Towards Sustainable Museum Conservation Practices: A Study on the Surface Cleaning of Contemporary Art and Design Objects with the Use of Biodegradable Agents
Abstract
:1. Introduction
1.1. Examples of Green Materials Used in Conservation
1.2. Cleaning Applications on Plastics
1.3. Green and Sustainable Approaches for the Cleaning Treatment of Plastics
2. Materials and Methods
2.1. Sample Types and Preparation
2.2. Cleaning Tests
2.2.1. Artificial Soiling
2.2.2. Cleaning Agents and Application Methods
2.3. Methodology for the Surface Examination
2.3.1. Microscopy Techniques
2.3.2. Raman Spectroscopy
2.3.3. Gloss Measurement
3. Results–Discussion
3.1. Cleaning Application Method Effect
3.2. Cleaning of Untreated and Artificially Soiled Samples
3.2.1. DI Water
3.2.2. Chelate MGDA Solution
3.2.3. Surfactant Solution (Plurafac® LF 900)
3.2.4. Agar Gel
3.2.5. Limonene
3.2.6. Ethyl Lactate
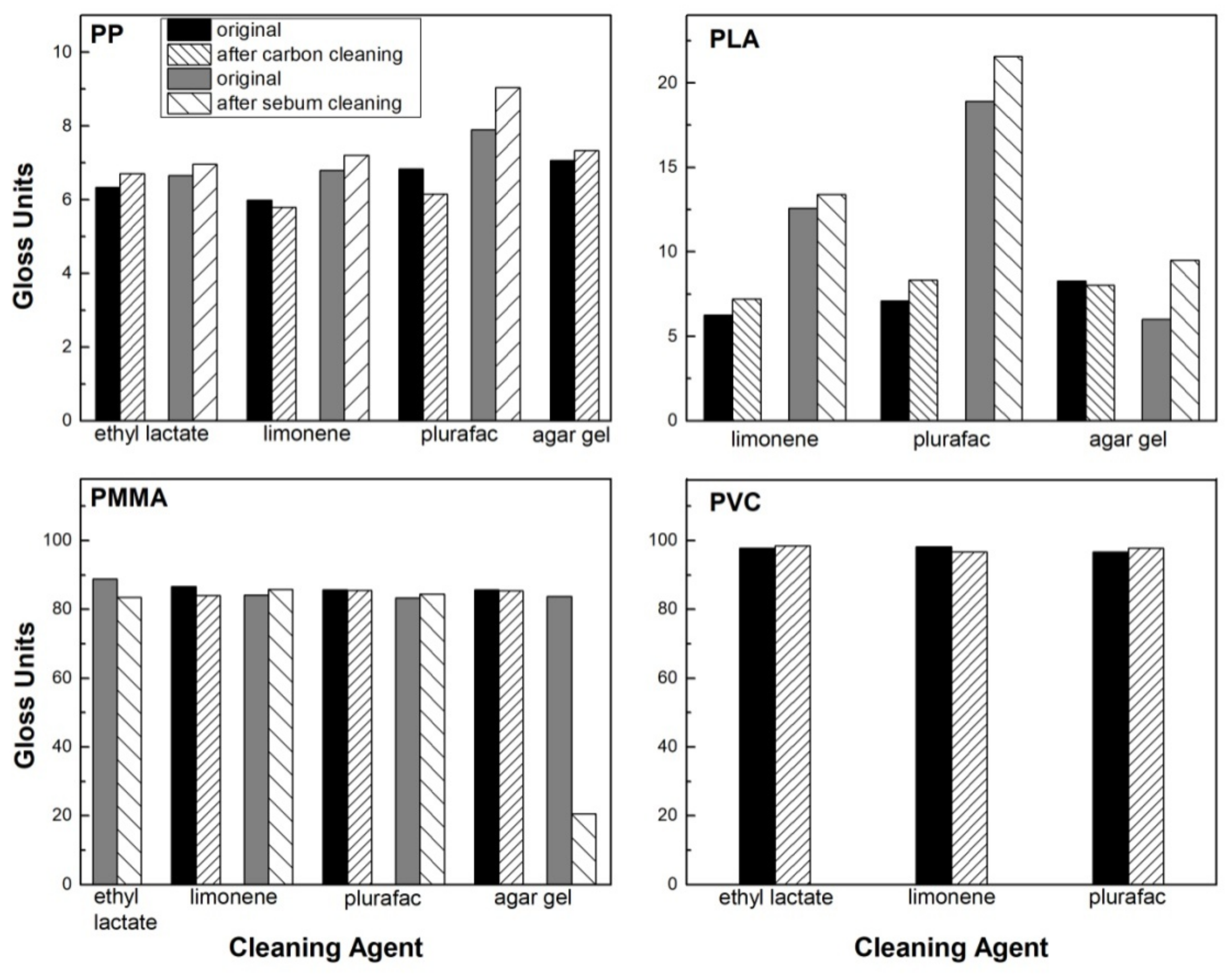
3.3. Gloss Measurements
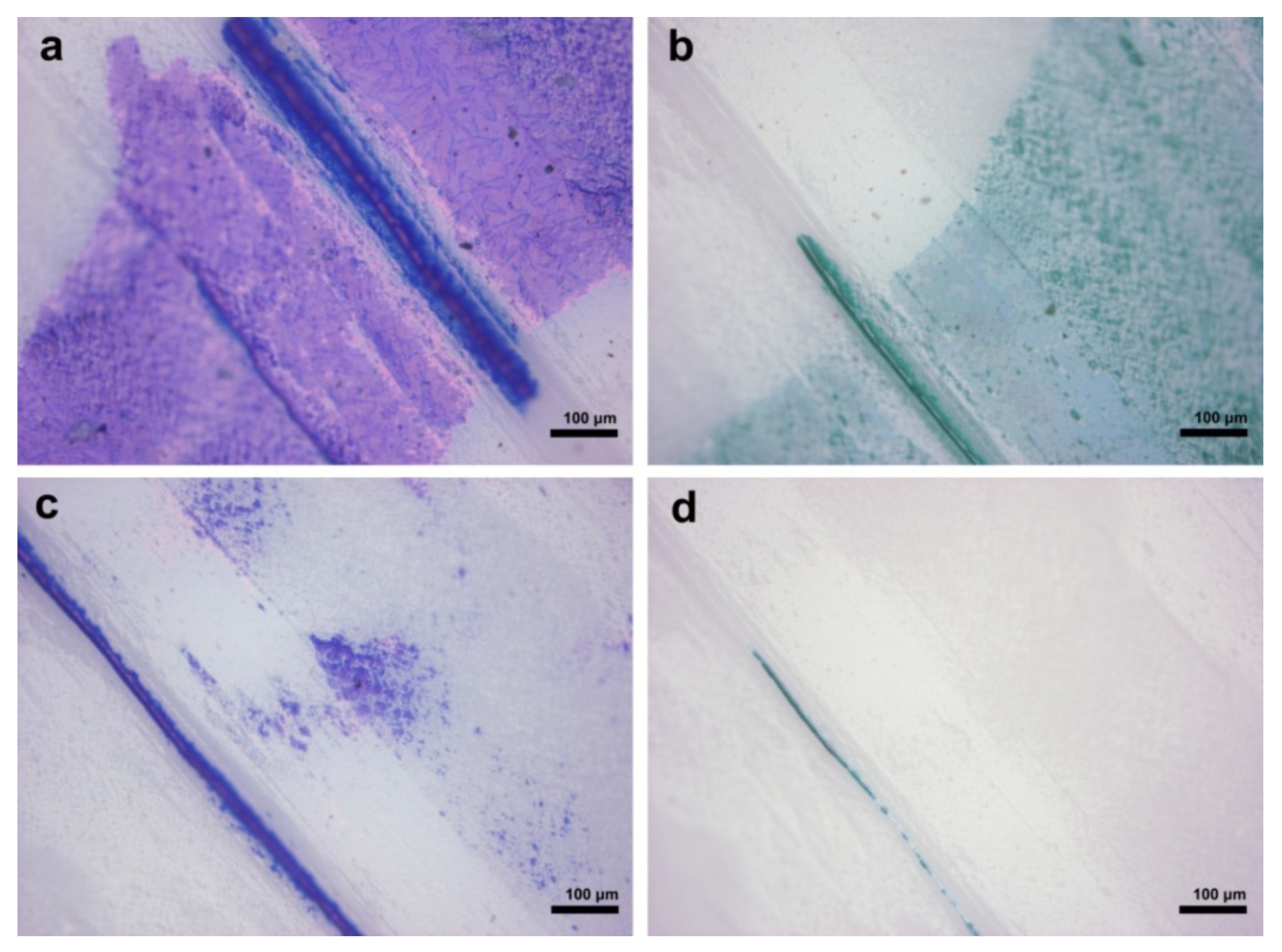
3.4. Ink Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McGhie, H. Evolving climate change policy and museums. Mus. Manag. Curatorship 2020, 35, 653–662. [Google Scholar] [CrossRef]
- Bickersteth, J. IIC and ICOM-CC 2014 Declaration on environmental guidelines. Stud. Conserv. 2016, 61, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Macchia, A.; Sacco, F.; Morello, S.; Prestileo, F.; La Russa, M.F.; Ruffolo, S.A.; Luvidi, L.; Settimo, G.; Rivaroli, L.; Laurenzi, M.; et al. Chemical Exposure in Cultural Heritage Restoration: Questionnaire