1. Introduction
A distinctive feature of cervids is antler, whose complex physiology, anatomy, and taphonomy differ from that of skeletal bone, even if their chemical compositions are identical. Grown by males in red deer, antler maintains a special position in archaeology as well. This paper is proceeding from the analysis of a pathological-looking stray find of red deer (Cervus elaphus Linnaeus, 1758) antler from Stockholm University (Sweden) toward a general evaluation of prehistoric antler racks against the background of human-induced changes in the conformation of antler in extant red deer in Hungary.
According to mtDNA studies [
1], the western lineage of red deer is believed to derive from a glacial refugium in Iberia, free of the glacial ice that covered the rest of Europe at the time. Western red deer are widely distributed in present-day Europe. A genetic signal congruent with these mtDNA results has recently also been identified in the nuclear genome, haplogroup A being most widely distributed from Iberia throughout continental Europe, the British Isles, and Scandinavia. In central/eastern Europe it co-occurs with haplogroup C of southeastern Europe, occurring in the Balkans and the Carpathians respectively [
2].
In principle, the
terminus post quem date of the Stockholm find is thus the end of the Last Glacial Maximum. According to the fossil record, the species reached western, central, and north-western Europe, including Scandinavia, by about 10,000 BP, mostly represented by haplogroup A [
3]. This time was the beginning of the Preboreal/Boreal chronozone that corresponds to the Mesolithic in archaeological terms [
4]. Red deer became increasingly common during the Atlantic chronozone (8000–5000 BP) with the emergence of closed woodland vegetation [
5]. Red deer distribution reached the Mälardalen region toward the north that includes present-day Stockholm. This game made up half or sometimes three quarters of the identifiable bones (i.e., not including antler fragments) from hunted artiodactyls at Mesolithic inland settlements in southern Sweden [
6,
7,
8,
9].
Due to hunting pressure, habitat loss, and climate change, red deer populations declined continuously in Scandinavia [
10]. Body size also decreased in correlation with the Holocene thermal maximum [
8] around 8000–7000 BP. Habitat loss has accelerated during the last four millennia, due to deforestation by humans [
3]. By the early 17th century, the red deer distribution area in Sweden shrank to a small refugium in Scania. That genetically intact residual population of the nominal subspecies
C. e. elaphus Linnaeus, 1758 is now categorized as Near Threatened in the red list of endangered species in Sweden [
11]. The genetics of “pure”
C. e. elaphus were not found to substantially differ from contemporary Swedish red deer of possibly heterogeneous sub-specific origins. The population established to preserve the genetic characteristics of the
C. e. elaphus subspecies appeared to have lost 36 per cent of the electrophoretically measurable heterozygosity [
12].
In Hungary, red deer has been a far more constant presence beginning with the Upper Pleistocene and throughout the entire Holocene [
13], although 19th century imports have supposedly strengthened its western character in the Carpathian Basin [
14]. In Sweden, there have also been continuous Modern Age introductions of red deer from Denmark [
15].
Although red deer has often been translocated by humans, it seems that its present-day phylogeographic pattern in Europe reflects predominantly natural processes, in response to environmental changes and natural replacement between mtDNA lineages [
2]. A major body of data on extant antlers has been used here to contextualize the morphometric characteristics of the stray find from Sweden. Contemporary stag antlers show signs of targeted breeding, distinct selection pressure governed largely by trophy hunting. It is this trend that makes “genuinely wild” archaeological finds, pre-dating massive human impact particularly interesting.
2. Materials and Methods
Central to this analysis is the deformed antler of a mature stag kept in the collections of the Osteoarchaeological Research Laboratory (ORL henceforth) at the Department of Archaeology and Classical Studies, Stockholm University. Its date of acquisition and place of origin are unknown; it was probably retained in the collection only as a curious pathological case. In the absence of archaeological information funding its radiocarbon dating could not be linked to any ongoing projects. The purpose of this osteological analysis has been to describe the size and shape of this antler in order to compare it to those of prehistoric and extant red deer.
