The Impacts of Ethanol and Freeze–Thaw Cycling on Arsenic Mobility in a Contaminated Boreal Wetland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Location and Procedure
2.2. Freeze–Thaw Experiments
2.3. Gas Chromatography
2.4. Spectrophotometric Sulfate Analysis
2.5. Sequential and Total Extraction of Wetland Soils
2.6. Arsenic Speciation
2.7. Determination of Metal Concentrations
2.8. DNA Extraction and 16S rRNA Gene Analyses
3. Results and Discussion
3.1. Distribution of Arsenic and Iron in Wetland Soil Impacted by Mine Water Discharge
3.2. Changes in the Distribution of As and Fe After FT Cycling
3.3. The Effects of Freeze–Thaw Cycling and Carbon Amendment on Microbial Activity and Water Chemistry
3.4. Microbial Communities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dybowska, A.; Farago, M.; Valsami-Jones, E.; Thornton, I. Remediation Strategies for Historical Mining and Smelting Sites. Sci. Prog. 2006, 89, 71–138. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.A.; Lehr, J.H.; Testa, S.M. Acid Mine Drainage, Rock Drainage, and Acid Sulfate Soils: Causes, Assessment, Prediction, Prevention, and Remediation; Wiley: Hoboken, NJ, USA, 2014; Volume 9780470487, ISBN 978-1-118-74919-7. [Google Scholar]
- Merkel, B.J.; Hasche-Berger, A. Uranium in Natural Wetlands: A Hydrogeochemical Approach to Reveal Immobilization Processes. In Uranium in the Environment: Mining Impact and Consequences; Merkel, B.J., Hasche-Berger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 389–397. ISBN 978-3-540-28363-0. [Google Scholar]
- Wang, S.; Mulligan, C.N. Occurrence of Arsenic Contamination in Canada: Sources, Behavior and Distribution. Sci. Total Environ. 2006, 366, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Szkokan-Emilson, E.J.; Watmough, S.A.; Gunn, J.M. Wetlands as Long-Term Sources of Metals to Receiving Waters in Mining-Impacted Landscapes. Environ. Pollut. 2014, 192, 91–103. [Google Scholar] [CrossRef]
- Williams, M. Arsenic in Mine Waters: An International Study. Environ. Geol. 2001, 40, 267–278. [Google Scholar] [CrossRef]
- Sarkar, B.; Wijesekara, H.; Mandal, S.; Singh, M.; Bolan, N.S. Characterization and Improvement in Physical, Chemical, and Biological Properties of Mine Wastes. In Spoil to Soil: Mine Site Rehabilitation and Revegetation; Bolan, N.S., Kirkham, M.B., Ok, Y.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 3–15. ISBN 978-1-138-19730-8. [Google Scholar]
- Vaughan, J.P. The Process Mineralogy of Gold: The Classification of Ore Types. JOM 2004, 56, 46–48. [Google Scholar] [CrossRef]
- Amos, R.T.; Blowes, D.W.; Bailey, B.L.; Sego, D.C.; Smith, L.; Ritchie, A.I.M. Waste-Rock Hydrogeology and Geochemistry. Appl. Geochem. 2015, 57, 140–156. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef]
- Sracek, O.; Mihaljevič, M.; Kříbek, B.; Majer, V.; Filip, J.; Vaněk, A.; Penížek, V.; Ettler, V.; Mapani, B. Geochemistry of Mine Tailings and Behavior of Arsenic at Kombat, Northeastern Namibia. Environ. Monit. Assess. 2014, 186, 4891–4903. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y.; Luo, J.; Xu, B.; Zhao, J. Geochemical Processes Controlling Fate and Transport of Arsenic in Acid Mine Drainage (AMD) and Natural Systems. J. Hazard. Mater. 2009, 165, 13–26. [Google Scholar] [CrossRef]
- Stollenwerk, K.G. Geochemical Processes Controlling Transport of Arsenic in Groundwater: A Review of Adsorption. In Arsenic in Ground Water: Geochemistry and Occurrence; Welch, A.H., Stollenwerk, K.G., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 67–100. ISBN 978-0-306-47956-7. [Google Scholar]