to Define the State of Art. In Proceedings of the Scienza e Beni Culturali, Quale Sostenibilita’ per il Restauro? Bressanone, Italy, 1–4 July 2014; Edizioni Arcadia Ricerche: Venezia, Italy, 2014; pp. 529–539. [Google Scholar]
- De Silva, M.; Henderson, J. Sustainability in conservation practice. J. Inst. Conserv. 2011, 34, 5–15. [Google Scholar] [CrossRef]
- Silence, P. How Are US Conservators Going Green? Results of Polling AIC Members. Stud. Conserv. 2010, 55, 159–163. [Google Scholar] [CrossRef]
- Balliana, E.; Ricci, G.; Pesce, C.; Zendri, E. Assessing the Value of Green Conservation Cultural Heritage: Positive Aspects already available methodologies. Int. J. Conserv. Sci. 2016, 7, 185–202. [Google Scholar]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Grimaldi, N.; Singer, B.; Richards, N. Testing limonene as a possible alternative solvent to petroleum based aromatic hydrocarbons for use in conservation. In Proceedings of the Going Green: Towards Sustainability in Conservation, London, UK, 24 April 2009. [Google Scholar]
- Ormsby, B.; Barker, R.; Keefe, M.; Tucker, C.; Donate, F.; Smithen, P. The removal of graffiti ink from Mark Rothko’s Untitled(Black on Maroon), 1958. A collaborative approach. In Proceedings of the ICOM-CC 17th Triennial Meeting Preprints, Melbourne, Australia, 19–23 September 2014; Pulido & Nunes, Ed.; Available online: https://www.icom-cc-publications-online.org/1435/The-removal-of-graffiti-ink-from-Mark-Rothkos-Black-on-Maroon-1958-a-collaborative-approach (accessed on 10 June 2021).
