Thermohygrometric Climate, Insects and Fungi in the Klosterneuburg Monastic Library
Abstract
1. Introduction
2. Method
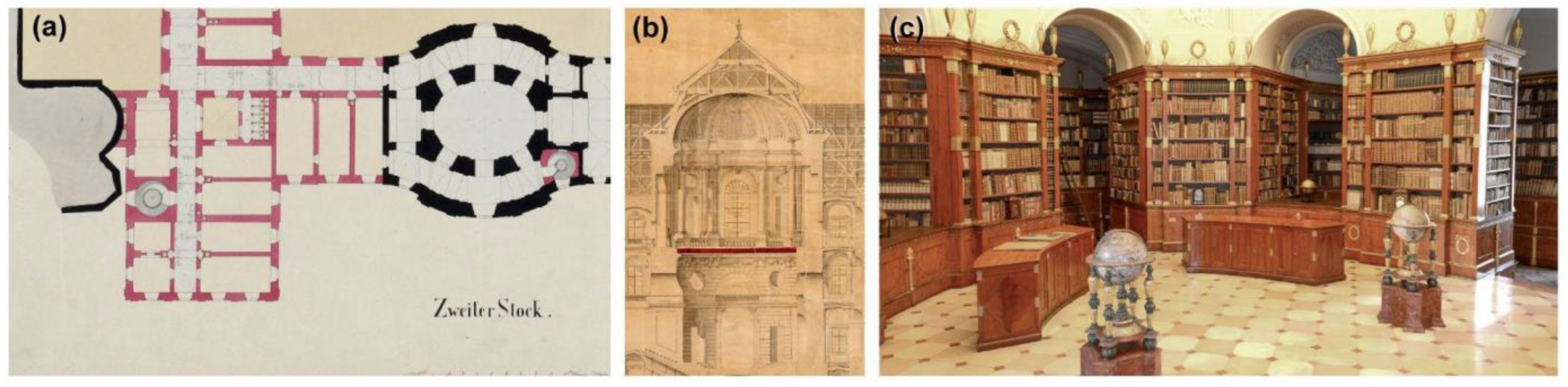