- Dworkin, M.; Falkow, S.; Rosenberg, E.; Schleifer, K.-H. Dissimilatory Sulfate- and Sulfur-Reducing Prokaryotes. In The Prokaryotes: A Handbook on the Biology of Bacteria; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 659–768. ISBN 978-0-387-30742-8. [Google Scholar]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of Passive Systems for Acid Mine Drainage Treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef]
- Hwang, S.K.; Jho, E.H. Heavy Metal and Sulfate Removal from Sulfate-Rich Synthetic Mine Drainages Using Sulfate Reducing Bacteria. Sci. Total Environ. 2018, 635, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Briones-Gallardo, R.; Escot-Espinoza, V.M.; Cervantes-González, E. Removing Arsenic and Hydrogen Sulfide Production Using Arsenic-Tolerant Sulfate-Reducing Bacteria. Int. J. Environ. Sci. Technol. 2017, 14, 609–622. [Google Scholar] [CrossRef]
- Campbell, K.M.; Malasarn, D.; Saltikov, C.W.; Newman, D.K.; Hering, J.G. Simultaneous Microbial Reduction of Iron(III) and Arsenic(V) in Suspensions of Hydrous Ferric Oxide. Environ. Sci. Technol. 2006, 40, 5950–5955. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rodríguez, A.M.; Durán-Barrantes, M.M.; Borja, R.; Sánchez, E.; Colmenarejo, M.F.; Raposo, F. Heavy Metals Removal from Acid Mine Drainage Water Using Biogenic Hydrogen Sulphide and Effluent from Anaerobic Treatment: Effect of PH. J. Hazard. Mater. 2009, 165, 759–765. [Google Scholar] [CrossRef]
- O’Day, P.A.; Vlassopoulos, D.; Root, R.; Rivera, N. The Influence of Sulfur and Iron on Dissolved Arsenic Concentrations in the Shallow Subsurface under Changing Redox Conditions. Proc. Natl. Acad. Sci. USA 2004, 101, 13703–13708. [Google Scholar] [CrossRef]
- Paikaray, S. Arsenic Geochemistry of Acid Mine Drainage. Mine Water Environ. 2015, 34, 181–196. [Google Scholar] [CrossRef]
- Slowey, A.J.; Johnson, S.B.; Newville, M.; Brown, G.E. Speciation and Colloid Transport of Arsenic from Mine Tailings. Appl. Geochem. 2007, 22, 1884–1898. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Jia, Y. Effect of Sulfide on As(III) and As(V) Sequestration by Ferrihydrite. Chemosphere 2017, 185, 321–328. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic Arsenic: Toxicity, Speciation, Transformations, and Remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Aguinaga, O.E.; White, K.N.; Dean, A.P.; Pittman, J.K. Addition of Organic Acids to Acid Mine Drainage Polluted Wetland Sediment Leads to Microbial Community Structure and Functional Changes and Improved Water Quality. Environ. Pollut. 2021, 290, 118064. [Google Scholar] [CrossRef]
- O’Sullivan, A.D.; Moran, B.M.; Otte, M.L. Accumulation and Fate of Contaminants (Zn, Pb, Fe and S) in Substrates of Wetlands Constructed for Treating Mine Wastewater. Water Air Soil Pollut. 2004, 157, 345–364. [Google Scholar] [CrossRef]
- El Bayoumy, M.A.; Bewtra, J.K.; Ali, H.I.; Biswas, N. Sulfide Production by Sulfate Reducing Bacteria with Lactate as Feed in an Upflow Anaerobic Fixed Film Reactor. Water Air Soil Pollut. 1999, 112, 67–84. [Google Scholar] [CrossRef]
- Lee, M.K.; Saunders, J.A.; Wilson, T.; Levitt, E.; Saffari Ghandehari, S.; Dhakal, P.; Redwine, J.; Marks, J.; Billor, Z.M.; Miller, B.; et al. Field-Scale Bioremediation of Arsenic-Contaminated Groundwater Using Sulfate-Reducing Bacteria and Biogenic Pyrite. Bioremediat. J. 2019, 23, 1–21. [Google Scholar] [CrossRef]