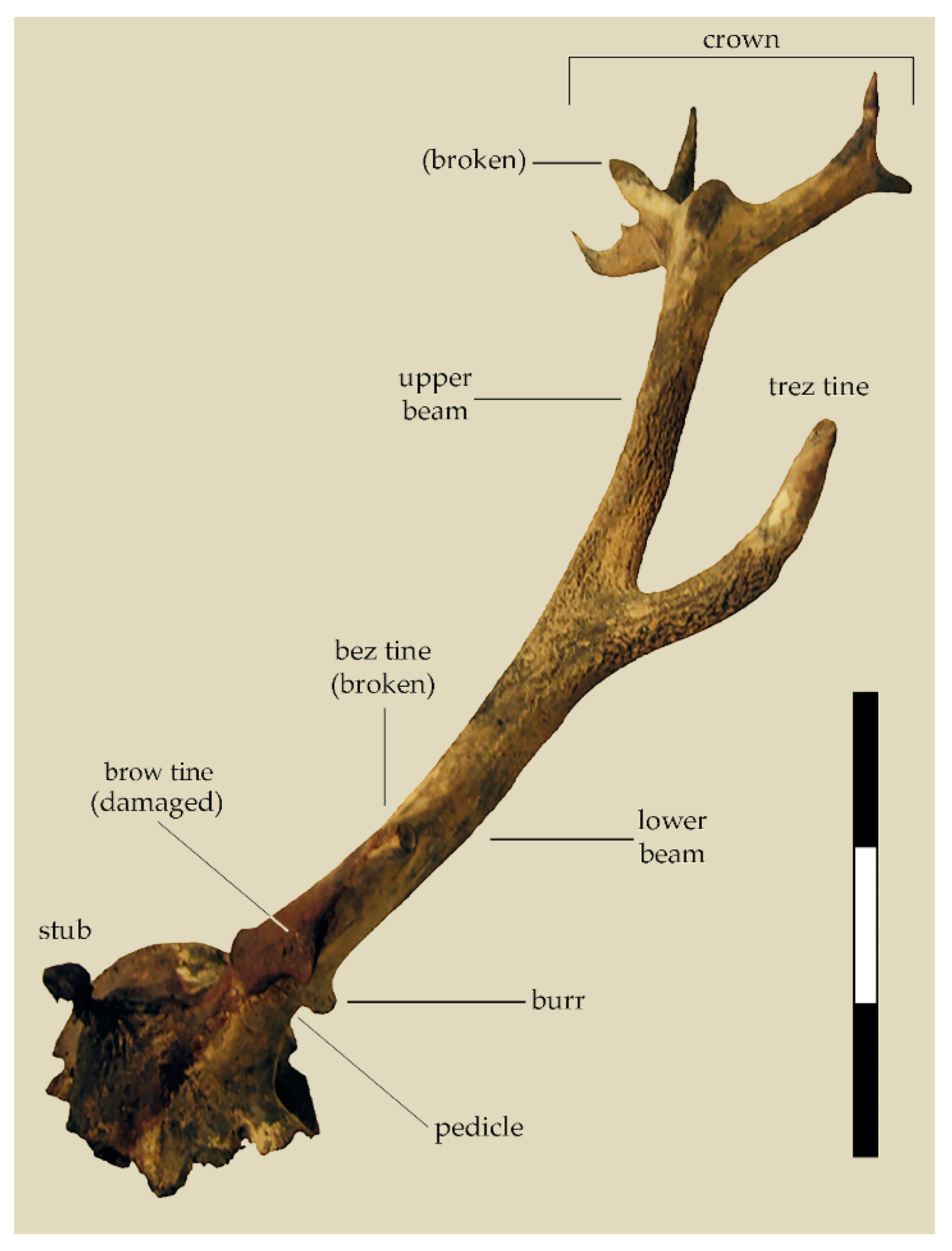
Altogether, the antler’s appearance and its degree of preservation (to be detailed later) are indicative of prehistoric origins. It consists of the left antler subject to metric analyses here, still firmly attached to the neurocranium which also holds the rudimentary right antler. The season of death of this stag must thus have been between the fall and early spring. The reason why this specimen deserves publication even in the absence of contextual information is that its right antler is represented only by a thumb-sized stub on the forehead, an unusual pathological formation.
Moreover, antlers preserved in full length are also rare among excavated remains. A complete pair of red deer antlers came to light from the seabed outside Verkö, southern Sweden. In the absence of human modification, it was considered a probable a natural deposit. That find was radiocarbon dated to the pre-Roman Iron Age (500–0 BCE) [
16].
