Comparative Investigation of Red and Orange Roman Tesserae: Role of Cu and Pb in Colour Formation
Abstract
1. Introduction
2. Materials and Methods
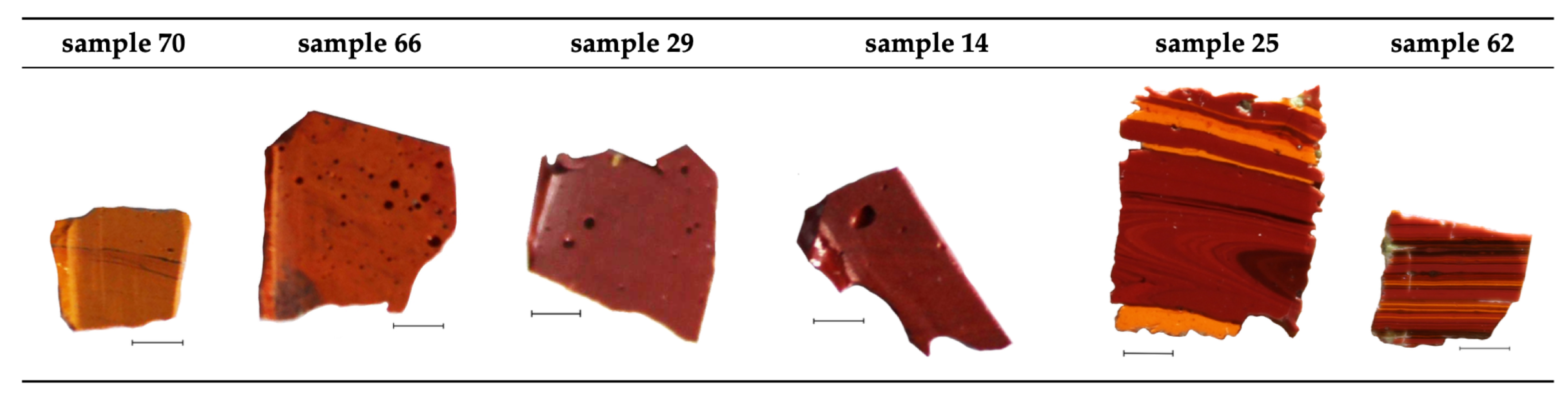
2.1. Samples Desciption
2.2. Composition Analyses
2.3. X-ray Absorption Spectroscopy
2.4. Electronic Imaging
3. Results
3.1. Monochrome Tesserae with Orange or Red Colour
3.1.1. XANES Spectra at Cu K-Edge
3.1.2. Microstructure
3.2. Striated Tesserae
3.2.1. Chemical Composition Variations
3.2.2. XANES Spectra at -Edges
3.2.3. Microstructure
4. Discussion
4.1. Compositional Classification of Red/Orange Glasses/Tesserae
- -
- Low-copper (CuO < 4 wt%) and low-lead (PbO < 10 wt%);
- -
- High-copper (5–12 wt% CuO) and high-lead (20–40 wt% PbO).
- -
- High-copper (5–15 wt% CuO) and low- to medium-lead (<13 wt% PbO).