- Luo, Q.; Tsukamoto, T.K.; Zamzow, K.L.; Miller, G.C. Arsenic, Selenium, and Sulfate Removal Using an Ethanol-Enhanced Sulfate-Reducing Bioreactor. Mine Water Environ. 2008, 27, 100–108. [Google Scholar] [CrossRef]
- Neculita, C.-M.; Zagury, G.J.; Bussière, B. Passive Treatment of Acid Mine Drainage in Bioreactors Using Sulfate-Reducing Bacteria. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oyekola, O.O.; van Hille, R.P.; Harrison, S.T.L. Kinetic Analysis of Biological Sulphate Reduction Using Lactate as Carbon Source and Electron Donor: Effect of Sulphate Concentration. Chem. Eng. Sci. 2010, 65, 4771–4781. [Google Scholar] [CrossRef]
- Santini, T.C.; Malcolm, L.I.; Tyson, G.W.; Warren, L.A. pH and Organic Carbon Dose Rates Control Microbially Driven Bioremediation Efficacy in Alkaline Bauxite Residue. Environ. Sci. Technol. 2016, 50, 11164–11173. [Google Scholar] [CrossRef]
- Gopi Kiran, M.; Pakshirajan, K.; Das, G. An Overview of Sulfidogenic Biological Reactors for the Simultaneous Treatment of Sulfate and Heavy Metal Rich Wastewater. Chem. Eng. Sci. 2017, 158, 606–620. [Google Scholar] [CrossRef]
- Gibert, O.; De Pablo, J.; Luis Cortina, J.; Ayora, C. Chemical Characterisation of Natural Organic Substrates for Biological Mitigation of Acid Mine Drainage. Water Res. 2004, 38, 4186–4196. [Google Scholar] [CrossRef]
- Ko, M.S.; Park, H.S.; Lee, J.U. Influence of Indigenous Bacteria Stimulation on Arsenic Immobilization in Field Study. Catena 2017, 148, 46–51. [Google Scholar] [CrossRef]
- Santamaria, B.; Strosnider, W.H.J.; Apaza Quispe, M.R.; Nairn, R.W. Evaluating Locally Available Organic Substrates for Vertical Flow Passive Treatment Cells at Cerro Rico de Potosí, Bolivia. Environ. Earth Sci. 2014, 72, 731–741. [Google Scholar] [CrossRef]
- Mattes, A.; Evans, L.J.; Douglas Gould, W.; Duncan, W.F.A.; Glasauer, S. The Long Term Operation of a Biologically Based Treatment System That Removes As, S and Zn from Industrial (Smelter Operation) Landfill Seepage. Appl. Geochem. 2011, 26, 1886–1896. [Google Scholar] [CrossRef]
- Nyquist, J.; Greger, M. A Field Study of Constructed Wetlands for Preventing and Treating Acid Mine Drainage. Ecol. Eng. 2009, 35, 630–642. [Google Scholar] [CrossRef]
- Henry, H.A.L. Climate Change and Soil Freezing Dynamics: Historical Trends and Projected Changes. Clim. Change 2008, 87, 421–434. [Google Scholar] [CrossRef]
- Coppolino, J.; Munford, K.E.; Macrae, M.; Glasauer, S. Shifts in Soil Phosphorus Fractions during Seasonal Transitions in a Riparian Floodplain Wetland. Front. Environ. Sci. 2022, 10, 983129. [Google Scholar] [CrossRef]
- Velasco, A.; Ramírez, M.; Volke-Sepúlveda, T.; González-Sánchez, A.; Revah, S. Evaluation of Feed COD/Sulfate Ratio as a Control Criterion for the Biological Hydrogen Sulfide Production and Lead Precipitation. J. Hazard. Mater. 2008, 151, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Kousi, P.; Remoundaki, E.; Hatzikioseyian, A.; Battaglia-Brunet, F.; Joulian, C.; Kousteni, V.; Tsezos, M. Metal Precipitation in an Ethanol-Fed, Fixed-Bed Sulphate-Reducing Bioreactor. J. Hazard. Mater. 2011, 189, 677–684. [Google Scholar] [CrossRef]
- Ministry of Energy, Northern Development and Mines. Long Lake Gold Mine Rehabilitation Project; Ministry of Energy, Northern Development and Mines: Sudbury, ON, Canada, 2019.
- Long Lake Gold Mine Rehabilitation Project. Available online: https://www.ontario.ca/page/long-lake-gold-mine-rehabilitation-project (accessed on 5 February 2025).
