Characterization of Barium Hydroxide Used as Consolidating Agent for Monumental Surfaces in Venice
Abstract
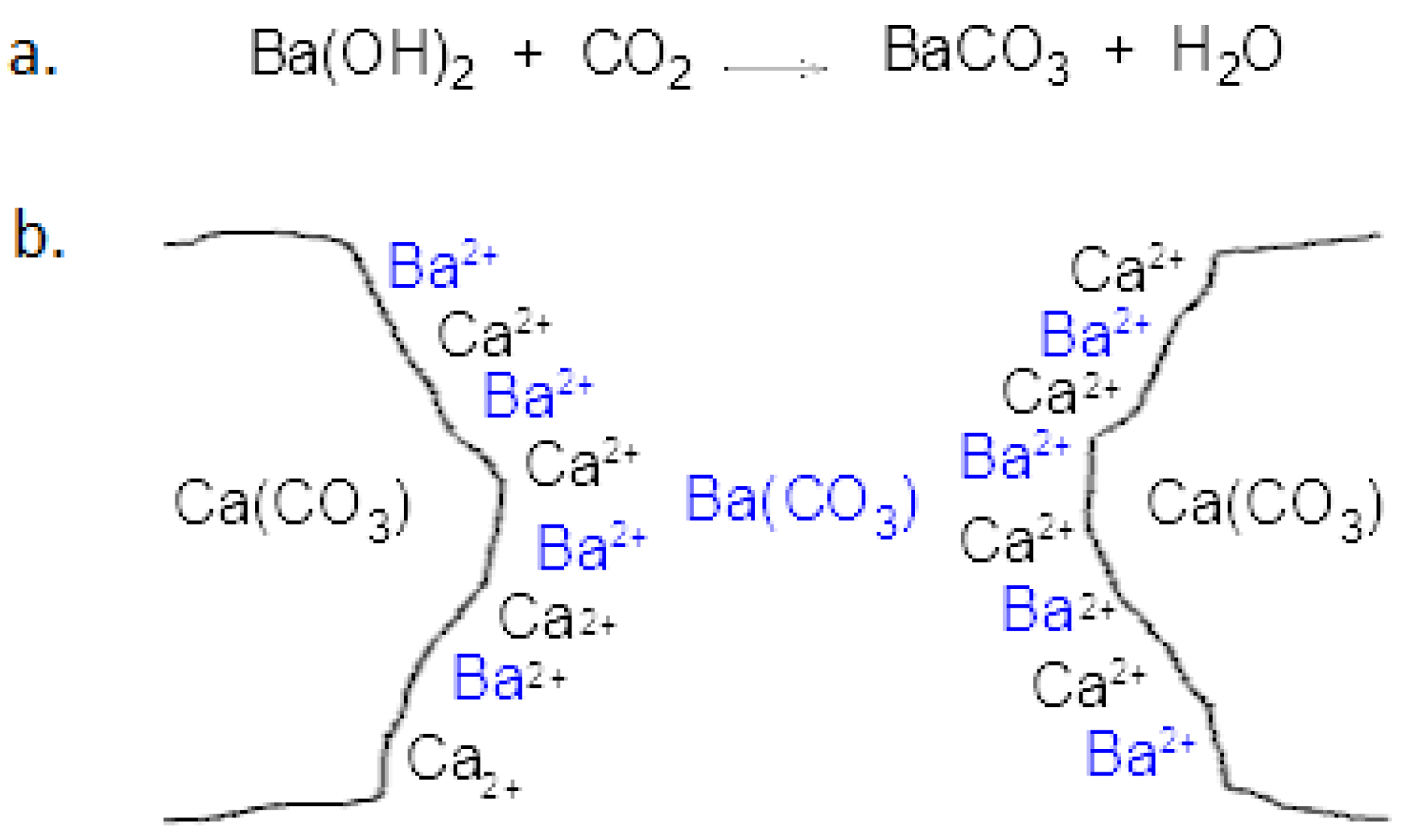
:1. Introduction
2. Materials and Methods
2.1. Experimental Samples and Related Weathering Methods
Analytical Program for Experimental Samples
2.2. Monitoring Procedure for Evaluating Past Conservative Treatments
2.2.1. Old Laboratory Samples
2.2.2. Samples from Historical Buildings
2.2.3. Analytical Characterization of Past Treatments
3. Results
3.1. Study of Ba(OH)2 Treatment Effects on Experimental Ad Hoc Samples
Study of Biological Growth
3.2. Evaluation of the Stability of Ba(OH)2 Treatments Applied in the Past Stone Materials
3.2.1. Old Laboratory Samples
3.2.2. Historical Venetian Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hansen, E.; Dohene, F.; Fidler, J.; Larson, J.; Martin, B.; Matteini, M.; Rodríguez-Navarro, C.; Sebastián Pardo, E.; Price, C.; de Tagle, A.; et al. A review of selected inorganic consolidants and protective treatments for porous calcareous materials. Rev. Conserv. 2003, 4, 13–25. [Google Scholar] [CrossRef]
- Bracci, S.; Sacchi, B.; Ferreira Pinto, A.P.; Delgado Rodrigues, J. Inorganic consolidants on stone artefacts: Optimisation of application procedures for marble and limestones. In Proceedings of the International Symposium, Stone Consolidation in Cultural Heritage, Research and Practice, Lisbon, Portugal, 6–7 May 2008; Rodrigues, J.D., Mimoso, J.M., Eds.; [Google Scholar]
- Ferroni, E.; Dini, D. Chemical-structural conservation of sulphatized marbles. In Proceedings of the Conservation of Stone II. Preprints of the Contribution to the International Symposium, Bologna, Italy, 27–30 October 1981; pp. 559–566. [Google Scholar]
- Matteini, M. In review: An assessment of Florentine methods of wall painting conservation based on the use of mineral treatments. In The Conservation of Wall Paintings, Proceedings of the Symposium Organized by the Courtauld Institute of Art and the Getty Conservation Institute, London, UK, 13–16 July 1987; Gather, S., Ed.; Getty Publications: Los Angeles, CA, USA, 1991; pp. 137–148. [Google Scholar]
- Matteini, M.; Moles, A. La “Metodologia del Bario” in relazione ai problemi di solfatazione e decoesione che interessano i dipinti murali. In Il Restauro Delle Opere D’arte; Accademia Nazionale Virgiliana: Mantova, Italy, 1987; pp. 33–41. [Google Scholar]
- Ferroni, E.; Malaguzzi, V.V.; Rovida, G. Experimental study by diffraction of heterogeneous systems as a preliminary to the proposal of a technique for the restoration of gypsum polpute murals. In Proceedings of the ICOM Conference, Amsterdam, Holland, 6–12 July 1969. [Google Scholar]
- Matteini, M.; Moles, A. Barium aluminates for the consolidation of mural paintings. In Proceedings of the ICOM Committee for Conservation, 5th Triennial Meeting, Zagreb, Croatia, 1–8 October 1978. [Google Scholar]
- Berlucchi, N.; Ginanni Corradini, R.; Bonomi, R.; Bemporad, E.; Tisato, M. “La fenice” theatre—Foyer and Apollinee rooms—Consolidation of fire-damaged stucco and marmorino decorations by means of combined applications of ion-exchange resins and Barium hydroxide. In Proceedings of the 9th International Congress on Deterioration and Conservation of Stone, Venice, Italy, 19–24 June 2000. [Google Scholar]