4.2. Role of Lead in the Colour Formation
4.3. Reducing Agents
4.4. Technological Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bring, T.; Högskolan, K.T.; Kemi, O. Red Glass Coloration: A Colorimetric and Structural Study. Ph.D. Thesis, Oorganisk Kemi, KTH Kemi, Stockholm, Sweden, 2006. [Google Scholar]
- Cãpãþînã, C. The Study of Copper Ruby Glass. Ceram. Silikàty 2005, 49, 283–286. [Google Scholar]
- Quaranta, A.; Ceccato, R.; Pederiva, L.; Capra, N.; Dal Maschio, R. Formation of copper nanocrystals in alkali-lime silica glass by means of different reducing agents. J. Non-Cryst. Solids 2004, 345&346, 671–675. [Google Scholar] [CrossRef]
- Cuvelier, P.A.; Andraud, C.; Chaudanson, D.; Lafait, J.; Nitsche, S. Copper red glazes: A coating with two families of particles. Appl. Phys. A 2012, 106, 915–929. [Google Scholar] [CrossRef]
- Colomban, P.; Schreiber, H.D. Raman signature modification induced by copper nanoparticles in silicate glass. J. Raman Spectrosc. 2005, 36, 884–890. [Google Scholar] [CrossRef]
- Wakamatsu, M.; Takeuchi, N.; Nagai, H.; Ishida, S. Chemical States of Copper and Tin in Copper Glazes Fired under Various Atmospheres. J. Am. Ceram. Soc. 1989, 72, 16–19. [Google Scholar] [CrossRef]
- Drünert, F.; Blanz, M.; Pollok, K.; Pan, Z.; Wondraczek, L.; Möncke, D. Copper-based opaque red glasses—Understanding the colouring mechanism of copper nanoparticles in archaeological glass samples. Opt. Mater. 2018, 76, 375–381. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ashour, G.M. Effect of melting conditions on the crystallisation of cuprous oxide and copper in glass. Elev. Congr. Glass 1977, 2, 177–187. [Google Scholar]
- Ahmed, A.A.; Ashour, G.M. Effect of heat treatment on the crystallisation of cuprous oxide in glass. Glass Technol. 1980, 22, 24–32. [Google Scholar]
- Cable, M.; Smedley, J.W. The Replication of an Opaque Red Glass from Nimrud. Br. Mus. Occas. Pap. 1987, 56, 151–164. [Google Scholar]
- Vandiver, P. Glass technology at the mid-second-millenium B.C. Hurrian site of Nuzi. J. Glass Stud. 1982, 25, 239–247. [Google Scholar]
- Barber, D.; Freestone, I.; Moulding, K. Ancient copper red glasses: Investigation and analysis by microbeam techniques. J. Archaeol. Sci. 2010, 37, 898–899. [Google Scholar] [CrossRef]
- Klysubun, W.; Thongkam, Y.; Pongkrapan, S.; Won-in, K.; T-Thienprasert, J.; Dararutana, P. XAS study on copper red in ancient glass beads from Thailand. Anal. Bioanal. Chem. 2011, 399, 3033–3040. [Google Scholar] [CrossRef]
- Gratuze, B.; Blet-Lemarquand, M.; Dussubieux, L. Innovation dans les techniques de coloration: Les verres rouges et orange en Asie du Sud. In Les Cahiers de L’Institut D’histoire Sociale du Verre et de la Céramique; IHS Verre et Céramique: Montreuil, France, 2013; pp. 11–20. [Google Scholar]
- Welter, N.; Schüssler, U.; Kiefer, W. Characterisation of inorganic pigments in ancient glass beads by means of Raman microspectroscopy, microprobe analysis and X-ray diffractometry. J. Raman Spectrosc. 2007, 38, 113–121. [Google Scholar] [CrossRef]
- Brun, N.; Mazerolles, L.; Pernot, M. Microstructure of opaque red glass containing copper. J. Mater. Sci. Lett. 1991, 10, 1418–1420. [Google Scholar] [CrossRef]
- Freestone, I.C.; Stapleton, C.P.; Rigby, V. The production of red glass and enamel in the Late Iron Age, Roman and Byzantine periods. In Through a Glass Brightly—Studies in Byzantine and Medieval Art and Archaeology Presented to David Buckton; Entwistle, C., Ed.; Oxbow: Oxford, UK, 2003; pp. 142–154. [Google Scholar]