- Munford, K.E.; Watmough, S.A.; Rivest, M.; Poulain, A.; Basiliko, N.; Mykytczuk, N.C.S. Edaphic Factors Influencing Vegetation Colonization and Encroachment on Arsenical Gold Mine Tailings near Sudbury, Ontario. Environ. Pollut. 2020, 264, 114680. [Google Scholar] [CrossRef]
- Emerson, D.; Tang, J. Methods for General and Molecular Microbiology, 3rd ed.; Marzluf, G.A., Reddy, C.A., Beveridge, T.J., Schmidt, T.M., Snyder, L.R., Breznak, J.A., Eds.; American Society of Microbiology: Washington, DC, USA, 2007; pp. 200–215. ISBN 9781555812232. [Google Scholar]
- Rodriguez-Freire, L.; Moore, S.E.; Sierra-Alvarez, R.; Root, R.A.; Chorover, J.; Field, J.A. Arsenic Remediation by Formation of Arsenic Sulfide Minerals in a Continuous Anaerobic Bioreactor. Biotechnol. Bioeng. 2016, 113, 522–530. [Google Scholar] [CrossRef]
- Alam, R.; McPhedran, K. Applications of Biological Sulfate Reduction for Remediation of Arsenic—A Review. Chemosphere 2019, 222, 932–944. [Google Scholar] [CrossRef]
- Tuominen, L.; Kairesalo, T.; Hartikainen, H. Comparison of Methods for Inhibiting Bacterial Activity in Sediment. Appl. Environ. Microbiol. 1994, 60, 3454–3457. [Google Scholar] [CrossRef] [PubMed]
- Lees, K.; Fitzsimons, M.; Snape, J.; Tappin, A.; Comber, S. Soil Sterilisation Methods for Use in OECD 106: How Effective Are They? Chemosphere 2018, 209, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lotrario, J.B.; Stuart, B.J.; Lam, T.; Arands, R.R.; O’Connor, O.A.; Kosson, D.S. Effects of Sterilization Methods on the Physical Characteristics of Soil: Implications for Sorption Isotherm Analyses. Bull. Environ. Contam. Toxicol. 1995, 54, 668–675. [Google Scholar] [CrossRef]
- Razavi Darbar, S.; Lakzian, A. Evaluation of Chemical and Biological Consequences of Soil Sterilization Methods. Casp. J. Environ. Sci. 2007, 5, 87–91. [Google Scholar]
- Powlson, D.S.; Jenkinson, D.S. The Effects of Biocidal Treatments on Metabolism in Soil-II. Gamma Irradiation, Autoclaving, Air-Drying and Fumigation. Soil Biol. Biochem. 1976, 8, 179–188. [Google Scholar] [CrossRef]
- Huang, J.H.; Kretzschmar, R. Sequential Extraction Method for Speciation of Arsenate and Arsenite in Mineral Soils. Anal. Chem. 2010, 82, 5534–5540. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package, R Package Version 2.6-4, 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 5 February 2025).
- Fernandes, A.D.; Macklaim, J.M.; Linn, T.G.; Reid, G.; Gloor, G.B. ANOVA-Like Differential Expression (ALDEx) Analysis for Mixed Population RNA-Seq. PLoS ONE 2013, 8, e67019. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Northern Development and Mines. Category C Environmental Assessment: Long Lake Gold Mine Rehabilitation Project, Geographic Township of Eden, Ontario; Ministry of Northern Development and Mines: Eden, ON, Canada, 2017.
- Canadian Council of Ministers of the Environment. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health: Arsenic (Inorganic); Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2001.
