Evaluation of the Photocatalytic Activity of Water-Based TiO2 Nanoparticle Dispersions Applied on Historical Painting Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colloidal Dispersion Preparation
2.2. Painting Mock-Ups Preparation
2.3. Photocatalytic Tests
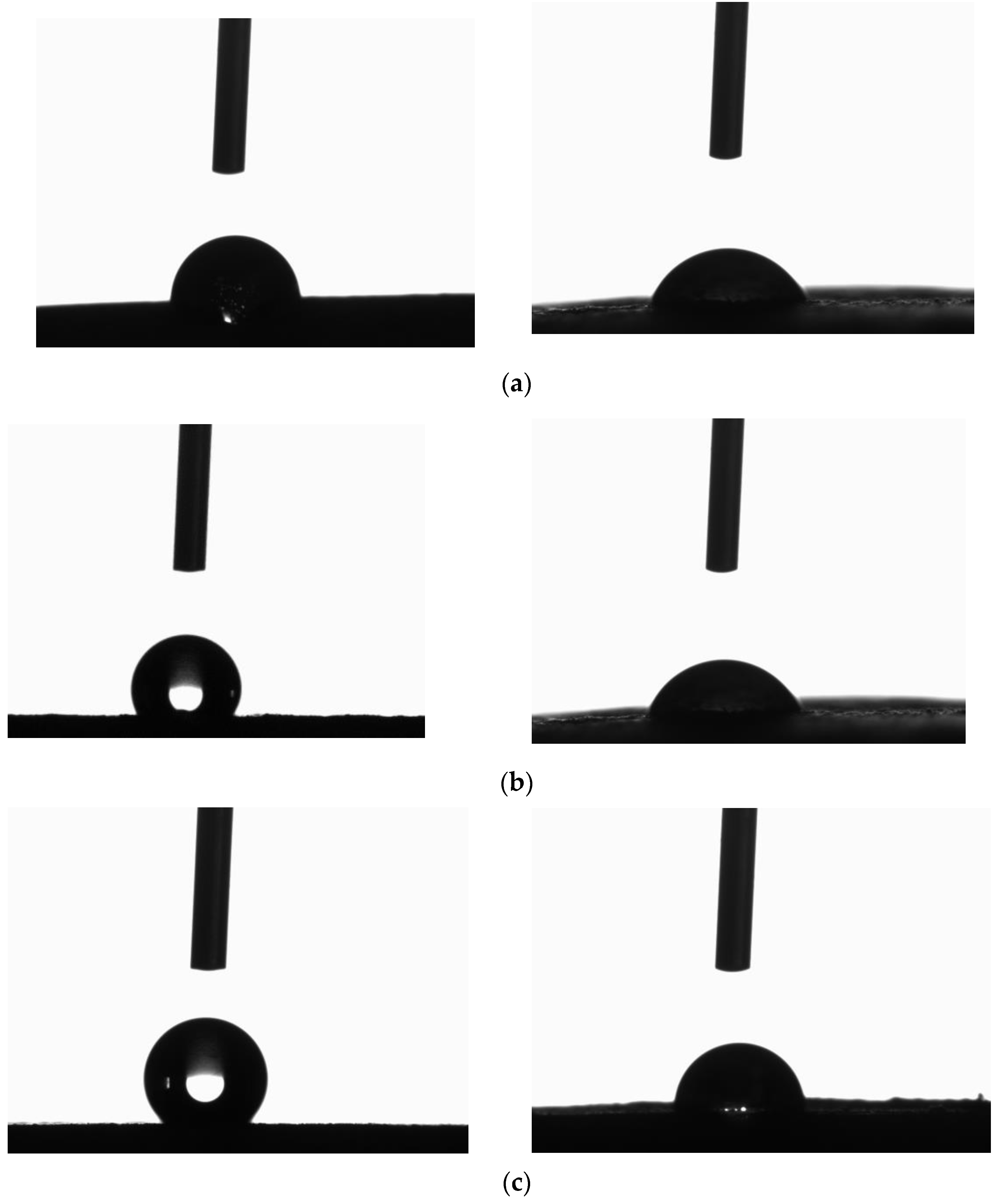
2.4. Contact Angle and Roughness Measurements
2.5. Optical and Colorimetric Characterization
3. Results and Discussion
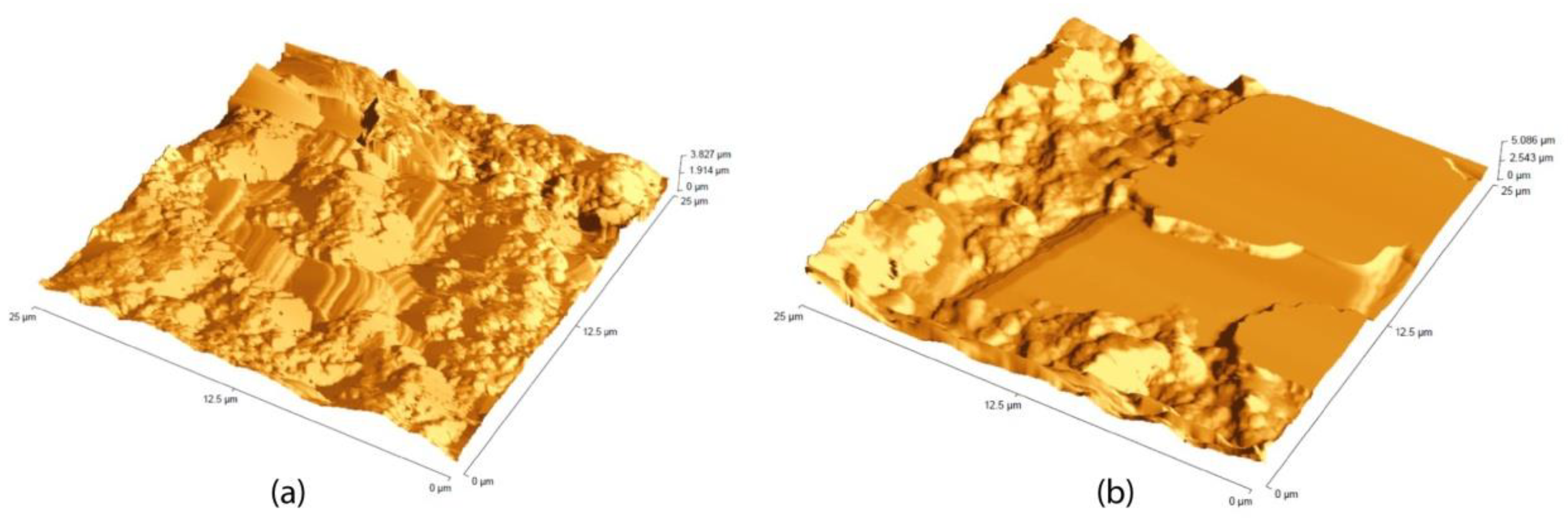
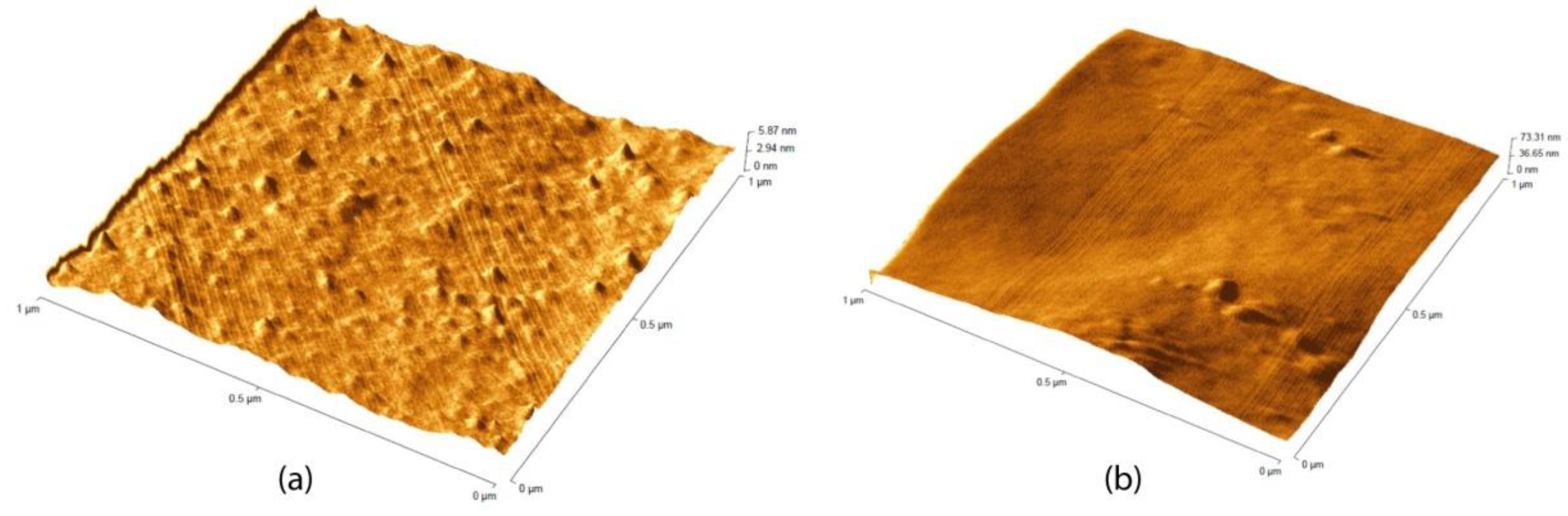
3.1. Painting Surface Characterization after Nanoparticles Application
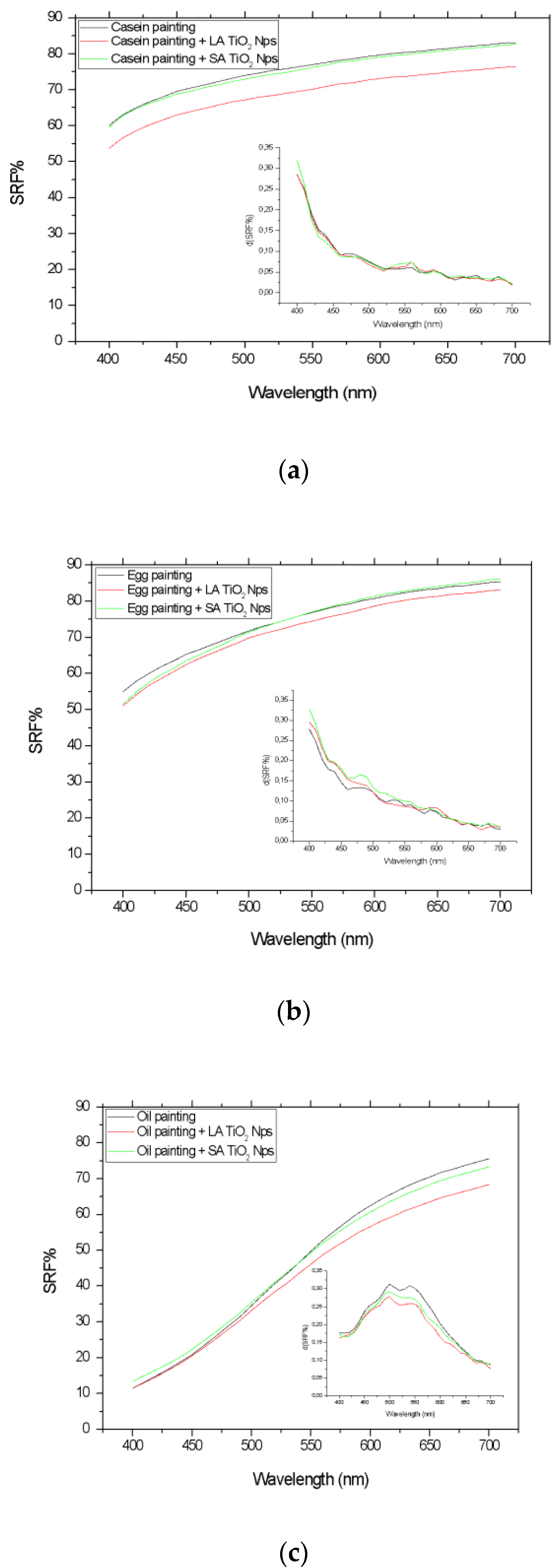
3.2. Photocatalytic Activity
3.3. Painting Surface Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage: A Compendium of Materials and Techniques; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Matteini, M.; Moles, A. Chimica Per l’Arte; Nardini: Firenze, Italy, 2007. [Google Scholar]
- Quagliarini, E.; Graziani, L.; Diso, D.; Licciulli, A.; D’Orazio, M. Is nano-TiO2 alone an effective strategy for the maintenance of stones in Cultural Heritage? J. Cult. Herit. 2018, 30, 81–91. [Google Scholar] [CrossRef]