- Lazzarini, L.; Laurenzi Tabasso, M. Il Restauro Della Pietra; Cedam: Padova, Italy, 1986. [Google Scholar]
- Slížková, Z.; Drdácký, M.; Viani, A. Consolidation of weak lime mortars by means of saturated solution of calcium hydroxide or barium hydroxide. J. Cult. Herit. 2015, 16, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, R.J. Weathering of Natural Building Stones; His Majesty’s Stationery, Office: London, UK, 1932; Reprinted in Building Research Establishment: Watford, UK, 1972. [Google Scholar] [CrossRef]
- Toniolo, L.; Colombo, C.; Realini, M.; Peraio, A.; Positano, M. Evaluation of barium hydroxide treatment efficacy on a dolomitic marble. In Annali Di Chimica; Wiley-VCH: Weinheim, Germany, 2001; Volume 91, pp. 813–821. [Google Scholar]
- Matteini, M.; Moles, A. Aspetti critici del trattamento fondato sull’impiego di idrato di bario. In Pitture Murali; Danti, C., Matteini, M., Moles, M., Eds.; Centro Di della Edifimi s.r.l.: Firenze, Italy, 1990; pp. 297–302. [Google Scholar]
- Graf, D.L.; Lamar, L.E. Properties of calcium and magnesium carbonates and their bearing on some uses of carbonate rocks. Econ. Geol. 1955, 50, 696–713. [Google Scholar]
- Lewin, S.Z.; Baer, N.S. Rationale of the barium hydroxide-urea treatment of decayed stone. Stud. Conserv. 1974, 19, 24–35. [Google Scholar] [CrossRef]
- Lewin, S.Z. Method of Preserving Limestone Structure. U.S. Patent 529,213, 25 February 1966. [Google Scholar]
- Lewin, S.Z. Method of Preserving Limestone Structure. U.S. Patent 3,577,244, 5 May 1971. [Google Scholar]
- Schnabel, L. Evaluation of the barium hydroxide-urea consolidation. In Proceedings of the 7th International Congress on Deterioration and Conservation of Stone, Lisbon, Portugal, 15–18 June 1992; Rodrigues, J.D., Henriques, F., Jeremias, F.T., Eds.; Laboratório Nacional de Engenharia Civil: Lisboa, Portugal, 1992; pp. 1063–1072. [Google Scholar] [CrossRef]
- Favaro, M.; Naccari, A.; Crivellari, F.; Magris, D.; Pigo, M.; Burtet, B.; Fumo, G.; Fassina, V. New findings on past treatment’s effects on the lunette of San Giovanni Evangelista in Venice. In Proceedings of the 9th International Congress on Deterioration and Conservation of Stone, Venice, Italy, 19–24 June 2000. [Google Scholar]
- Delgado Rodrigues, J.; Ferreira Pinto, A.P. Laboratory and onsite study of barium hydroxide as a consolidant for high porosity limestones. J. Cult. Herit. 2016, 19, 467–476. [Google Scholar] [CrossRef]
- Joy, C.D. Monte Carlo Modelling for Electron Microscopy and Microanalysis; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Internazionale Marmi E Macchine. The Tuscan Marble Identities; Signa: Florence, Italy, 2010. [Google Scholar]
- Berto, L.; Favaretto, T.; Saetta, A.; Antonelli, F.; Lazzarini, L. Assessment of seismic vulnerability of art objects: The Galleria dei Prigioni sculptures at the Accademia Gallery in Florence. J. Cult. Herit. 2012, 13, 7–21. [Google Scholar] [CrossRef]