2.1. Library
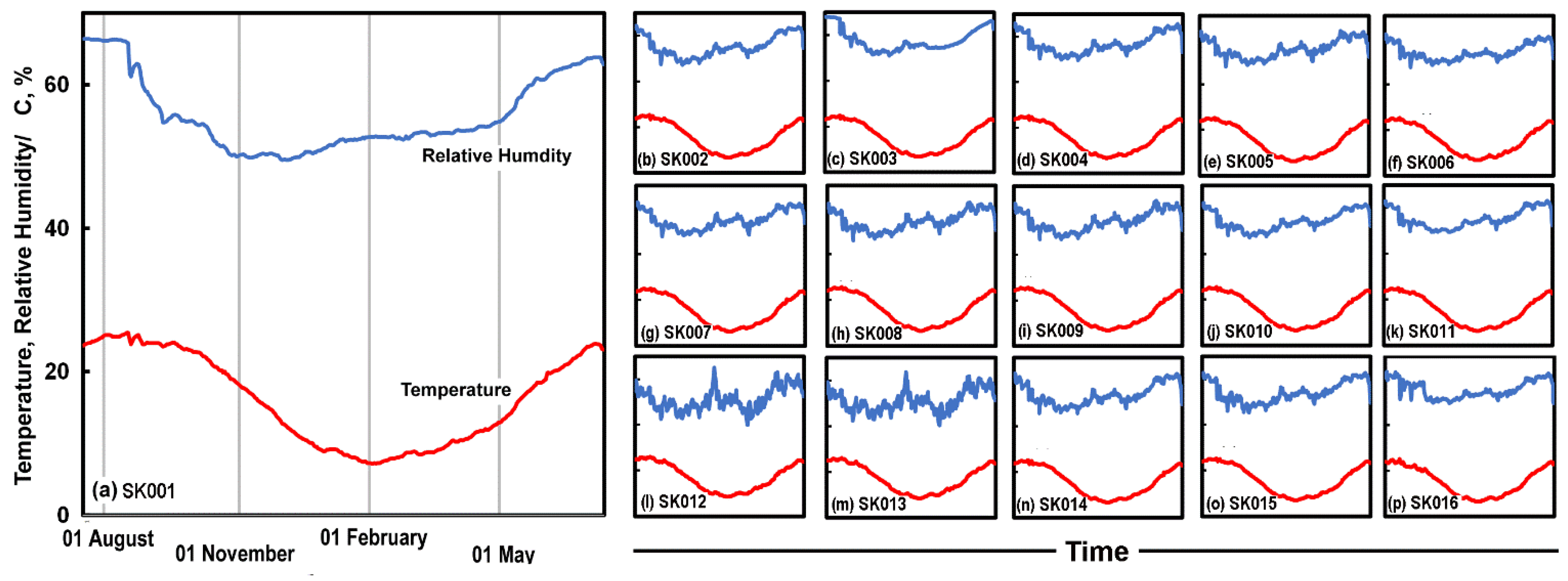
2.2. Climate Monitoring
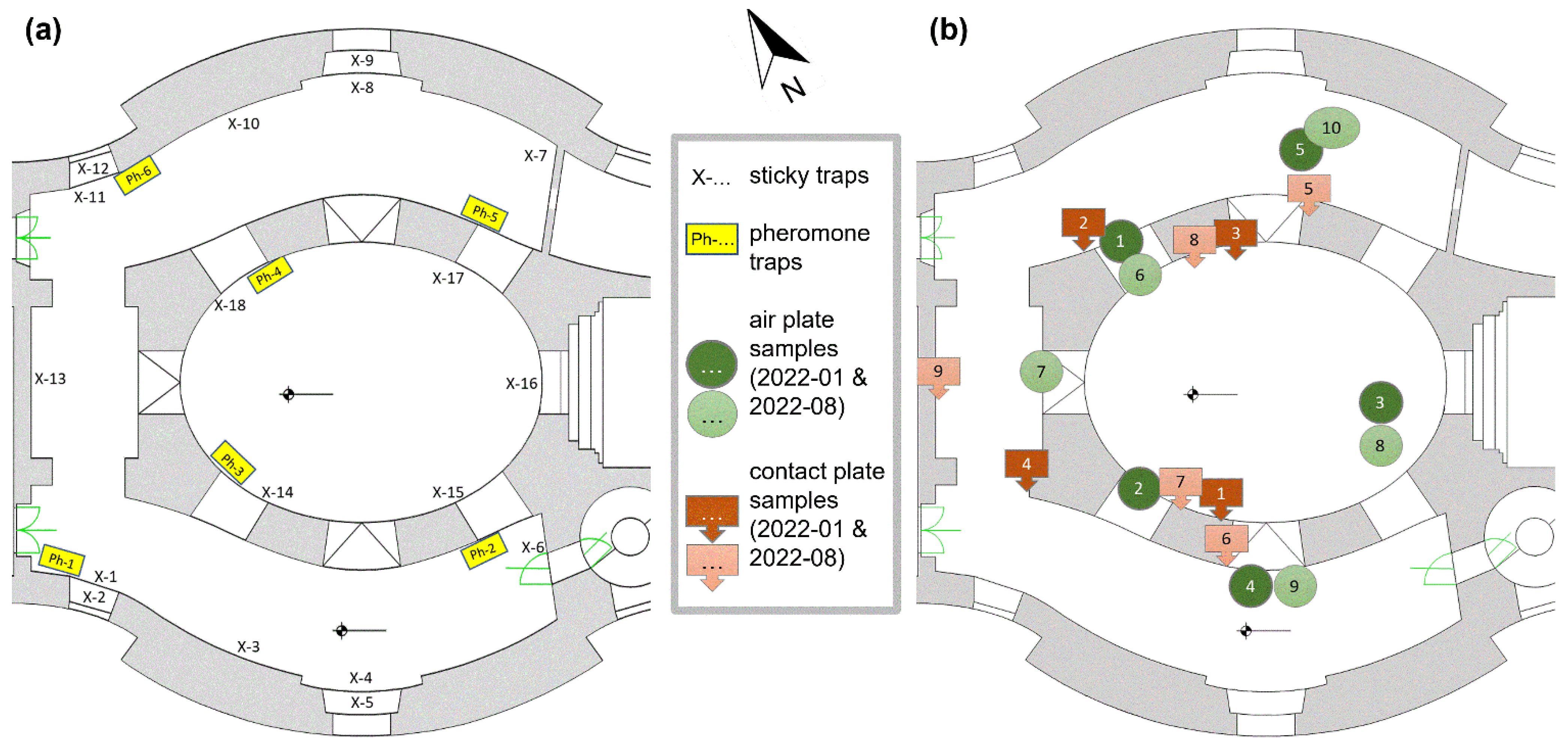
2.3. Insect Monitoring with Traps