- Baglioni, P.; Carretti, E.; Chelazzi, D. Nanomaterials in art conservation. Nat. Nanotechnol. 2015, 10, 287–290. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; de Buergo, M.A.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Bergamonti, L.; Predieri, G.; Paz, Y.; Fornasini, L.; Lottici, P.P.; Bondioli, F. Enhanced self-cleaning properties of N-doped TiO2 coating for Cultural Heritage. Microchem. J. 2017, 133, 1–12. [Google Scholar] [CrossRef]
- Ion, R.M.; Doncea, S.M.; Țurcanu-Caruțiu, D. Nanotechnologies in Cultural Heritage. Materials and Instruments for Diagnosis and Treatment. Laser Technology and Its Application; Harbin Institute of Technology: Harbin, China, 2019. [Google Scholar]
- Baglioni, P.; Giorgi, R. Soft and hard nanomaterials for restoration and conservation of cultural heritage. Soft Matter 2006, 4, 293–303. [Google Scholar] [CrossRef]
- Giorgi, R.; Baglioni, M.; Berti, B.P. New methodologies for the conservation of cultural heritage: Micellar solutions. microemulsions. and hydroxide nanoparticles. Acc. Chem. Res. 2010, 43, 695–704. [Google Scholar] [PubMed]
- Giorgi, R.; Dei, L.; Ceccato, M.; Schettino, C.; Baglioni, P. Nanotechnologies for Conservation of Cultural Heritage: Paper and Canvas Deacidification. Langmuir 2002, 18, 8198–8203. [Google Scholar] [CrossRef]