- Cattaneo, A.; De Vecchi, G.P.; Menegazzo Vitturi, L. Le pietre tenere dei Colli Berici. In Atti e Memorie Dell’accademia Patavina di Scienze, Lettere ed Arti, V.LXXXVIII, Parte II, Classe di Scienze Matematiche e Naturali; R. Accademia di Scienze, Lettere ed Arti: Padua, Italy, 1976; pp. 69–100. [Google Scholar]
- Tesser, E.; Lazzarini, L.; Bracci, S. Investigation on the chemical structure and ageing transformations of the cycloaliphatic epoxy resin EP2101 used as stone consolidant. J. Cult. Herit. 2018, 31, 72–82. [Google Scholar] [CrossRef]
- Tesser, E.; Antonelli, F. Evaluation of silicone based products used in the past as today for the consolidation of Venetian monumental stone surfaces. Mediterr. Archaeol. Archaeom. 2018, 18, 159–170. [Google Scholar] [CrossRef]
- Manganelli Del Fà, C. La Porosità Nei Materiali Lapidei Naturali e Artificiali–Problematiche di Determinazione Della Porosità. Correlazione Tra Caratteristiche Fisiche Dei Materiali, Porosità, Dinamica dei Fluidi, Degrado e Trattamenti Conservativi. Supplemento al n.; 10 di Fist Geoitalia: Modena, Italy, 2002. [Google Scholar]
- UNI 10925; Beni Culturali Materiali Lapidei Naturali ed Artificiali. Metodologia per L’irraggiamento Con Luce Solare Artificiale: Milan, Italy, 2001.
- ASTM D 3273-76; Standard Test Method For: Resistance to Growth of Mold on the Surface of Interior Coatings in an Environmental Chamber. ASTM International: West Conshohocken, PA, USA, 2000.
- UNI 11432; Beni Culturali. Materiali Lapidei Naturali ed Artificiali. Misura Della Capacità di Assorbimento di Acqua Mediante Spugna di Contatto: Milan, Italy, 2011.
- EN 15886; 2010 Conservation of Cultural Property—Test Methods—Colour Measurement of Surfaces. European Standard: Brussels, Belgium, 2010.
- Normal 8/81; Esame Delle Caratteristiche Morfologiche al Microscopio Elettronico a Scansione (SEM). CNR Milano e Roma—ICR: Rome, Italy, 1981.
- Caneva, G.; Nugari, M.P.; Salvadori, O. La Biologia Vegetale per i Beni Culturali; Nardini Ed.: Florence, Italy, 2007. [Google Scholar]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: London, UK, 1971. [Google Scholar]
- Hawksworth, D.L.; Sutton, B.C.; Ainsworth, G.C. Ainsworth and Bisby’s Dictionary of the Fungi, 7th ed.; Commonwealth Mycological Institute: London, UK; CAB Direct: Glasgow, UK, 1983. [Google Scholar]
- Ellis, M.B. More Dematiaceous Hyphomycetes; CABI Publishing: Wallingford, UK, 1976. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; Burgess Publishing, Co.: London, UK, 1969. [Google Scholar]
- Lazzarini, L.; Mariottini, M. A first study of some lumachelle (fossiliferous stones) used in Roman antiquity. In Proceedings of the Interdisciplinary Studies on Ancient Stone. Proceedings of the IX ASMOSIA Conference—Tarragona 2009; Garcia-M, A.G., Lapuente, P., Rodà., I., Eds.; Institut Català d’Arqueologia Classica: Tarragona, Spain, 2012; pp. 445–451. [Google Scholar]
- Tesser, E.; Lazzarini, L.; Ganzerla, R.; Antonelli, F. The decay of the polysiloxane resin Sogesil XR893 applied in the past century for consolidating monumental marble surfaces. J. Cult. Herit. 2017, 27, 107–115. [Google Scholar] [CrossRef]
- Normal 3/80, Materiali Lapidei: Campionamento, CNR Milano e Roma–ICR. 1980. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1296207416303442 (accessed on 15 September 2022).