The largely intact left antler of the ORL stag possessing a total of ten points may be of help in viewing this stray find in zoological context, revealing changes in the size and shape of red deer antler through time. Although the brow tine of the ORL specimen was damaged and the small bez tine broke off, its trez tine is complete. The fourth and fifth tines–forming the somewhat flattened crown–branch out into what is called the “composite” shape in trophy nomenclature [
14]. One of the points in the crown was broken (
Figure 1).
Characteristics of the two complete antler finds from Sweden would have been too few for detailed statistical evaluation. Their measurements were therefore analyzed against the backdrop of trophies from late 20th century Hungary. Summarized in
Table 1, the body of data selected from the set of measurements taken on trophies between 1956 and 1977 [
17] included four lengths and three circumferences in addition to the weight of the antler as well as the number of points in the crown. Although the numbers of individuals vary between sets of extant measurements, they fall within a range of 15,700 to 18,250 individuals, offering a solid standard population against which antler dimensions of prehistoric stags can be reliably compared.
Relative age estimations were carried out comparing the total number of points of prehistoric antlers as well as their pedicle lengths to the growth characteristics of 1208 extant red deer antlers of various ages studied in Germany [
18].
Diachronic changes in antler size in Sweden were reviewed using smaller sets of fossil and extant burr measurements in relation to the Verkö find [
15,
16] and comparing them to the parameters of extent red deer trophies in Hungary (
Table 1).
Antler and neurocranium dimensions on osteological finds were taken following archeozoological protocols [
19,
20], in accordance with the 1954 guidelines of the
Conseil International de la Chasse et du Conservation du Gibier (CIC) [
21], an international system using specific, unified trophy parameters. In light of the great variety of methods used in pooling different measurements in fragmented archaeological assemblages [
22], standard scores (z-scores) were calculated for each prehistoric antler measurement and pooled in relation to the mean values and standard deviations of corresponding antler dimensions in extant red deer (
Table 1).
A cultural construct dominated by antler size, the CIC scoring system for red deer is based on the arbitrary balancing of weighed quantitative characteristics (given in cm and kg; antler weight × 2, five circumferences × 1 each, total number of points × 1, lateral beam length × 0.5, brow and trez tines × 0.25 each). In addition, a maximum of 21 scores can be added on aesthetical variables such as color, spread of the beam, and surface texture. Finally, scores are reduced by perceived faults relative to the contemporary ideal of antler conformation [
23].
Linear regression analyses were used in the assessment of relative age based on antler measurements and detecting diachronic trends in contemporary CIC scores and antler weights in an effort to add emphasis to the morphological observations made in prehistoric deer. The strengths of linear correlation between the studied variables were appraised following standard criteria in statistics [
24]. The statistical significance of reported results reached the
p ≤ 0.05 level of probability. Quantitative similarities between antler finds and the mean values of extant red deer were studied using a cluster analysis. Ward’s method was chosen as in its steps the increase in within-group variance is minimized. In this calculation, the Euclidean distance measure is inherent to the algorithm [
25].
3. Results
As shown in
Table 1, the measurements available on the ORL specimen are quite similar to those of the pre-Roman Iron Age antler find from Verkö [
16]. The only difference that stands out is the more robust upper beam of the ORL stag. Based on the fragment surface, the missing bez tine of this individual was smaller than in the Verkö antlers. On the other hand, the estimated distance between the eye tine and broken brow tine was somewhat greater in the ORL antler find. Antler weights are difficult to reliably compare in archaeological specimens as they are influenced by both the chance size of the attached neurocranium fragment and various degrees of mineralization.
Measurements of the ORL antler will also be compared to three pairs of complete antlers recovered at the turn of the 19th–20th century in Austria-Hungary, kept in the Hungarian Natural History Museum, Budapest. Two of them came to light during river regulation in the Middle Tisza river valley near the town of Csongrád. The third was found downstream, some 250 km towards the south near Zemun (present-day Serbia), located at the confluence between the Danube and Sava rivers. Similar to the fragment in the ORL collections, these antlers were still attached to the neurocranium. Based on their stratigraphic positions in alluvial deposits they have been dated to the Upper Pleistocene [
26]. Their individual measurements are summarized in
Table 2.
The weight of mounted prehistoric antlers in the Hungarian Natural History Museum could not be reliably appraised. However, the ORL specimen, having only one full-grown antler, weighed 3310 g. This value was doubled in attempted comparisons with modern trophy weights from Hungary.
Figure 2 offers a visual comparison between the antlers analyzed. All prehistoric pairs appear rather symmetric; therefore, the mirror image of the ORL specimen was used to virtually replace the atrophied right antler.