- Chen, X.; Zeng, X.C.; Kawa, Y.K.; Wu, W.; Zhu, X.; Ullah, Z.; Wang, Y. Microbial Reactions and Environmental Factors Affecting the Dissolution and Release of Arsenic in the Severely Contaminated Soils under Anaerobic or Aerobic Conditions. Ecotoxicol. Environ. Saf. 2020, 189, 109946. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Drahota, P.; Machovič, V.; Böhmová, V.; Mihaljevič, M. Arsenic Mineralogy and Mobility in the Arsenic-Rich Historical Mine Waste Dump. Sci. Total Environ. 2015, 536, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Rickard, D.; Morse, J.W. Acid Volatile Sulfide (AVS). Mar. Chem. 2005, 97, 141–197. [Google Scholar] [CrossRef]
- Watts, M.P.; Lloyd, J.R. Bioremediation via Microbial Metal Reduction. In Microbial Metal Respiration: From Geochemistry to Potential Applications; Lloyd, J.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 161–201. ISBN 978-3-642-32867-1. [Google Scholar]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate Reduction at Low pH to Remediate Acid Mine Drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef]
- Saalfield, S.L.; Bostick, B.C. Changes in Iron, Sulfur, and Arsenic Speciation Associated with Bacterial Sulfate Reduction in Ferrihydrite-Rich Systems. Environ. Sci. Technol. 2009, 43, 8787–8793. [Google Scholar] [CrossRef]
- Glasauer, S.; Weidler, P.G.; Langley, S.; Beveridge, T.J. Controls on Fe Reduction and Mineral Formation by a Subsurface Bacterium. Geochim. Cosmochim. Acta 2003, 67, 1277–1288. [Google Scholar] [CrossRef]
- Vaxevanidou, K.; Christou, C.; Kremmydas, G.F.; Georgakopoulos, D.G.; Papassiopi, N. Role of Indigenous Arsenate and Iron(III) Respiring Microorganisms in Controlling the Mobilization of Arsenic in a Contaminated Soil Sample. Bull. Environ. Contam. Toxicol. 2015, 94, 282–288. [Google Scholar] [CrossRef]
- Drahota, P.; Mikutta, C.; Falteisek, L.; Duchoslav, V.; Klementová, M. Biologically Induced Formation of Realgar Deposits in Soil. Geochim. Cosmochim. Acta 2017, 218, 237–256. [Google Scholar] [CrossRef]