- Bandiera, M.; Verità, M.; Lehuédé, P.; Vilarigues, M. The Technology of Copper-Based Red Glass Sectilia from the 2nd Century AD Lucius Verus Villa in Rome. Minerals 2020, 10, 875. [Google Scholar] [CrossRef]
- Boschetti, C.; Henderson, J.; Evans, J. Mosaic tesserae from Italy and the production of Mediterranean coloured glass (4th century BCE—4th century CE). Part II: Isotopic provenance. J. Archaeol. Sci. Rep. 2017, 11, 647–657. [Google Scholar] [CrossRef]
- Maltoni, S.; Silvestri, A. A Mosaic of Colors: Investigating Production Technologies of Roman Glass Tesserae from Northeastern Italy. Minerals 2018, 8, 255. [Google Scholar] [CrossRef]
- Santagostino Barbone, A.; Gliozzo, E.; D’Acapito, F.; Memmi Turbanti, I.; Turchiano, M.; Volpe, G. The Sectilia Panels of Faragola (Ascoli Satriano, Southern Italy): A Multi-Analytical Study of the Red, Orange and Yellow Glass Slabs. Archaeometry 2008, 50, 451–473. [Google Scholar] [CrossRef]
- Tesser, E.; Verità, M.; Lazzarini, L.; Falcone, R.; Saguì, L.; Antonelli, F. Glass in imitation of exotic marbles: An analytical investigation of 2nd century AD Roman sectilia from the Gorga collection. J. Cult. Herit. 2020, 42, 202–212. [Google Scholar] [CrossRef]
- Barca, D.; Basso, E.; Bersani, D.; Galli, G.; Invernizzi, C.; La Russa, M.F.; Lottici, P.P.; Malagodi, M.; Ruffolo, S.A. Vitreous tesserae from the calidarium mosaics of the Villa dei Quintili, Rome. Chemical composition and production technology. Microchem. J. 2016, 124, 726–735. [Google Scholar] [CrossRef]
- Schibille, N.; Boschetti, C.; Valero Tévar, M.A.; Veron, E.; de Juan Ares, J. The Color Palette of the Mosaics in the Roman Villa of Noheda (Spain). Minerals 2020, 10, 272. [Google Scholar] [CrossRef]
- Fiori, C. Production technology of Byzantine red mosaic glasses. Ceram. Int. 2015, 41, 3152–3157. [Google Scholar] [CrossRef]
- Silvestri, A.; Tonietto, S.; Molin, G.; Guerriero, P. The palaeo-Christian glass mosaic of St. Prosdocimus (Padova, Italy): Archaeometric characterisation of tesserae with copper- or tin-based opacifiers. J. Archaeol. Sci. 2014, 42, 51–67. [Google Scholar] [CrossRef]
- Shugar, A.N. Byzantine Opaque Red Glass Tesserae from Beit Shean, Israel. Archaeometry 2000, 42, 375–384. [Google Scholar] [CrossRef]
- Adlington, L.; Ritter, M.; Schibille, N. Production and Provenance of Architectural Glass from the Umayyad Period. PLoS ONE 2020, 15, e0239732. [Google Scholar] [CrossRef]
- Farges, F.; Etcheverry, M.P.; Scheidegger, A.; Grolimund, D. Speciation and weathering of copper in “copper red ruby” medieval flashed glasses from the Tours cathedral (XIII century). Appl. Geochem. 2006, 21, 1715–1731. [Google Scholar] [CrossRef]
- Kunicki-Goldfinger, J.J.; Freestone, I.C.; McDonald, I.; Hobot, J.A.; Gilderdale-Scott, H.; Ayers, T. Technology, production and chronology of red window glass in the medieval period—Rediscovery of a lost technology. J. Archaeol. Sci. 2014, 41, 89–105. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Zhu, J.; Zhang, X.; Jiang, S.; Zhang, Z.; Yao, Z.; Solbrekken, G. Colour-generating mechanism of copper-red porcelain from Changsha Kiln (A.D. 7th–10th century), China. Ceram. Int. 2016, 42, 8495–8500. [Google Scholar] [CrossRef]
- Maxwell, M.J. The Reception and Adaptation of Oriental Ceramics in Britain, with Particular Reference to Imperial Chinese Copper—Red Wares. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2017. [Google Scholar]