- Hosseini, M.; Karapanagiotis, I. Advanced Materials for the Conservation of Stone; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Van Driel, B.A.; Kooyman, J.; Van den Berg, K.J.; Schmidt-Ott, A.; Dik, J. A Quick Assessment of the Photocatalytic Activity of TiO2 Pigments. From lab to Conservation Studio. Microchem. J. 2016, 126, 162–171. [Google Scholar] [CrossRef]
- Van Driel, B.A.; Van den Berg, K.J.; Kooyman, J.; Gascon, J.; Dik, J. Determination of Early Warning Signs for Photocatalytic Degradation of Titanium White Oil Paints by Means of Surface Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 172, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Driel, B.A.; Van den Berg, K.J.; Smout, M.; Dekker, N.; Kooyman, J.; Dik, J. Investigating the Effect of Artists’ Paint Formulation on Degradation Rates of TiO2-Based Oil Paints. Herit. Sci. 2018, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Van Driel, B.A.; Van der Meer, S.R.; van den Berg, K.J.; Dik, J. Determining the Presence of Photocatalytic Titanium White Pigments via Embedded Paint Sample Staining: A Proof of Principle. Stud. Conserv. 2019, 64, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Haick, H.; Paz, Y. Long-range effects of noble metals on the photocatalytic properties of titanium dioxide. J. Phys. Chem. B 2003, 107, 2319. [Google Scholar] [CrossRef]