- Macchia, A.; Rivaroli, L.; Gianfreda, B. The GREEN RESCUE: A ‘green’ experimentation to clean old varnishes on oil paintings. Nat. Prod. Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Pacheco, M.F.; Pereira, A.I.; Branco, L.C.; Parola, A.J. Varnish removal from paintings using ionic liquids. J. Mater. Chem. A 2013, 1, 7016–7018. [Google Scholar] [CrossRef]
- Hrdlickova Kuckova, S.; Crhova Krizkova, M.; Cortes Pereira, C.L.; Hynek, R.; Lavrova, O.; Busani, T.; Branco, L.C.; Sandu, I.C.A. Assessment of Green Cleaning Effectiveness on Polychrome Surfaces by MALDI-TOF Mass Spectrometry and Microscopic Imaging. Microsc. Res. Tech. 2014, 77, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Prati, S.; Volpi, F.; Fontana, R.; Galletti, P.; Giorgini, L.; Mazzeo, R.; Mazzocchetti, L.; Samorì, C.; Sciutto, G.; Tagliavini, E. Sustainability in art conservation: A novel bio-based organogel for the cleaning of water sensitive works of art. Pure Appl. Chem. 2018, 90, 239–251. [Google Scholar] [CrossRef]
- Cremonesi, P.; Casoli, A. Thermo-reversible rigid agar hydrogels: Their properties and action in cleaning. In Gels in the Conservation of Art, Preprints; Angelova, L., Ormsby, B., Townsend, J.H., Wolbers, R., Eds.; Archetype Publications: London, UK, 2017; pp. 19–27. [Google Scholar]
- Hughes, A.; Sullivan, M. Targeted Cleaning of Works on Paper: Rigid Polysaccharide Gels and Conductivity in Aqueous Solutions. Book Pap. Group Annu. 2016, 35, 30–41. [Google Scholar]
- Mazzuca, A.; Micheli, L.; Cervelli, E.; Basoli, F.; Cencetti, C.; Coviello, T.; Iannuccelli, S.; Sotgiu, S.; Palleschi, A. Cleaning of Paper Artworks: Development of an Efficient Gel-Based Material Able to Remove Starch Paste. ACS Appl. Mater. Interfaces 2014, 6, 16519. [Google Scholar] [CrossRef] [Green Version]
- Volk, A.; Van der Berg, K.J. Agar—A new tool for the surface cleaning of water sensitive oil paint? In Issues in Contemporary Oil Paint; Van der Berg, K.J., Burnstock, A., De Keijzer, M., Krueger, J., Learner, T., De Tagle, A., Heydenreich, G., Eds.; Springer: Cham, Switzerland, 2014; pp. 389–406. [Google Scholar] [CrossRef]
- Ormsby, B.; Soldano, A.; Keefe, M.H.; Phenix, A.; Learner, T. An Empirical Evaluation of a Range of Cleaning Agents for Removing Dirt from Artists’ Acrylic Emulsion Paints. AIC Spec. Group Postprints 2013, 23, 77–87. [Google Scholar]
- Fardi, T.; Pintus, V.; Kampasakali, E.; Pavlidou, E.; Papaspyropoulos, K.G.; Schreiner, M.; Kuriacou, G. A novel methodological approach for the assessment of surface cleaning of acrylic emulsion paints. Microchem. J. 2018, 141, 25–39. [Google Scholar] [CrossRef]
- Macchia, A.; Ruffolo, S.A.; Rivaroli, L.; La Russa, M.F. The treatment of iron-stained marble: Toward a “green” solution. Int. J. Conserv. Sci. 2016, 7, 323–332. [Google Scholar]
- Solbes-García, Á.; Miranda-Vidales, J.M.; Nieto-Villena, A.; Hernández, L.S.; Narváez, L. Evaluation of the oxalic and tartaric acids as an alternative to citric acid in aqueous cleaning systems for the conservation of contemporary acrylic paintings. J. Cult. Herit. 2017, 25, 127–134. [Google Scholar] [CrossRef]
- Rapti, S.; Boyatzis, S.C.; Rivers, S.; Pournou, A. Siderophores and their Applications in Wood, Textile, and Paper Conservation. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021; pp. 301–339. [Google Scholar] [CrossRef]
- Cremonesi, P. L’uso Degli Enzimi Nella Pullitutra di Opera Policrome; Il Prato Editrize: Padova, Italy, 2002. [Google Scholar]
- Vokic, A.; Berovic, M. Use of lipase to remove oil-based overpaints. In Proceedings of the ICOM 14th Triennial Meeting, The Hague, The Netherlands, 12–16 September 2005; Verger, I., Ed.; James & James (Science Publishers): London, UK, 2005; pp. 862–868. [Google Scholar]
- Belluci, R.; Cremonesi, P.; Pignagnoli, G. A preliminary note on the use of enzymes in conservation: The removal of aged acrylic resin coatings with lipase. Stud. Conserv. 1999, 44, 278–281. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Migliore, G.; Marconi, P.; Tasso, F. Sustainable Restoration Through Biotechnological Processes: A Proof of Concept. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021; pp. 235–261. [Google Scholar] [CrossRef]
