | 3.90 Bb ± 0.54 | |
| 9.0 | * | 0.12 Bb ± 0.02 | 33.00 Db ± 0.81 | 3.41 Bb ± 0.02 | |
| 0 | 5.36 Ba ± 0.49 | 2.95 Aa ± 0.54 | 119.00 Aa ± 9.01 | 5.75 Aa ± 0.23 | |
| 1.5 | 6.17 Aa ± 0.64 | 2.69 Aa ± 0.51 | 103.00 Ba ±17.52 | 6.47 Aa ± 0.30 | |
| 3.0 | 5.38 Ba ± 1.31 | 2.02 Ba ± 0.25 | 102.50 Ba ± 26.68 | 6.41 Aa ± 1.02 | |
| Alfisol | 4.5 | 4.23 Ca ± 0.93 | 1.82 Ba ± 0.38 | 95.50 Ba ± 13.30 | 5.99 Aa ± 0.20 |
| 6.0 | 4.65 Ca ± 0.71 | 1.90 Ba ± 0.21 | 96.00 Ba ± 23.30 | 5.79 Aa ± 1.33 | |
| 9.0 | 4.03 Ca ± 1.00 | 1.54 Ca ± 0.35 | 63.25 Ca ± 15.52 | 6.70 Aa ± 1.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, J.R.M.d.; Leal, L.Y.d.C.; Paulino, M.K.S.S.; Nunes, J.A.; Medeiros, R.L.S.d.; Santos, M.A.d.; Lins, C.M.T.; Souza Júnior, V.S.d.; Schaffer, B.; Souza, E.R.d. Cowpea (Vigna unguiculata) Water Relations, Growth, and Productivity as Affected by Salinity in Two Soils with Contrasting Mineralogies. Soil Syst. 2025, 9, 36. https://doi.org/10.3390/soilsystems9020036
Souza JRMd, Leal LYdC, Paulino MKSS, Nunes JA, Medeiros RLSd, Santos MAd, Lins CMT, Souza Júnior VSd, Schaffer B, Souza ERd. Cowpea (Vigna unguiculata) Water Relations, Growth, and Productivity as Affected by Salinity in Two Soils with Contrasting Mineralogies. Soil Systems. 2025; 9(2):36. https://doi.org/10.3390/soilsystems9020036
Chicago/Turabian StyleSouza, Jaciane Rosa Maria de, Lucas Yago de Carvalho Leal, Martha Katharinne Silva Souza Paulino, José Alfredo Nunes, Rafael Luís Silva de Medeiros, Monaliza Alves dos Santos, Cintia Maria Teixeira Lins, Valdomiro Severino de Souza Júnior, Bruce Schaffer, and Edivan Rodrigues de Souza. 2025. "Cowpea (Vigna unguiculata) Water Relations, Growth, and Productivity as Affected by Salinity in Two Soils with Contrasting Mineralogies" Soil Systems 9, no. 2: 36. https://doi.org/10.3390/soilsystems9020036
APA StyleSouza, J. R. M. d., Leal, L. Y. d. C., Paulino, M. K. S. S., Nunes, J. A., Medeiros, R. L. S. d., Santos, M. A. d., Lins, C. M. T., Souza Júnior, V. S. d., Schaffer, B., & Souza, E. R. d. (2025). Cowpea (Vigna unguiculata) Water Relations, Growth, and Productivity as Affected by Salinity in Two Soils with Contrasting Mineralogies. Soil Systems, 9(2), 36. https://doi.org/10.3390/soilsystems9020036