- Gramp, J.P.; Bigham, J.M.; Jones, F.S.; Tuovinen, O.H. Formation of Fe-Sulfides in Cultures of Sulfate-Reducing Bacteria. J. Hazard. Mater. 2010, 175, 1062–1067. [Google Scholar] [CrossRef]
- Saunders, J.A.; Lee, M.K.; Shamsudduha, M.; Dhakal, P.; Uddin, A.; Chowdury, M.T.; Ahmed, K.M. Geochemistry and Mineralogy of Arsenic in (Natural) Anaerobic Groundwaters. Appl. Geochem. 2008, 23, 3205–3214. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Karlsson, J.; Giesler, R. Freeze-Thaw Cycles of Arctic Thaw Ponds Remove Colloidal Metals and Generate Low-Molecular-Weight Organic Matter. Biogeochemistry 2018, 137, 321–336. [Google Scholar] [CrossRef]
- Cummings, D.E.; March, A.W.; Bostick, B.; Spring, S.; Caccavo, F.; Fendorf, S.; Rosenzweig, R.F. Evidence for Microbial Fe(III) Reduction in Anoxic, Mining-Impacted Lake Sediments (Lake Coeur d’Alene, Idaho). Appl. Environ. Microbiol. 2000, 66, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Caccavo, F.; Lonergan, D.J.; Lovley, D.R.; Davis, M.; Stolz, J.F.; McInerney, M.J. Geobacter sulfurreducens sp. nov., a Hydrogen- and Acetate-Oxidizing Dissimilatory Metal-Reducing Microorganism. Appl. Environ. Microbiol. 1994, 60, 3752–3759. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Holmes, D.E.; Woodard, T.L.; Hinlein, E.S.; Ostendorf, D.W.; Lovley, D.R. Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., Two Novel Fe(III)-Reducing Subsurface Isolates. Int. J. Syst. Evol. Microbiol. 2005, 55, 1667–1674. [Google Scholar] [CrossRef]
- Akob, D.M.; Mills, H.J.; Gihring, T.M.; Kerkhof, L.; Stucki, J.W.; Anastácio, A.S.; Chin, K.J.; Küsel, K.; Palumbo, A.V.; Watson, D.B.; et al. Functional Diversity and Electron Donor Dependence of Microbial Populations Capable of U(VI) Reduction in Radionuclide-Contaminated Subsurface Sediments. Appl. Environ. Microbiol. 2008, 74, 3159–3170. [Google Scholar] [CrossRef]
- Das, S.; Liu, C.C.; Jean, J.S.; Lee, C.C.; Yang, H.J. Effects of Microbially Induced Transformations and Shift in Bacterial Community on Arsenic Mobility in Arsenic-Rich Deep Aquifer Sediments. J. Hazard. Mater. 2016, 311, 11–19. [Google Scholar] [CrossRef]