- Ranalli, G.; Alfano, G.; Belli, C.; Lustrato, G.; Colombini, M.P.; Bonaduce, I.; Zanardini, E.; Abbruscato, P.; Cappitelli, F.; Sorlini, C. Biotechnology applied to cultural heritage: Biorestoration of frescoes using viable bacterial cells and enzymes. J. Appl. Microbiol. 2005, 98, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Roig, P.; Regidor-Ros, J.L.; Montes-Estellés, R. Biocleaning of nitrate alterations on wall paintings by Pseudomonas stutzeri. Int. Biodeter Biodegrad. 2013, 84, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.; Salvador, C.; Candeias, A.; Caldeira, A.T.; Pereira, A. Methodology for the synthesis of chitosan derivatives to be applied in Cultural. Int. J. Conserv. Sci. 2020, 11, 867–874. [Google Scholar]
- Silva, M.; Rosado, T.; Teixeira, D.; Candeias, A.; Caldeira, A.T. Green mitigation strategy for Cultural Heritage: Bacterial potential for biocide production. Environ. Sci. Pollut. Res. 2017, 24, 4871–4881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, A.; Santos, S.; Caetano, J.; Pintado, M.; Vieira, E.; Moreira, P.R. Basil essential oil as an alternative to commercial biocides against fungi associated with black stains in mural painting. Build. Environ. 2020, 167, 106459. [Google Scholar] [CrossRef]
- Sakr, A.A.; Ghaly, M.F.; Helal, G.E.; Abdel Haliem, M.E.F. Effect of Thymol against Fungi Deteriorating Mural Paintings at Tell Basta Tombs, Lower Egypt. Int. J. Res. Stud. Biosci. 2018, 6, 8–23. [Google Scholar] [CrossRef]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential Oils as Natural Biocides in Conservation of Cultural Heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavédrine, B.; Fournier, A.; Martin, G. (Eds.) Preservation of Plastic Artefacts in Museum Collections (POPART); Comité des Travaux Historiques et Scientifiques (CTHS): Paris, France, 2012.
- Shashoua, Y.; Segel, K.; Van Oosten, T.; Laganá, A.; Wagena, M.; Keneghan, B.; Barabant, G.; Bollard, C.; Kuperholc, S. Wiping away the dirt—A safe option for plastics? In Proceedings of the ICOM Committee for Conservation 16th Triennial Meeting, Lisbon, Portugal, 19–23 September 2011; Bridgland, J., Ed.; ICOM-CC Lisbon Preprints: Lisbon, Portugal, 2011. Available online: https://www.icom-cc-publications-online.org/1242/Wiping-away-the-dirt--a-safe-option-for-plastics (accessed on 10 June 2021).
- Fricker, A. The Conservation of Polymeric Materials in Museum Collections Using Advanced Surface Science and Surface Analysis Techniques. Ph.D. Thesis, Imperial College London, London, UK, 2016. [Google Scholar]
- Fricker, A.L.; McPhail, D.S.; Keneghan, B.; Pretzel, B. Investigating the impact of cleaning treatments on polystyrene using SEM, AFM and ToF–SIMS. Herit. Sci. 2017, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Kavda, S.; Dhopatkar, N.; Angelova, L.V.; Richardson, E.; Golfomitsou, S.; Dhinojwala, A. Surface behaviour of PMMA: Is gel cleaning the way to go? Stud. Conserv. 2016, 61, 297–299. [Google Scholar] [CrossRef]
- Kavda, S.; Richardson, E.; Golfomitsou, S. The Use of Solvent-Gel Systems for the Cleaning of PMMA. MRS Adv. 2017, 2, 2179–2187. [Google Scholar] [CrossRef]
- Hackett, J. Cleaning PVC with Microemulsions. W A Conserv. J. 2014, 62. Available online: http://www.vam.ac.uk/content/journals/conservation-journal/autumn-2014-issue-62/cleaning-pvc-with-microemulsions-joanne-hackett/ (accessed on 17 May 2021).
- Morales Muñoz, A. Surface modification of plasticized PVC by dry cleaning methods: Consequences for artworks C. Appl. Surf. Sci. 2010, 256, 3567–3572. [Google Scholar] [CrossRef]
- Morales Muñoz, A.; Egsgaard, H.; Landaluze, J.S.; Dietz, C. A Model for finding cleaning solutions for plasticized PVC surfaces of collections objects. J. Am. Inst. Conserv. 2014, 53, 236–251. [Google Scholar] [CrossRef]
- Morrison, J. Should we clean plastics like we clean paintings? A study in cleaning plasticised poly (vinyl chloride). In Proceedings of the Plastic in Peril: Focus on Conservation of Polymeric Materials in Cultural Heritage, Virtual Conference. 16–19 November 2020. [Google Scholar]
- Ormsby, B.; Keefe, M.; Phenix, A.; Von Aderkas, E.; Learner, T.; Tucker, C.; Kozak, C. Mineral Spirits-Based Microemulsions: A Novel Cleaning System for Painted Surfaces. J. Am. Inst. Conserv. 2016, 55, 12–31. [Google Scholar] [CrossRef]
- Available online: https://sites.fct.unl.pt/plasco2/home (accessed on 15 June 2021).