- PCC. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- La Russa, M.F.; Comite, V.; Aly, N.; Barca, D.; Fermo, P.; Rovella, N.; Antonelli, F.; Tesser, E.; Aquino, M.; Ruffolo, S.A. Black crusts on Venetian built heritage, investigation on the impact of pollution sources on their composition. Eur. Phys. J. Plus 2018, 133, 370. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 82nd ed.; CRC Press LLC.: Boca Raton, FL, USA, 2001–2002. [Google Scholar]
- Ariano, R.; Bonifazi, F. Aerobiologia ed Allergeni Stagionali: Il Campionamento Aerobiologico Applicato Alla Pratica Clinica; ECIG: Genova, Switzerland, 2006; p. 240. [Google Scholar]
- ISPRA. Pollini Allergenici in Italia: Analisi dei Trend 2010–2019; Rapporti: Ispra, Italy, 2020; p. 335. [Google Scholar]
- Matteini, M.; Scuto, S. Consolidamento di manufatti lapidei con Idrossido di Bario. Test colorimetrici per la verifica della diffusione del consolidante. Arkos Sci. Restauro 2001, 1, 28–31. [Google Scholar]










| Stone Materials | Type and Number of Samples | Conservation Treatment | Application Method | Conservation Conditions | |
|---|---|---|---|---|---|
| Experimental ad hoc samples |
| 12 Specimens measuring 5 × 5 × 2 cm (6 specimens was treated, 6 specimens remained untreated) | saturated Ba(OH)2 solution | Brushing up to rejection |
|
| Old laboratory samples |
| 2 Specimens measuring 10 × 10 × 1.7 cm | warm solution of Ba(OH)2 | Immersion |
|
| Samples from historical buildings | Carrara marble | 1 micro fragment sampled from St. Fantin church—VE | cold mixture of barium hydroxide, urea and glycerin | Capillary absorption |
|
| Lumachella del Tasso | 1 micro fragment sampled from Scuola di San Giovanni Evangelista—VE | cold mixture of barium hydroxide, urea and glycerin | Capillary absorption |
| |
| medium-grained Greek marble from the island of Paros (Cyclades) | 1 micro fragment sampled from St. Alvise church—VE | unknown | unknown |
|
| After Treatment | After Aging | ||||
|---|---|---|---|---|---|
| ∆E | ∆Wa | ∆E | ∆Wa | ||
| Natural weathering | Carrara marble | 3.11 ± 0.25 | −9.72 × 10−6 ± 1.00 × 10−6 | 2.96 ± 0.18 | 7.46 × 10−6 ± 0.49 × 10−6 |
| Vicenza limestone | 4.44 ± 0.20 | −1.07 × 10−3 ± 0.05 × 10−3 | 3.48 ± 0.10 | 2.87 × 10−4 ± 0.40 × 10−4 | |
| Xenon test | Carrara marble | 2.74 ± 0.39 | −9.06 × 10−6 ± 0.70 × 10−6 | 0.19 ± 0.05 | 3.85 × 10−6 ± 0.70 × 10−6 |
| Vicenza limestone | 3.51 ± 0.35 | −9.85 × 10−4 ± 1.66 × 10−4 | 0.30 ± 0.03 | 1.36 × 10−4 ± 0.23 × 10−4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesser, E.; Conventi, A.; Majerle, F. Characterization of Barium Hydroxide Used as Consolidating Agent for Monumental Surfaces in Venice. Heritage 2022, 5, 3280-3297. https://doi.org/10.3390/heritage5040168
Tesser E, Conventi A, Majerle F. Characterization of Barium Hydroxide Used as Consolidating Agent for Monumental Surfaces in Venice. Heritage. 2022; 5(4):3280-3297. https://doi.org/10.3390/heritage5040168
Chicago/Turabian StyleTesser, Elena, Alberto Conventi, and Floriana Majerle. 2022. "Characterization of Barium Hydroxide Used as Consolidating Agent for Monumental Surfaces in Venice" Heritage 5, no. 4: 3280-3297. https://doi.org/10.3390/heritage5040168