- Lu, Z.X.; Zhou, L.; Zhang, Z.L.; Shi, W.L.; Xie, Z.X.; Xie, H.Y.; Pang, D.W.; Shen, P. Cell damage induced by photocatalysis of TiO2 thin films. Langmuir 2013, 19, 87. [Google Scholar]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Sadrolhosseini, A.R.; Mahdi, M.A.; Alizadeh, F.; Rashid, S.A. Laser Ablation Technique for Metal Nanoparticles in Liquid, Laser Technology, and Its Application; Harbin Institute of Technology: Harbin, China, 2019. [Google Scholar]
- Thompson, D.V. The Craftsman’s Handbook: Il Libro dell’Arte by Cennino d’Andrea Cennini; Dover: New York, NY, USA, 1959. [Google Scholar]
- Mayer, R. The Artist’s Handbook of Materials and Techniques; Viking: New York, NY, USA, 1991. [Google Scholar]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Oleari, C. Standard Colorimetry: Definitions. Algorithms and Software; John Wiley & Sons: Chichester, UK, 2016. [Google Scholar]
- Judd, D.B.; Wyszecki, G. Color in Business. Science and Industry; John Wiley: New York, NY, USA, 1952. [Google Scholar]
- Clarke, F.J.J.; McDonald, R.; Rigg, B. Modification to the JPC79 colour difference formula. J. Soc. Dye. Colour. 1984, 100, 128–132. [Google Scholar] [CrossRef]
- UNI 10829:1999 Works of Art of Historical Importance. Ambient Conditions for the Conservation. Measurement and Analysis; IOS: Rome, Italy, 1999.