- Bartoletti, A.; Ferreira, J.L.; Casimiro, T.; Ferreira, J.T.; Soares, I.; França de Sá, S.; Babo, S.; Ramos, A.M.; Aguiar-Ricardo, A.; Quye, A.; et al. Sustainable strategies using supercritical carbon dioxide for the conservation of plastics: Insights from the PlasCO2 project. In Proceedings of the Plastic in Peril: Focus on Conservation of Polymeric Materials in Cultural Heritage, Virtual Conference, Online. 16–19 November 2020. [Google Scholar]
- Shashoua, Y. Conservation of Plastics: Materials Science, Degradation and Preservation; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Sato, S.; Gondo, D.; Wada, T.; Kanehashi, S.; Nagai, K. Effects of Various Liquid Organic Solvents on Solvent-Induced Crystallization of Amorphous Poly(lactic acid) Film. J. Appl. Polym. Sci. 2013, 129, 1607–16017. [Google Scholar] [CrossRef]
- Cai, Y.; Lv, J.; Feng, J. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Polym. Environ. 2013, 21, 108–114. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Coordination of cations in TiNb2O7 by Raman spectroscopy. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- De Groot, S. The Cultural Heritage Agency of the Netherlands: RSR00005, Polyvinyl chloride plasticized; Ed. Beth A. Price, Boris Pretzel and Suzanne Quillen Lomax. Infrared and Raman Users Group Spectral Database. Infrared and Raman Users Group. 2007. Available online: www.irug.org (accessed on 13 July 2021).
- Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/quality/spectra/396/367/RAIR010011.pdf (accessed on 13 July 2021).
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658. [Google Scholar] [CrossRef]
- Cirimina, R.; Lomeli-Rodriguez, M.; Carà, P.D.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288. [Google Scholar] [CrossRef]
- Walsh, J.; Tse, N. Cleaning of Painted surfaces with green chemicals: Investigating the use of sustainable removal of natural and synthetic resin varnishes from oil-based painted surfaces. In Proceedings of the Making Conservation AICCM National Conference Prepritns, Melbourne, Australia, 13–15 November 2019; p. 39. [Google Scholar]
- Garlotta, A. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Cremonesi, P. Surface cleaning? Yes, freshly grated Agar gel, Please. Stud. Conserv. 2016, 61, 1–6. [Google Scholar] [CrossRef]
- Available online: https://deffner-johann.de/en/evolon-cr-en.html (accessed on 15 June 2021).
- Available online: https://www.getty.edu/conservation/our_projects/education/caps/pH_water_recipes.pdf (accessed on 6 July 2021).
- Hanson, A.R. A National Measurement Godd Practice Guide No. 94, Good Practice Guide for the Measurement of Gloss; National Physical Laboratory: Middlesex, UK, 2006; p. 20.
- Vergeer, M.; Jan van den Berg, K.; Van Oudheusden, S.; Stols-Witlox, M. (Eds.) Evolon® CR microfiber cloth as a tool for varnish removal. In Conservation of Modern Oil Paintings; Springer: Cham, Switzerland, 2020; pp. 587–596. [Google Scholar] [CrossRef]
- Rocco, F. A multisciplinary approach to the study and conservation of the contemporary Sarah lucas’ papier-maché sculpture Love Me. CeRoArt 2016. [Google Scholar] [CrossRef]
- Ecker, J.V.; Haider, A.; Burzic, I.; Huber, A.; Eder, G.; Hild, S. Mechanical properties and water absorption behaviour of PLA and PLA/wood composites prepared by 3D printing and injection moulding. Rapid Prototyp. J. 2019, 25, 672–678. [Google Scholar] [CrossRef]
- Baran, E.H.; Erbil, H.Y. Surface modification of 3D printed PLA objects by fused deposition modelling: A review. Colloids Interfaces 2019, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Zhao, H.; Jiag, P. Poly(vinyl-chloride) films plasticized with novel Poly-nadic-anhydride polyester plasticizers. J. Vinyl Addit. Technol. 2017, 23, 321–332. [Google Scholar] [CrossRef]
- Morrison, R.; Van den Berg, K.J.; Burnstock, A.; Van Keulen, H.; Bagley-Young, A. An investigation of parameters for the use of citrate solutions for surface cleaning unvarnished paintings. Stud. Conserv. 2007, 52, 255–270. [Google Scholar] [CrossRef]
- Kampasakali, E.; Fardi, T.; Pavlidou, E.; Chatzidakis, M. Green Conservation Approaches for Contemporary Art and Design Objects: The use of microemulsions and agar gels for the cleaning treatments of acrylic paint and PLA surfaces. In Proceedings of the 3rd International Conference in Green Conservation of Cultural Heritage, Porto, Portugal, 10–11 October 2019. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters, A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 29. [Google Scholar]
- Stefanis, E.; Panayiotou, C. Prediction of Hansen Solubility Parameters with a New Group-Contribution Method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Fardi, T.; Stefanis, E.; Panayiotou, C.; Abbott, S.; Van Loon, S. Artwork conservation materials and Hansen solubility parameters: A novel methodology towards critical solvent selection. J. Cult. Herit. 2014, 15, 583–594. [Google Scholar] [CrossRef]
- Stefanis, E.; (Interdepartmental Postgraduate Program for Protection, Conservation and Restoration of Cultural Monuments, Faculty of Engineering, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece, em.stefanis@gmail.com). Personal communication, 2021.