- Jones, E.J.P.; Nadeau, T.L.; Voytek, M.A.; Landa, E.R. Role of Microbial Iron Reduction in the Dissolution of Iron Hydroxysulfate Minerals. J. Geophys. Res. Biogeosci. 2006, 111, G01012. [Google Scholar] [CrossRef]
- Williams, K.; Long, P.E.; Davis, J.; Wilkins, M.; N’Guessan, A.L.; Steefel, C.; Yang, L.; Newcomer, D.; Spane, F.; Kerkhof, L.; et al. Acetate Availability and Its Influence on Sustainable Bioremediation of Uranium-Contaminated Groundwater. Geomicrobiol. J. 2011, 28, 519–539. [Google Scholar] [CrossRef]
- Alazard, D.; Joseph, M.; Battaglia-Brunet, F.; Cayol, J.L.; Ollivier, B. Desulfosporosinus acidiphilus sp. nov.: A Moderately Acidophilic Sulfate-Reducing Bacterium Isolated from Acid Mining Drainage Sediments. Extremophiles 2010, 14, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Balk, M.; Altinbaş, M.; Rijpstra, W.I.C.; Damsté, J.S.S.; Stams, A.J.M. Desulfatirhabdium butyrativorans gen. nov., sp. nov., a Butyrate-Oxidizing, Sulfate-Reducing Bacterium Isolated from an Anaerobic Bioreactor. Int. J. Syst. Evol. Microbiol. 2008, 58, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Romanek, C.S.; Wiegel, J. Desulfosporosinus youngiae sp. nov., a Spore-Forming, Sulfate-Reducing Bacterium Isolated from a Constructed Wetland Treating Acid Mine Drainage. Int. J. Syst. Evol. Microbiol. 2009, 59, 2743–2746. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Sass, H.; Langner, H.; Schumann, P.; Kroppenstedt, R.M.; Spring, S.; Overmann, J.; Rosenzweig, R.F. Desulfosporosinus lacus sp. nov., a Sulfate-Reducing Bacterium Isolated from Pristine Freshwater Lake Sediments. Int. J. Syst. Evol. Microbiol. 2006, 56, 2729–2736. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Singh, S.K.; Mudhoo, A. A Comprehensive Overview of Elements in Bioremediation. Rev. Environ. Sci. Biotechnol. 2010, 9, 215–288. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid Mine Drainage Remediation Options: A Review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