- EN 1586:2010. Conservation of Cultural Property—Test Methods—Color Measurement of Surfaces; UNI Ente Nazionale Italiano di Unificazione: Rome, Italy, 2010.
- Zimbone, M.; Buccheri, M.A.; Cacciato, G.; Sanz, R.; Rappazzo, G.; Boninelli, S.; Reitano, R.; Romano, L.; Privitera, V.; Grimaldi, M.G. Photocatalytical and antibacterial activity of TiO2 nanoparticles obtained by laser ablation in water. Appl. Catal. B Environ. 2015, 165, 487–494. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Buccheri, M.A.; Sanz, R.; Piluso, N.; Reitano, R.; La Via, F.; Grimaldi, M.G.; Privitera, V. Photocatalytical activity of amorphous hydrogenated TiO2 obtained by pulsed laser ablation in liquid. Mater. Sci. Semicond. Process. 2016, 42, 28–31. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Sanz, R.; Carles, R.; Gulino, A.; Privitera, V.; Grimaldi, M.G. Black TiO x photocatalyst obtained by laser irradiation in water. Catal. Commun. 2016, 84, 11–15. [Google Scholar] [CrossRef]
- Cacciato, G.; Zimbone, M.; Ruffino, F.; Grimaldi, M.G. TiO2 Nanostructures and Nanocomposites for Sustainable Photocatalytic Water Purification. In Green Nanotechnology-Overview and Further Prospects; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Zecchi Official Site. Available online: http://www.zecchi.it (accessed on 8 August 2021).
- Lyklema, J. Fundamental Soft Interface and Colloid Science; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Gueli, A.M.; Pasquale, S.; Politi, G.; Stella, G. The Role of Scale Adjustment in Color Change Evaluation. Instruments 2019, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods. Quantitative Data and Formulas; John Wiley and Sons Inc.: New York, NY, USA, 1967. [Google Scholar]
- CIE 15:2004. Colorimetry, 3rd ed.; Commission Internationale de l’Éclairage: Vienna, Austria, 2004. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Meiron, S.; Marmur, A.; Saguy, I.S. Contact angle measurement on rough surface. J. Colloid Interface Sci. 2004, 274, 637–644. [Google Scholar] [CrossRef]
- Marmur, A. Apparent contact angle on rough surfaces: The Wenzel equation revisited. Colloids Surf. A Photochem. Eng. Asp. 1999, 156, 381–388. [Google Scholar]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Self-cleaning materials on Architectural Heritage: Compatibility of photoinduced hydrophilicity of TiO2 coatings on stone surfaces. J. Cult. Herit. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Feller, R.F. Accelerated Aging. Photochemical and Thermal Aspects; Getty Publications: Los Angeles, CA, USA, 1994. [Google Scholar]
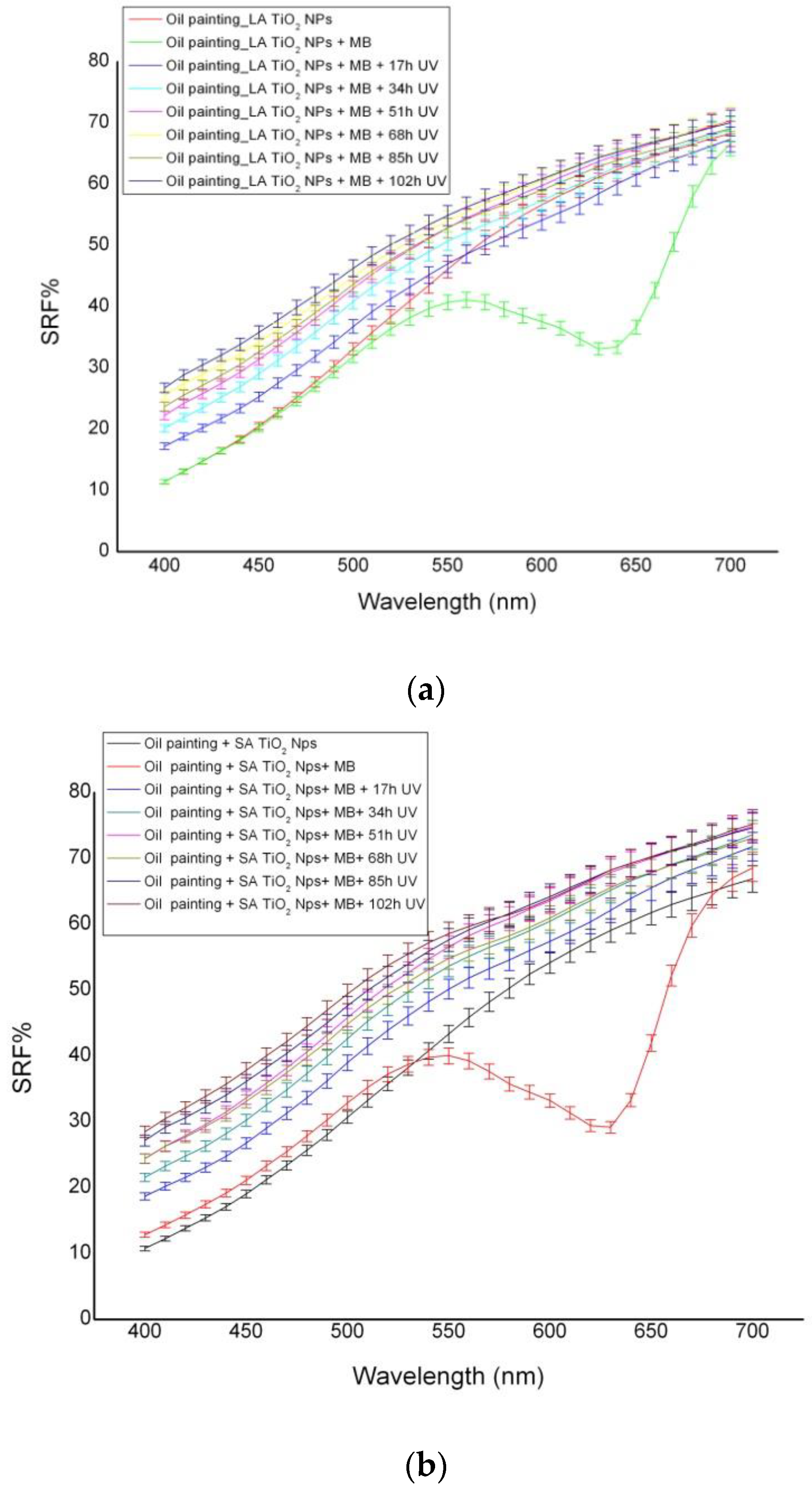
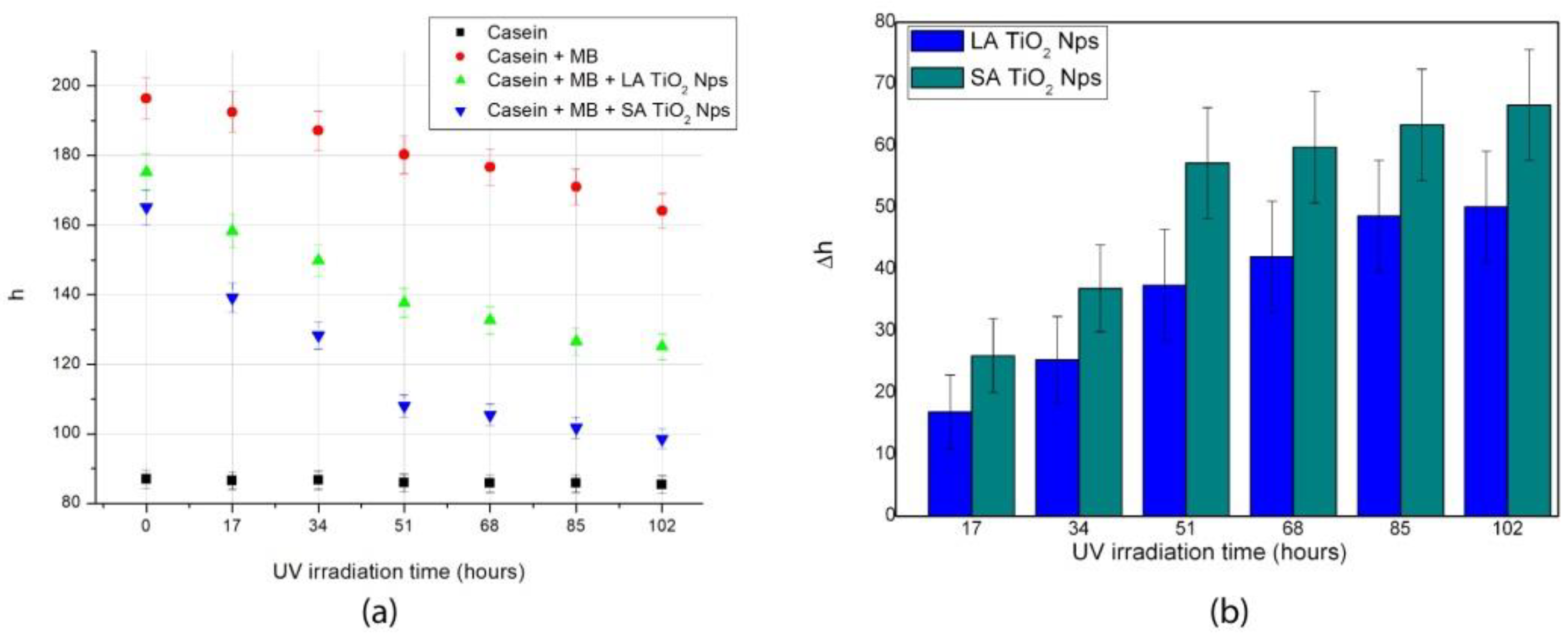
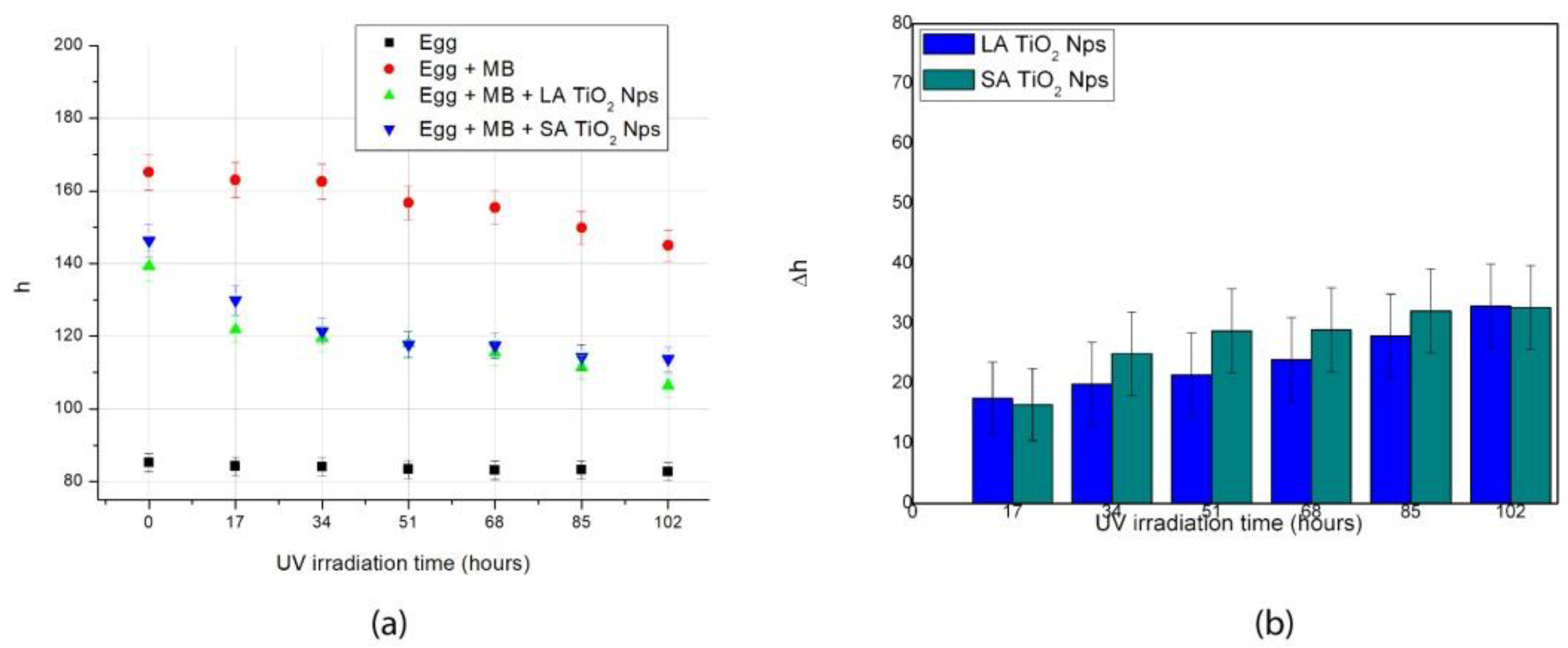


| Painting | a | b | c | R2 |
|---|---|---|---|---|
| Casein | 0.50 ± 0.02 | 116.46 ± 68.48 | 0.95 ± 0.02 | 0.969 |
| Casein + LA TiO2 | 0.52 ± 0.02 | 40.38 ± 5.52 | 0.49 ± 0.02 | 0.987 |
| Casein + SA TiO2 | 0.71 ± 0.05 | 75.64 ± 11.56 | 0.31 ± 0.05 | 0.994 |
| Egg Tempera | 0.20 ± 0.04 | 144.59 ± 44.24 | 0.81 ± 0.04 | 0.994 |
| Egg Tempera + LA TiO2 | 0.23 ±0.02 | 32.32 ± 7.49 | 0.77 ± 0.02 | 0.973 |
| Egg Tempera + SA TiO2 | - | - | - | 0.953 |
| Linseed Oil | 0.37 ± 0.02 | 45.86 ± 5.34 | 0.62 ± 0.02 | 0.992 |
| Linseed Oil + LA TiO2 | 0.53 ± 0.09 | 161.32 ± 39.54 | 0.47 ± 0.09 | 0.997 |