- Shashoua, Y. Effect of indoor climate at the rate and degradation mechanism of plasticized poly (vinyl chloride). Polym. Degrad. Stab. 2003, 81, 29–36. [Google Scholar] [CrossRef]
- Harrison, M. Labelling and Marking Museum Objects Booklet; Collections Trust: London, UK, 2008; p. 5. Available online: https://collectionstrust.org.uk/wp-content/uploads/2016/11/labelling-and-marking-booklet-2020.pdf (accessed on 20 June 2021).
- Braun, T.J. An alternative technique for applying accession numbers to museum artifacts. J. Am. Inst. Conserv. 2007, 46, 91. [Google Scholar] [CrossRef]

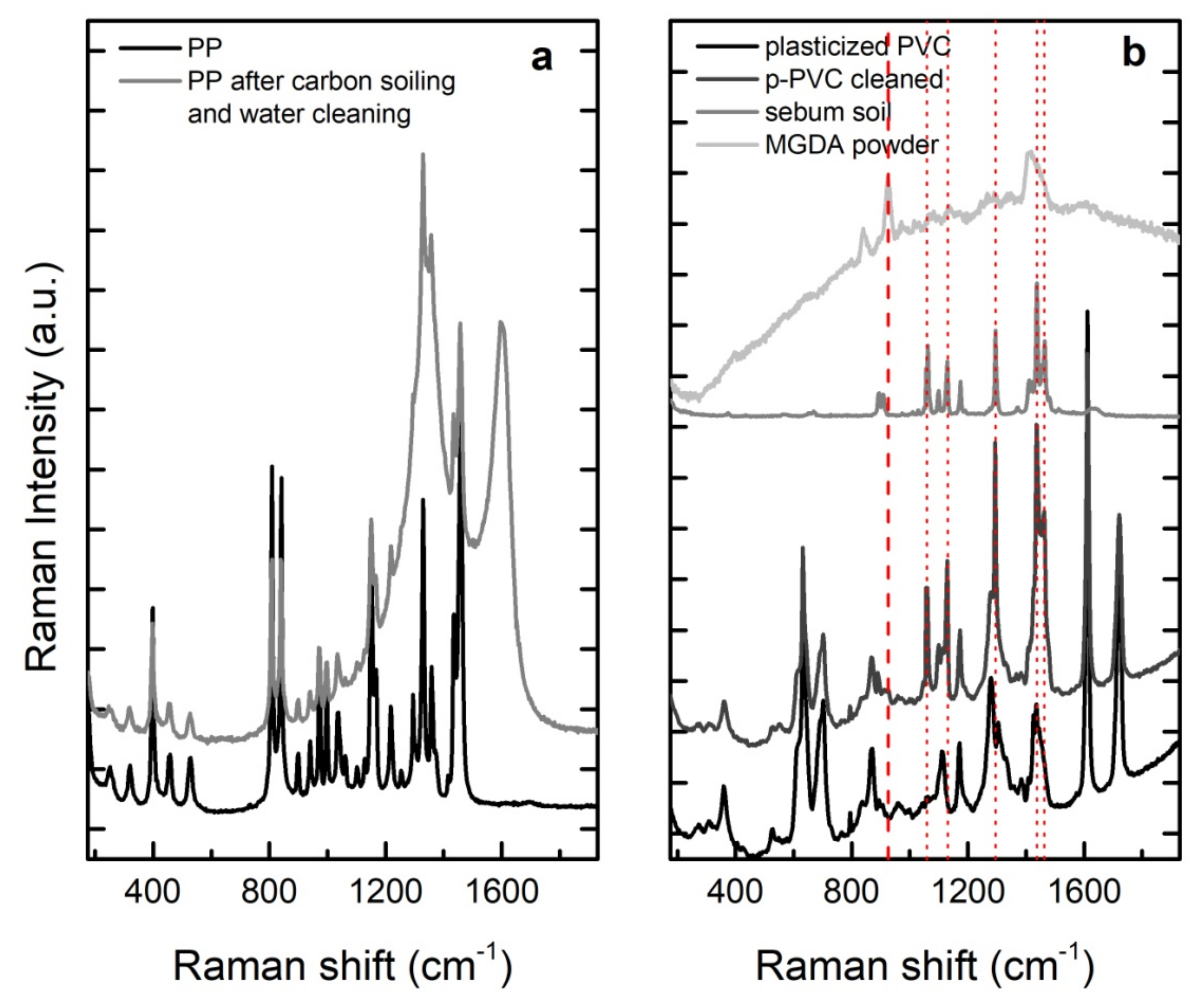
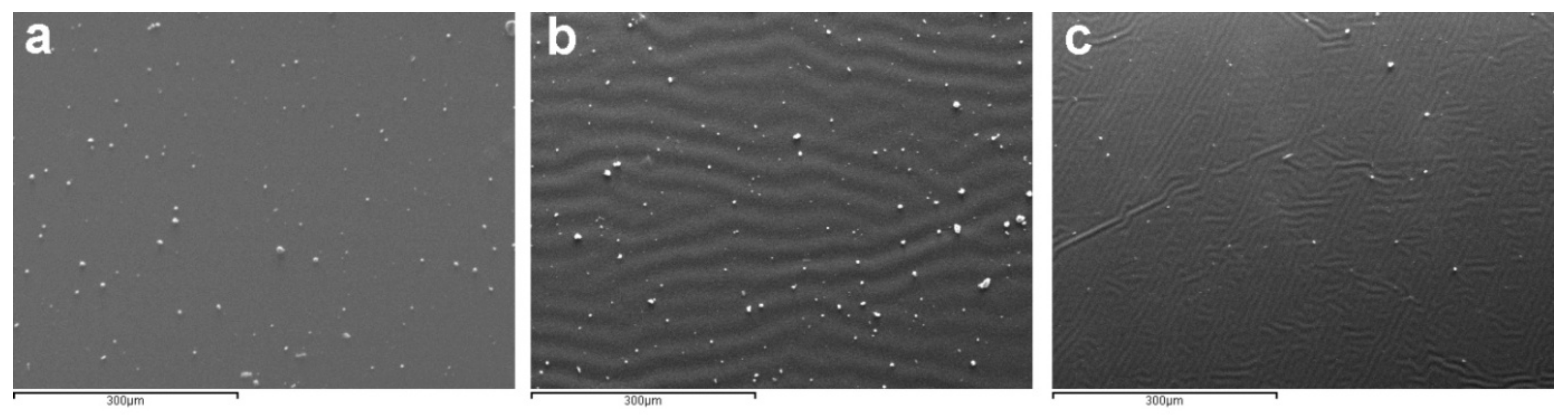
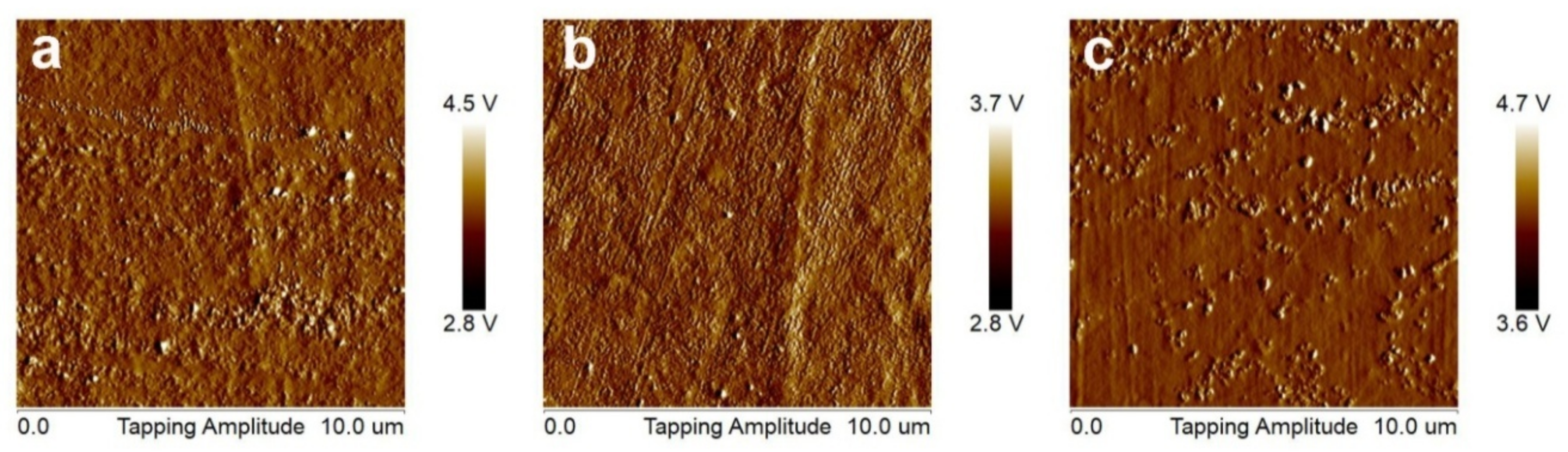
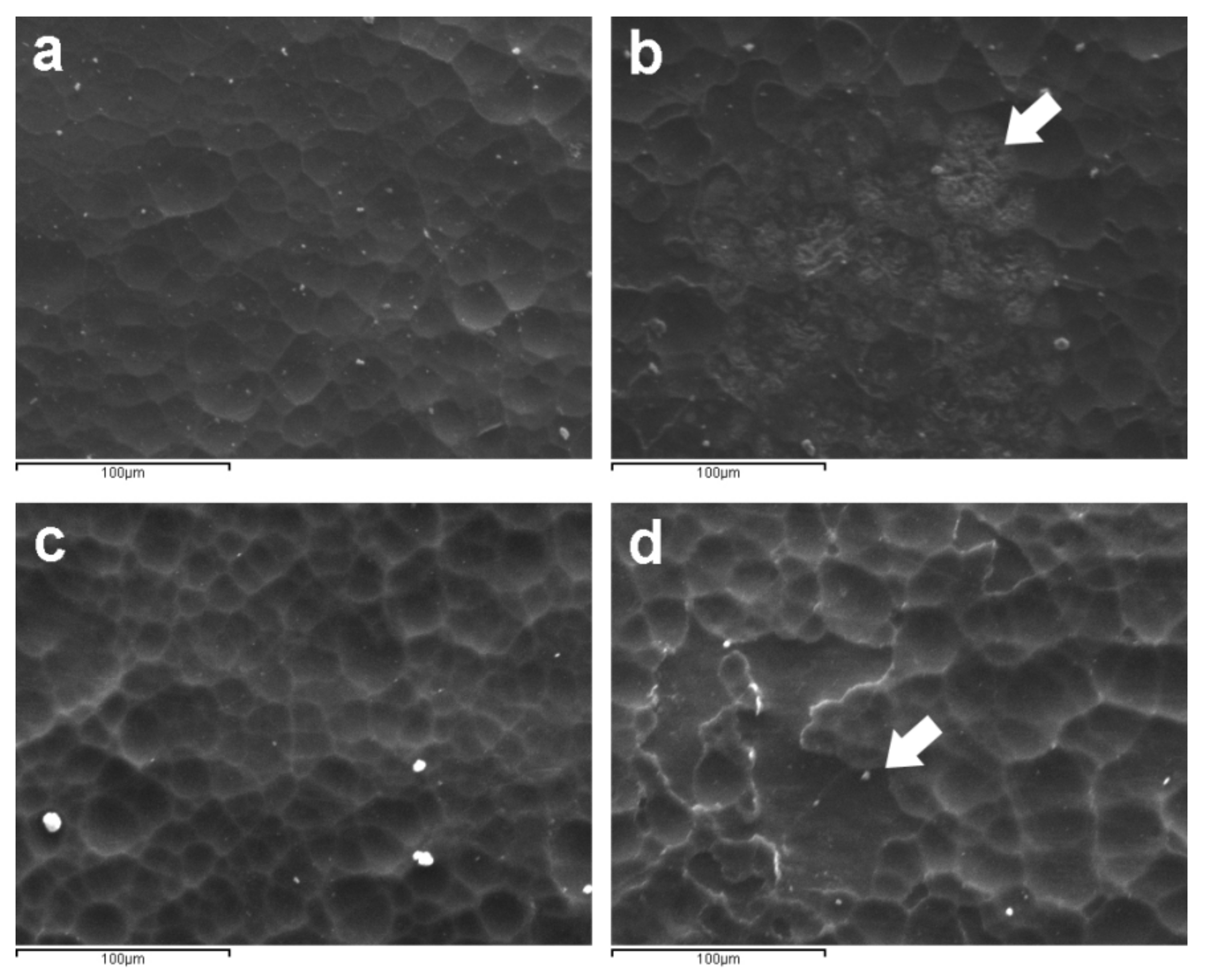


| Cleaning Agent | Cleaning System Details | Application Mode |
|---|---|---|
| Deionised water | buffered to pH 6 and conductivity 6 mS/cm (ammonium acetate) [59] | |
| Agar powder (ACROS) | 4% agar in DI water |
|