- Peak, J.R.N. Early Anglo—Saxon Glass Beads: Composition and Origines Based on the Finds from RAF Lakenheat, Suffolk. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2013. [Google Scholar]
- Lilyquist, C.; Brill, R. Studies in Early Egyptian Glass; The Metropolitan Museum of Art: New York, NY, USA, 1993. [Google Scholar]
- Fonda, E.; Rochet, A.; Ribbens, M.; Barthe, L.; Belin, S.; Briois, V. The SAMBA quick—EXAFS monochromator: XAS with edge jumping. J. Synchrotron Radiat. 2012, 19, 417–424. [Google Scholar] [CrossRef]
- Bearden, J.A.; Burr, A.F. Reevaluation of X-ray Atomic Energy Levels. Rev. Mod. Phys. 1967, 39, 125–142. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA ARTEMIS HEPHAESTUS: Data Anal. X-Ray Absorpt. Spectrosc. Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Vantelon, D.; Trcera, N.; Roy, D.; Moreno, T.; Mailly, D.; Guilet, S.; Metchalkov, E.; Delmotte, F.; Lassalle, B.; Lagarde, P.; et al. The LUCIA beamline at SOLEIL. J. Synchrotron Radiat. 2016, 23, 635–640. [Google Scholar] [CrossRef]
- Thakur, P.; Bisogni, V.; Cezar, J.C. Electronic structure of Cu-doped ZnO thin films by X-ray absorption, magnetic circular dichroism, and resonant inelastic X-ray scattering. J. Appl. Phys. 2010, 107, 103915. [Google Scholar] [CrossRef]
- Rothe, J.; Hormes, J.; Bönnemann, H.; Brijoux, W.; Siepen, K. In Situ X-ray Absorption Spectroscopy Investigation during the Formation of Colloidal Copper. J. Am. Chem. Soc. 1998, 120, 6019–6023. [Google Scholar] [CrossRef]
- Bandiera, M.; Lehuédé, P.; Verità, M.; Alves, L.; Biron, I.; Vilarigues, M. Nanotechnology in Roman Opaque Red Glass from the 2nd Century AD. Archaeometric Investigation in Red Sectilia from the Decoration of the Lucius Verus Villa in Rome. Heritage 2019, 2, 2597–2611. [Google Scholar] [CrossRef]
- Malerba, C.; Biccari, F.; Leonor Azanza Ricardo, C.; D’Incau, M.; Scardi, P.; Mittiga, A. Absorption coefficient of bulk and thin film Cu2O. Sol. Energy Mater. Sol. Cells 2011, 95, 2848–2854. [Google Scholar] [CrossRef]
- Freestone, I.C. Composition and Microstructure of Early Opaque Red Glass. Br. Mus. Occas. Pap. 1987, 56, 173–191. [Google Scholar]
- Edward, R.; Paul, A.; Douglas, R. Spectroscopy and oxidationreduction ofiron and copper inNa20-PbO-Si02 glasses. Phys. Chem. Glasses 1972, 13, 131. [Google Scholar]
- Shevchenko, M.; Jak, E. Experimental Liquidus Studies of the Binary Pb-Cu-O and Ternary Pb-Cu-Si-O Systems in Equilibrium with Metallic Pb-Cu Alloys. J. Phase Equilibria Diffus. 2019, 40, 671–685. [Google Scholar] [CrossRef]
- Brill, D.R.; Cahill, N. A Red Opaque Glass from Sardis and Some Thoughts on Red Opaques in General. J. Glass Stud. 1988, 30, 16–27. [Google Scholar]
- Shortland, A.J.; Eremin, K. The Analysis of Second Millenium Glass from Egypt and Mesopotamia, Part 1: New WDS Analyses. Archaeometry 2006, 48, 581–603. [Google Scholar] [CrossRef]
- Grioni, M.; Goedkoop, J.B.; Schoorl, R.; de Groot, F.M.F.; Fuggle, J.C.; Schäfers, F.; Koch, E.E.; Rossi, G.; Esteva, J.-M.; Karnatak, R.C. Studies of copper valence states with CuL3x-ray-absorption spectroscopy. Phys. Rev. B 1989, 39, 1541–1545. [Google Scholar] [CrossRef]
- Tanaka, Y.; Karppinen, M.; Lee, J.M.; Liu, R.S.; Chen, J.M.; Yamauchi, H. Systematic Cu L2,3-edge and O K -edge XANES spectroscopy study on the infinite-layer superconductor system, (Sr,La)CuO2. Solid State Commun. 2008, 147, 370–373. [Google Scholar] [CrossRef]