| Linseed Oil + SA TiO2 | 0.53 ± 0.03 | 67.51 ± 10.11 | 0.48 ± 0.04 | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquale, S.; Zimbone, M.; Ruffino, F.; Stella, G.; Gueli, A.M. Evaluation of the Photocatalytic Activity of Water-Based TiO2 Nanoparticle Dispersions Applied on Historical Painting Surfaces. Heritage 2021, 4, 1854-1867. https://doi.org/10.3390/heritage4030104
Pasquale S, Zimbone M, Ruffino F, Stella G, Gueli AM. Evaluation of the Photocatalytic Activity of Water-Based TiO2 Nanoparticle Dispersions Applied on Historical Painting Surfaces. Heritage. 2021; 4(3):1854-1867. https://doi.org/10.3390/heritage4030104
Chicago/Turabian StylePasquale, Stefania, Massimo Zimbone, Francesco Ruffino, Giuseppe Stella, and Anna Maria Gueli. 2021. "Evaluation of the Photocatalytic Activity of Water-Based TiO2 Nanoparticle Dispersions Applied on Historical Painting Surfaces" Heritage 4, no. 3: 1854-1867. https://doi.org/10.3390/heritage4030104
APA StylePasquale, S., Zimbone, M., Ruffino, F., Stella, G., & Gueli, A. M. (2021). Evaluation of the Photocatalytic Activity of Water-Based TiO2 Nanoparticle Dispersions Applied on Historical Painting Surfaces. Heritage, 4(3), 1854-1867. https://doi.org/10.3390/heritage4030104