| Trisodium salt of methylglycinediacetic acid (MGDA—Trilon® M by BASF) | 2% in DI water |
|
| Non-ionic surfactant based on alkoxylated fatty alcohols (Plurafac®LF900 by BASF) | 1% in DI water | |
| (R)-(+)- Limonene (Sigma-Aldrich) | ||
| (-)-Ethyl L-lactate (Sigma-Aldrich) |
| DI Water | Agar Gel Film | Agar Gel Eraser | Plurafac® Solution | MGDA Solution | Ethyl Lactate | Limonene | ||
|---|---|---|---|---|---|---|---|---|
| PLA | SS | - | * | - | *** | - | - | **** |
| CS | - | *** | **** | |||||
| PMMA | SS | - | * | - | *** | - | ** | *** |
| CS | * | *** | **** | - | ||||
| PP | SS | - | - | - | *** | ** | **** | **** |
| CS | * | *** | **** | *** | ||||
| p-PVC | SS | - | - | - | *** | ** | **** | **** (†) |
| CS | - | * | ** | **** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampasakali, E.; Fardi, T.; Pavlidou, E.; Christofilos, D. Towards Sustainable Museum Conservation Practices: A Study on the Surface Cleaning of Contemporary Art and Design Objects with the Use of Biodegradable Agents. Heritage 2021, 4, 2023-2043. https://doi.org/10.3390/heritage4030115
Kampasakali E, Fardi T, Pavlidou E, Christofilos D. Towards Sustainable Museum Conservation Practices: A Study on the Surface Cleaning of Contemporary Art and Design Objects with the Use of Biodegradable Agents. Heritage. 2021; 4(3):2023-2043. https://doi.org/10.3390/heritage4030115
Chicago/Turabian StyleKampasakali, Elli, Theodora Fardi, Eleni Pavlidou, and Dimitrios Christofilos. 2021. "Towards Sustainable Museum Conservation Practices: A Study on the Surface Cleaning of Contemporary Art and Design Objects with the Use of Biodegradable Agents" Heritage 4, no. 3: 2023-2043. https://doi.org/10.3390/heritage4030115
APA StyleKampasakali, E., Fardi, T., Pavlidou, E., & Christofilos, D. (2021). Towards Sustainable Museum Conservation Practices: A Study on the Surface Cleaning of Contemporary Art and Design Objects with the Use of Biodegradable Agents. Heritage, 4(3), 2023-2043. https://doi.org/10.3390/heritage4030115