3.1. Relative Ages of Antler Finds
First, relationships between antler dimensions were studied to assess the relative age of the stray antler kept at ORL and prehistoric stags. In
Figure 3, changes in two age-related quantitative characteristics are shown as a function of lateral beam length. Very high correlations [
24] in both cases are in part caused by the use of mean values for annual age cohorts for 1208 extant red deer in this case. In calendar terms, those age groups ranged between 2 and 13 years based on dental ageing. Forty 13-year-old stags with a mean lateral beam length of only 856 mm [
18] represent the data point of oldest extant red deer in these graphs in close proximity of other individuals older than ten years.
The consecutive development of antler tines is poorly fit for exact ageing as the number of points in fully grown stags is not characteristic of any particular year of life [
27] although a clear, age-related trend is evident (
Figure 3a). All prehistoric antlers are longer than the mean value for extant red deer; however, the total numbers of their points vary. In this regard, of all prehistoric specimens the Verkö stag falls closest to the expected number of points. The plot also indicates that in spite of being larger, the antlers of the ORL and Cs1 stags are less complex than those of their modern counterparts in the sample from Germany. A factor complicating this pattern must be selection pressure in game management for more numerous points in extant deer trophies as the total number of points in trophies increases the final score in the CIC system.
Pedicle length is one of the most reliable cranial indicators of a stag’s age [
28]. In old stags it becomes much reduced as new antlers grow larger, stouter, and thus heavier each year. With the exception of individual Cs 2, the evident decline in pedicle length is also visible in all large prehistoric antlers continuing the trend in extant red deer (
Figure 3b). Using solely this criterion, stag Cs 2, estimated to be 7–8 years old, looks even “younger” than the other prehistoric individuals. This measurement was not available for the Verkö antler.
3.2. Sizes and Shapes of Antler Finds
Sizes of antler finds (two from Sweden and three from Hungary) were appraised by calculating the standard (z) scores of each of their individual measurements using the means and standard deviations of extant red deer trophies from Hungary (
Table 1). These contemporary parameters are not supposed to serve for direct comparisons, but rather a standard against which prehistoric measurements can be evaluated. In
Figure 4, data on lengths, circumferences, and crown points were explored as three separate groups due to the fundamentally different geometrical nature and growth characteristics of longitudinal and transversal dimensions as well as numerically different discrete antler points.
Figure 4a shows each individual measurement available on all prehistoric specimens. As a gross trend, most lengths (blue units, tending left) are shorter and most circumferences (pink units, right) are larger in the five antler finds than the mean values of extant red deer: their overall shape is thicker and stouter than contemporary trophies. The biological age of prehistoric stags may play some role here which may be higher than the mean age of extant trophies from Hungary. In consecutive years antlers develop relatively shorter tines and thicker beams [
29]. However, the age-related number of points in the crown corresponds to the modern average and most measurements (including those of the two finds from Sweden) fall within the ±1 standard deviation range of extant red deer. Negative outliers include one of the short bez tines each in the Cs 1 and Z specimens. The two positive outliers consist of both burr circumferences of Cs 1, which thus looks the “most prehistoric” in comparison to extant trophies.
In
Figure 4b the shape of the ORL antler (also included in
Figure 4a) can be appraised separately. Its measurements reflect the trend seen in
Figure 4a, falling within the ±1 standard deviation interval of extant red deer in Hungary. In this case antler weight was included as well, although the surviving part of the neurocranium is probably larger and heavier that the standard section of the skull retained by contemporary taxidermists. According to the CIC grading system, when antlers are attached to a complete skull, trophy weights should be reduced by 0.7 kg before scoring when the total length of skull is less than 48 cm and by 0.8 kg if total cranial length exceeds 50 cm [
23]. Still, the estimated weight of this stray specimen looks lighter than the modern average. This result may also be influenced by differences between fresh and dry subfossil bone the latter having lost all water and most organic content, but not yet fossilized. The similarity seen in
Figure 4b may thus be coincidental and should not be taken at face value.
All antlers in this study (prehistoric or extant) represent the western lineage of red deer that extends far into Eastern Europe with the exception of the Carpathians, Ukraine, and Russia where only eastern haplotypes (
C. elaphus maral Ogilby, 1840) were identified [
1,
3]. An antlered braincase with the complete left antler from a Holocene gravel deposit in the eastern Carpathians near Răchiteni (Romania) was assigned to this latter subspecies [
30]. That antler indeed represents a different, more gracile morphotype: its published length measurements are longer and circumferences smaller than those of the prehistoric antlers under discussion here.