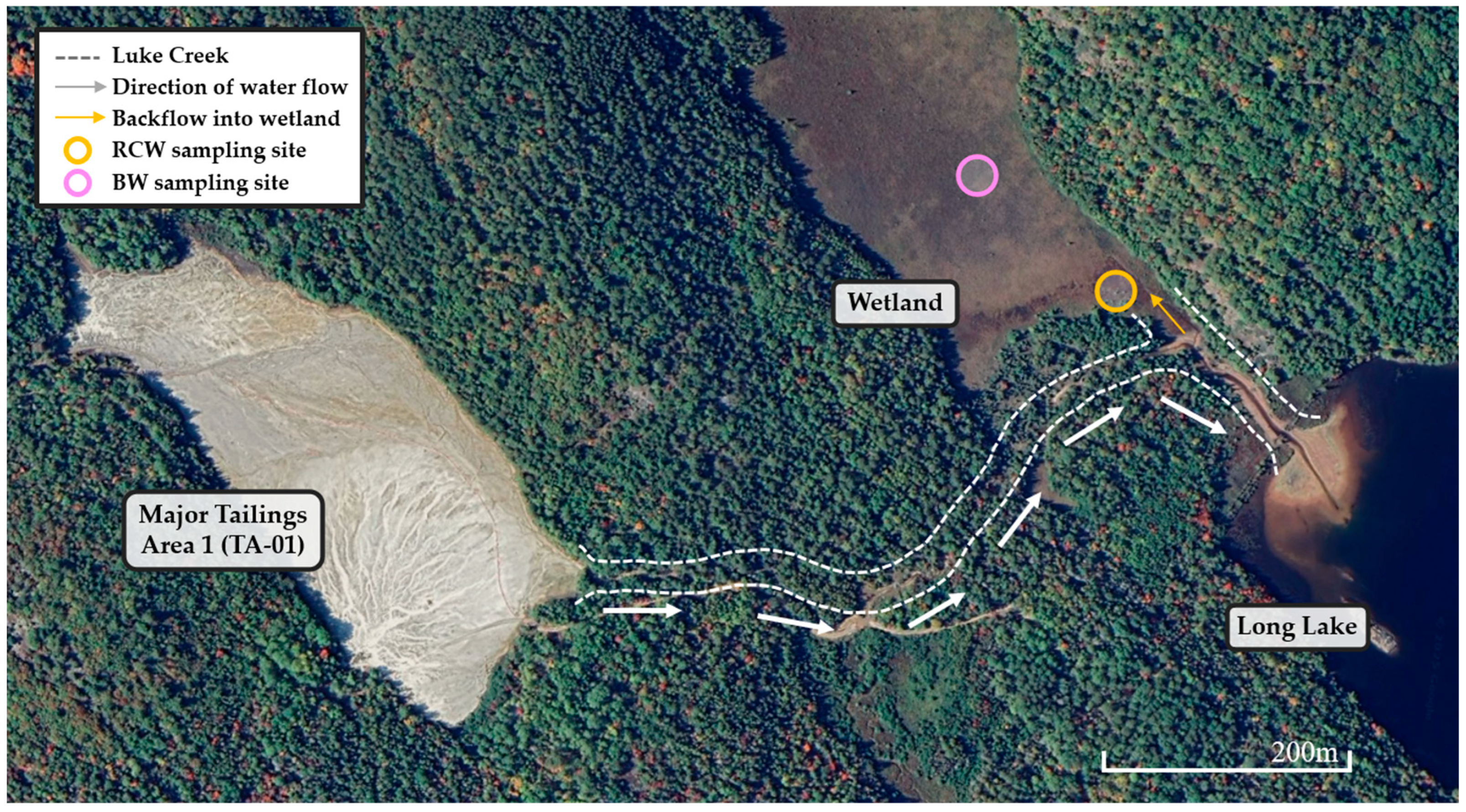



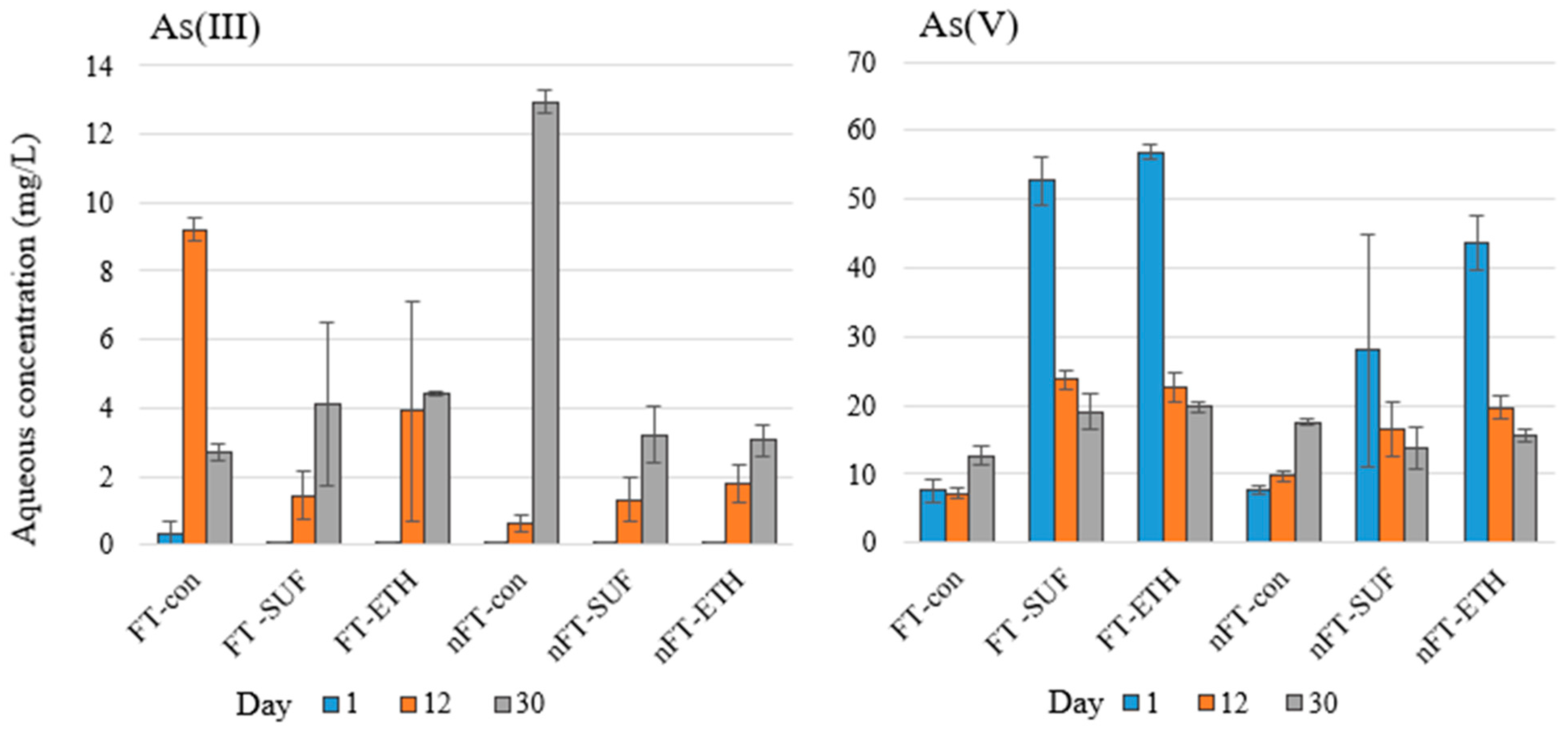

| Constituents | Sample Codes and Constituent Concentrations 1 | ||
|---|---|---|---|
| ARS | SUF | ETH | |
| Sodium arsenate | 1 mM | 1 mM | 1 mM |
| Sodium sulfate | - | 10 mM | 10 mM |
| Ethanol | - | - | 15 mM |
| Fraction | As (mg/kg) | Avg. As (%) | Fe (mg/kg) | Avg. Fe (%) |
|---|---|---|---|---|
| Soluble and exchangeable | 430 (±14) | 3.30% | 70 (±2.7) | 0.15% |
| Organic | 2195 (±119) | 16.89% | 8425 (±452) | 17.82% |
| AV sulfides *, VPC Fe/Al ** | 4674 (±486) | 35.97% | 20,584 (±1721) | 43.55% |
| Poorly crystalline Fe/Al | 3127(±165) | 24.07% | 8514 (±279) | 18.01% |
| Sulfides | 2380 (±225) | 18.31% | 6820 (±1029) | 14.43% |
| Crystalline Fe/Al | 189 (±46) | 1.46% | 1461 (±143) | 3.09% |
| Residual | 0 (±0) | 0.00% | 1394 (±68) | 2.95% |
| Total (sum of fractions) | 12,994 | 100.00% | 47,268 | 100.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radford, J.; Munford, K.E.; Mykytczuk, N.; Glasauer, S. The Impacts of Ethanol and Freeze–Thaw Cycling on Arsenic Mobility in a Contaminated Boreal Wetland. Soil Syst. 2025, 9, 37. https://doi.org/10.3390/soilsystems9020037
Radford J, Munford KE, Mykytczuk N, Glasauer S. The Impacts of Ethanol and Freeze–Thaw Cycling on Arsenic Mobility in a Contaminated Boreal Wetland. Soil Systems. 2025; 9(2):37. https://doi.org/10.3390/soilsystems9020037
Chicago/Turabian StyleRadford, Joseph, Kimber E. Munford, Nadia Mykytczuk, and Susan Glasauer. 2025. "The Impacts of Ethanol and Freeze–Thaw Cycling on Arsenic Mobility in a Contaminated Boreal Wetland" Soil Systems 9, no. 2: 37. https://doi.org/10.3390/soilsystems9020037
APA StyleRadford, J., Munford, K. E., Mykytczuk, N., & Glasauer, S. (2025). The Impacts of Ethanol and Freeze–Thaw Cycling on Arsenic Mobility in a Contaminated Boreal Wetland. Soil Systems, 9(2), 37. https://doi.org/10.3390/soilsystems9020037