- Meulenberg, R.W.; van Buuren, T.; Hanif, K.M.; Willey, T.M.; Strouse, G.F.; Terminello, L.J. Structure and Composition of Cu-Doped CdSe Nanocrystals Using Soft X-ray Absorption Spectroscopy. Nano Lett. 2004, 4, 2277–2285. [Google Scholar] [CrossRef][Green Version]
- van der Laan, G.; Pattrick, R.; Henderson, C.; Vaughan, D. Oxidation state variations in copper minerals studied with Cu 2p X-ray absorption spectroscopy. J. Phys. Chem. Solids 1992, 53, 1185–1190. [Google Scholar] [CrossRef]
- Lafait, J.; Berthier, S.; Andraud, C.; Reillon, V.; Boulenguez, J. Physical colors in cultural heritage: Surface plasmons in glass. Comptes Rendus. Phys. 2009, 10, 649–659. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Madasu, M.; Huang, M.H. Modified Semiconductor Band Diagrams Constructed from Optical Characterization of Size-Tunable Cu2O Cubes, Octahedra, and Rhombic Dodecahedra. J. Phys. Chem. C 2018, 122, 13027–13033. [Google Scholar] [CrossRef]
- Thoka, S.; Lee, A.-T.; Huang, M.H. Scalable Synthesis of Size-Tunable Small Cu2O Nanocubes and Octahedra for Facet-Dependent Optical Characterization and Pseudomorphic Conversion to Cu Nanocrystals. ACS Sustain. Chem. Eng. 2019, 7, 10467–10476. [Google Scholar] [CrossRef]










| Cuprite | Cu Foil | Cu Colourless Glass | R Factor of Fit | |
|---|---|---|---|---|
| Noheda 70 | 1 | - | - | 0.0027 |
| Noheda 66 | 1 | - | - | 0.0008 |
| Noheda 29 | - | 0.75 | 0.25 | 0.0013 |
| Noheda 14 | - | 0.82 | 0.18 | 0.0009 |
| Oxide wt% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 25 | ||||||||||||
| Red zones | 12.5(2) | 58.2(4) | 1.98(7) | 9.1(1) | 2.1(3) | 0.29(4) | 2.81(8) | 2.36(4) | 0.14(5) | 1.3(1) | 0.11(3) | 9.1(6) |
| Base glass | 14.3 | 66.7 | 2.3 | 10.4 | - | - | 3.2 | 2.7 | 0.2 | - | 0.1 | - |
| Orange zones | 10.8(7) | 51(3) | 1.78(9) | 7.3(8) | 10(3) | 0.9(3) | 2.8(1) | 2.2(1) | 0.13(4) | 1.6(2) | 0.4(1) | 10(2) |
| Base glass | 14.1 | 66.7 | 2.3 | 9.6 | - | - | 3.7 | 2.9 | 0.2 | - | 0.5 | - |
| Sample 62 | ||||||||||||
| Red zones | 15.0(4) | 60.2(5) | 1.80(5) | 11.2(4) | 3.6(3) | 0.20(5) | 3.3(1) | 2.31(9) | 0.15(5) | 1.2(1) | 0.18(5) | 0.7(2) |
| Base glass | 14.3 | 66.7 | 2.3 | 10.4 | - | - | 3.2 | 2.7 | 0.2 | - | 0.1 | - |
| Orange zones | 15(1) | 52(1) | 1.9(1) | 10(4) | 11(3) | 0.89(5) | 2.2(2) | 1.45(8) | 0.15(4) | 2.3(2) | 0.31(8) | 2.0(3) |
| Base glass | 14.1 | 66.7 | 2.3 | 9.6 | - | - | 3.7 | 2.9 | 0.2 | - | 0.5 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noirot, C.; Cormier, L.; Schibille, N.; Menguy, N.; Trcera, N.; Fonda, E. Comparative Investigation of Red and Orange Roman Tesserae: Role of Cu and Pb in Colour Formation. Heritage 2022, 5, 2628-2645. https://doi.org/10.3390/heritage5030137
Noirot C, Cormier L, Schibille N, Menguy N, Trcera N, Fonda E. Comparative Investigation of Red and Orange Roman Tesserae: Role of Cu and Pb in Colour Formation. Heritage. 2022; 5(3):2628-2645. https://doi.org/10.3390/heritage5030137
Chicago/Turabian StyleNoirot, Cécile, Laurent Cormier, Nadine Schibille, Nicolas Menguy, Nicolas Trcera, and Emiliano Fonda. 2022. "Comparative Investigation of Red and Orange Roman Tesserae: Role of Cu and Pb in Colour Formation" Heritage 5, no. 3: 2628-2645. https://doi.org/10.3390/heritage5030137
APA StyleNoirot, C., Cormier, L., Schibille, N., Menguy, N., Trcera, N., & Fonda, E. (2022). Comparative Investigation of Red and Orange Roman Tesserae: Role of Cu and Pb in Colour Formation. Heritage, 5(3), 2628-2645. https://doi.org/10.3390/heritage5030137