Complementing univariate statistics in
Figure 4, the similarity between antlers was summarized entering the six measurements available on all of them into a cluster analysis (
Figure 5). These measurements included lengths of the beam and the trez tine, circumferences of the burr and lower as well as upper beam, and the number of points in the crown.
The resulting dendrogram confirms that prehistoric individuals Cs 1 and Z stand apart (cluster 1), while Cs 2 is quite close to the mean value of extant trophies from Hungary in terms of the studied dimensions. Although pedicle length was not included in the cluster analysis, it is worth remembering that Cs 2 also stood out by its unusually “youthful” look in this regard (
Figure 3b). The ORL specimen is understandably different from red deer in Hungary. However, it shows more affinity to the latter group, including the mean values of extant trophies (cluster 2). The Verkö antler is most different from the others in terms of the studied six measurements.
3.3. Antler Deformation
Although the focus of this paper is not paleopathology, in addition to metric evaluation, the anomalous ORL specimen deserves brief discussion here. In contrast to its complete left antler, it is the small stub in the place of the right antler that makes this find special (
Figure 6). Barely larger than a thumb, this outgrowth is 43.2 mm long with greatest diameters of 31.1 × 23.6 mm. The retarded pedicle is 28.2 mm long. It is mushroom-shaped in its current form, with the tip being round and compact. However, part of the pedicle seems to have been heavily damaged by an interplay between pathological and taphonomic factors [
31].
Importantly, no cutmarks or scavenger gnawing could be identified on the fragment. However, judged by the dark discoloration and coarse surface of the bone material, this section of the skull spent some time in an erosive environment rich in organic compounds. These may have added to the deformation and in vivo bone loss caused by a necrotic process. It seems that the right antler of the ORL stag suffered from arrested growth, possibly due to disturbed early development during the spring/early summer.
4. Discussion
The most distinctive feature of the ORL find is the pathological deformation of the right antler. Major summaries in animal paleopathology [
32,
33,
34] do not discuss antler lesions. This may in part be explained by the short life cycle of antler: if it is not damaged by disease during its annual growth, it will show no pathological lesions. Thus, the probability of antler finds displaying any symptoms is much smaller than is the case with pathological skeletal bone possibly developing for years. In contemporary game management some antler lesions have been recorded [
35,
36,
37,
38]. However, contemporary stags growing anomalous antlers tend to be culled before the development of major deformities to prevent their procreation or on humane grounds.
When antler is shed, the open surface of the pedicle is soon covered by new skin. Antler growth below starts by the development of a fibro-cartilaginous process, pushing the newly formed skin outwards. While the cortical layer of antler is formed by desmogene ossification, the primary peg tissue inside this process is cartilage-like and is of true hyaline cartilage in its inner layers. During antler growth the interior of the peg shows endosteal apposition and perpetual bone remodeling [
39]. It may have been the early stage of this delicate process that was disrupted by trauma or infection, impeding normal antler development. The atrophied pedicle would not have been strong enough to support the weight of a fully-grown antler.
Magnell noted that the left trez tine of the Verkö stag showed something that looks like minor trauma inflicted during the spring or summer. This lesion healed as the antler was still in velvet and growing at the time [
16]. The anomaly is visible only on the antler surface and did not cause major deformation.
Pawłowska et al. [
40] reported a healed antler fracture in giant deer (
Megaloceros giganteus Blumenbach, 1799) from the proximity of Barycz, Poland dated to 40,000 BP. That right antler features a downward bend above the burr and a bony outgrowth is visible just above the brow tine in the posterior part of the bend. Detailed histological and radiographical studies have confirmed that new bone formation and callus resorption took place in the affected area.
Another example of antler fracture in red deer was reported from Croatia [
41]. It is of particular interest as symptoms could be recorded on the extant individual, thus tapho-nomic changes do not interfere with the diagnosis. That antler broke between the beam and the brow tine, due to a loss of mechanical stability impaired by purulent inflammation, likely resulting from an injury to the velvet still covering the antler. The antler could develop to normal size and shape prior to fracturing, suggesting that inflammation did not begin during its early growth. In that case the trauma would have caused some sort of malformation as is likely with the ORL specimen.
Although the possibly ancient origin of the ORL specimen cannot be directly proven on a morphometric basis, circumstantial evidence is well worth considering. By the 19th century, the red deer population in Sweden sunk to between 50–100 individuals [
10], in 1907 it consisted of only about 50 [
11]. Recently, there has been an increase thanks to the spread of deer from Scania, infiltration from Norway [
5], and the introduction of non-native stock. In 2007 there were 10,000–13,000 red deer in Sweden, by 2014 that estimate doubled. Still, in 2012/13 only 6000 red deer were hunted (27% stags), while elk and roe deer were killed almost 100,000 each [
42]. It is impossible to date the ORL specimen based on these trends, but given its degree of fossilization and—to some extent—size, it is likely that it pre-dated the dramatic decline of red deer stocks.
A long-term decline in antler size may be appraised when the burr circumferences (indicative of the carrying capacity of the burr, a proxy to antler weight) of subfossil and extant (20th century) red deer in Sweden [
15,
16] are compared with the parameters of the same measurement in extant red deer in Hungary (
Figure 7).
The Verkö antler differs from those of extant red deer in that it is larger and that the brow and trez tines are relatively thicker than in some contemporary stags in Sweden [
16]. In this sense it fits the mean of the subfossil record.
Figure 7a suggests a similarity in the distribution of burr circumferences between subfossil antlers from Sweden and extant trophies in Hungary. In spite of its slightly different proportions previously shown in
Figure 4b, the ORL antler (as well as the Verkö find) falls near the average of both data sets. Antler size distribution of extant red deer in Sweden clearly reflects this historical size decrease, illustrating that both antler finds would be unusually large by today’s local standards (
Figure 7b). In light of the completeness of the Verkö antler, Magnell could calculate the CIC score for that individual as 153 points [
16]. In recognition of extant red deer being smaller in the north of Europe (c.f.
Figure 7), the minimum CIC score for gold medal trophies is set to 180 for Scottish, Swedish and Norwegian red deer, while stags in Central Europe must score at least 210 [
43]. The Verkö “trophy” almost reaches the bronze medal threshold of 160 for present-day Sweden (170 in Central Europe).
World record CIC scores (representing the
crème de la crème of red deer trophies) showed an accelerating increase in Hungary between 1885 and 1985. During the last two decades of that period over a dozen individuals started exceeding the previously unprecedented 250 CIC score threshold [
44]. During a single human generation (1960–1985) CIC scores for these medal-winning antlers [
21] displayed a clearly increasing tendency, approaching the culturally determined mental template of the “ideal antler”. Although the effect of human preferences would be difficult to separate from natural factors,
a priori knowledge of the trophy evaluation system suggests that a classical phenomenon known in cultural anthropology is at play: “… while details vary with each individual… all conform to a cultural ‘ideal’ or mental template and … proportions, space separating significant features, and other features can be measured to discover this mental template” [
45]. The mental template embodied in the CIC scoring system has continuously shaped antlers through selective hunting.
Could similar forces have influenced the form of the ORL specimen? Building on the information on world record CIC scores used in 1999 [
44], the updated 2005 list was consulted, thus 85 additional cases were included in
Figure 8a [
46,
47].
CIC scores show a low correlation with time during the studied period, indicative of a definite but small relationship, i.e., diachronic “improvement” in terms of the mental template associated with the ideal trophy. This scoring system, however, relies heavily on quantitative characters, antler weight being weighed by 22% among the variables used in the calculation. Oscillations in
Figure 8a are largely consonant with the predicted patterns in the number of antler tines and beam length of 2926 antlers shown in exhibitions during 1881–2008 [
48]. Consequently, when only antler weight itself is plotted against the year of the kill a higher, moderate correlation can be detected, confirming a substantial diachronic increase in the largest record antlers during the 45 years interval studied (
Figure 8b).
A more detailed analysis of data on these trophies displayed a weak decline in size between 1881 and 1958 in Hungary, followed by an increase in trophy size between 1958 and 1974 (detected in my previous analysis if CIC scores [
44]), culminating in a period of stable antler tine numbers and a weak decline in beam length until 2008 [
48]. This stagnation after 1990 is also visible in
Figure 8. These trends illustrate the dynamic anthropogenic changes brought about by contemporary game management in Central Europe.
The initial, 1960–2005 list of world records in
Figure 8 included no trophy from Sweden. In May 2016, however, a stag with the largest ever antler rack in the country was killed near Karlshamn in the south. The 13.1 kg trophy scored 244.6 on the CIC scale [
49]. A “gigantic” dying stag, wounded in fight and shot in 2017 (almost 500 km north, near Kil in Värmland) scored even higher, 246.6 [
50] (red dots in
Figure 8), even above the gold medal minimum of 210 required in Central Europe [
43]. In short: as a result of conscious game management and recently developed culling regimes for stags, since the early 2000s some individuals began reaching the size and quality of world record trophies.
These trends are a stark warning that given red deer’s phenotypic variability across regions and its dynamic oscillations through time, one should be cautious in attributing the ORL specimen to any particular epoch on the basis of its appearance. All we can state is that this individual falls well within the range of average antlers of extant red deer in Hungary, which today are larger than their brethren in Sweden. Thus, while by local 20th century standards the ORL antler may be considered large, it fits within the general size distribution of subfossil antlers in Sweden (
Figure 7).
However, recently “bred” stags in Sweden have started reaching record parameters in a very short time as a result of applying strict selection criteria and “real values” of trophies in contemporary deer. These practical efforts are related to the desire of maintaining and improving some species traits in reared animals not only among livestock, but also in likewise managed game [
39]. The tight, scientific organization of breeding work in the hunting-ground has yielded a dramatic turn in recent red deer evolution, reflecting the aspirations of contemporary hunters within about a human generation.
5. Conclusions
Red deer have played important roles both in both subsistence and social hunting through time and space [
51]. Their antlers were an important raw material in prehistoric Europe [
52,
53,
54]. (Thanks to annual re-growth, shed antler may be the only “secondary product”
sensu Sherratt [
55] offered by game: a renewable resource which requires no killing.) Antler headdresses [
56] worn in various rituals carried a meaning beyond decoration [
57], making antlers a recurring feature in prehistoric “shaman” burials [
58]. Given this rich cognitive repertoire, it is unsurprising that there have been efforts to acquire large and beautiful antlers through the hunt and that for centuries anomalous antlers have also attracted special attention [
44].
The deformed right antler of the ORL stray specimen is such a curiosity that must have played a part in its acquisition. The asymmetry resulting from this grave condition, affecting the weight distribution of the head, must have had a detrimental effect on the stag’s hierarchical position among his peers, negating his reproductive role and likely even impeded his coordinated movement. Competitive disadvantage could have resulted in the deterioration of this individual’s condition. One may argue that by the standards of contemporary game management this stag would have been culled by game wardens way before full antler growth was completed on the left side.
This stray antler find is of unknown context, lacking exact chronological age. It was hypothesized that it pre-dated major changes in antler conformation due to human impact in the form of specialized trophy hunting. Recent strategies are in sharp contrast with Middle and Late Mesolithic (ca 9000–6000 cal BP) subsistence hunting in Sweden whose rich osteological evidence shows no specific conservational or management concerns but displays site-specific, territorial variations rather than regional or chronological patterns in the age and sex distributions of the red deer felled [
59].
Metric comparisons including the pre-Roman Iron Age specimen from Veksö in Sweden and three Upper Pleistocene antlers from Hungary showed that prehistoric specimens, as well as the ORL stray find, were stouter than the mean values of over 15,000 extant trophies from Hungary. The ORL antler fell near the average antler size in this comparison. While this may be considered quite large by 20th century Swedish standards, it falls around the average of subfossil stags in Sweden.
Antler weight is correlated with body size, an advantage in reproductive success among stags. Phenotypic size, however, is a complex biological phenomenon, equally influenced by inheritance, natural environment, and human manipulation both through selective hunting and landscape changes. Present day hunters collect “aesthetic” antler trophies and their strong selection pressure on extant red deer populations is shown in more-or-less steadily increasing CIC scores both in Central Europe and Sweden. Although stags use their antlers in dominance fighting during the rut, they also show them off to win access to females by display in ritualized combat. This behavior is especially important in large cervids whose “statement fights” (
Kommentkampf) may be more important than clashes that cause actual damage (
Beschädigungskampf), the latter being more typical in small-bodied deer species [
39]. Thus, in addition to recent game management, the appearance of antlers (e.g., size and bilateral symmetry) has also been subject to natural selection, making the diachronic evaluation of antler conformation a complex task.