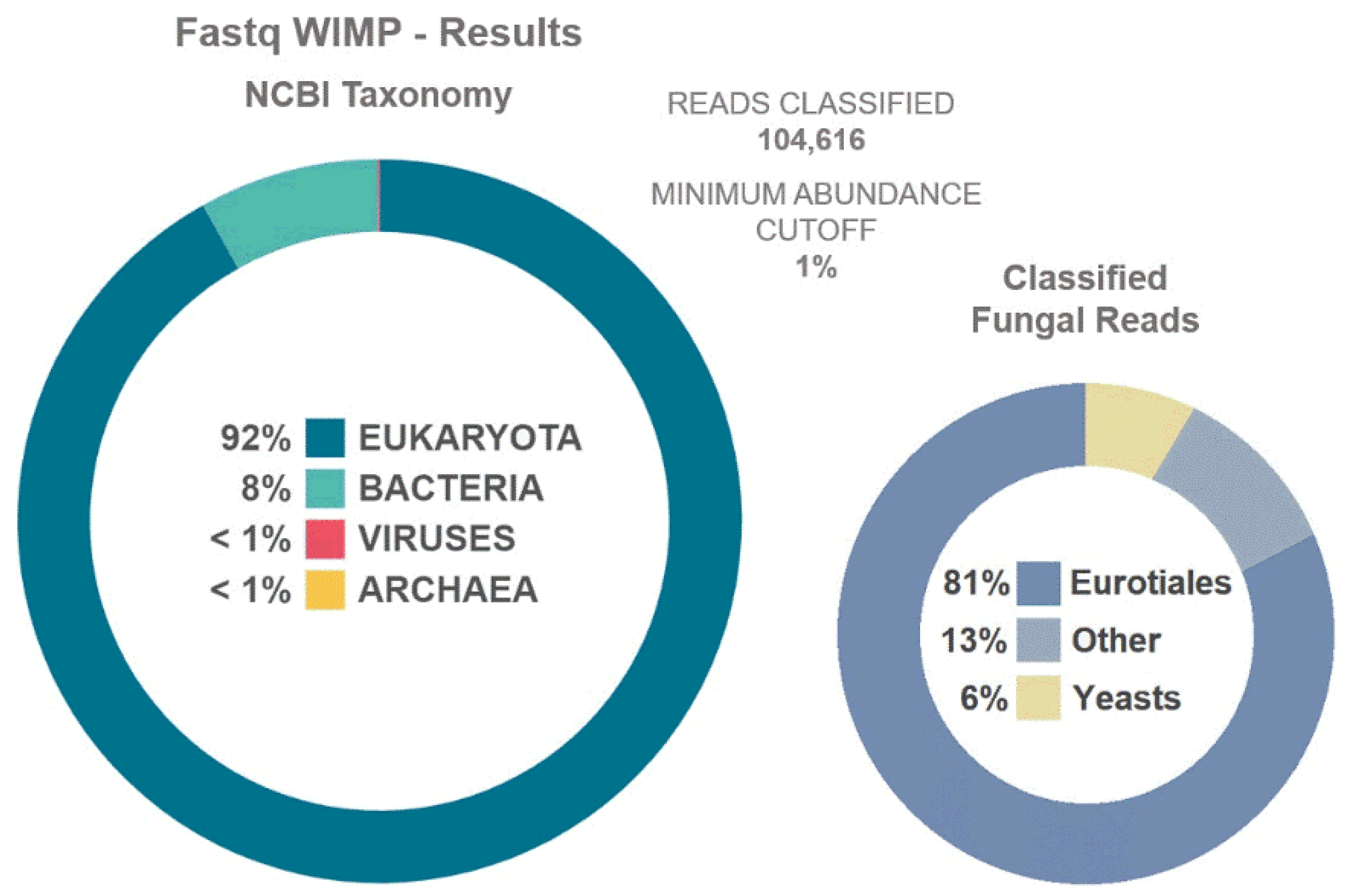
2.4. Fungal Monitoring with Cultivation Samples and Metagenomics
2.5. Statistical Analysis
3. Results
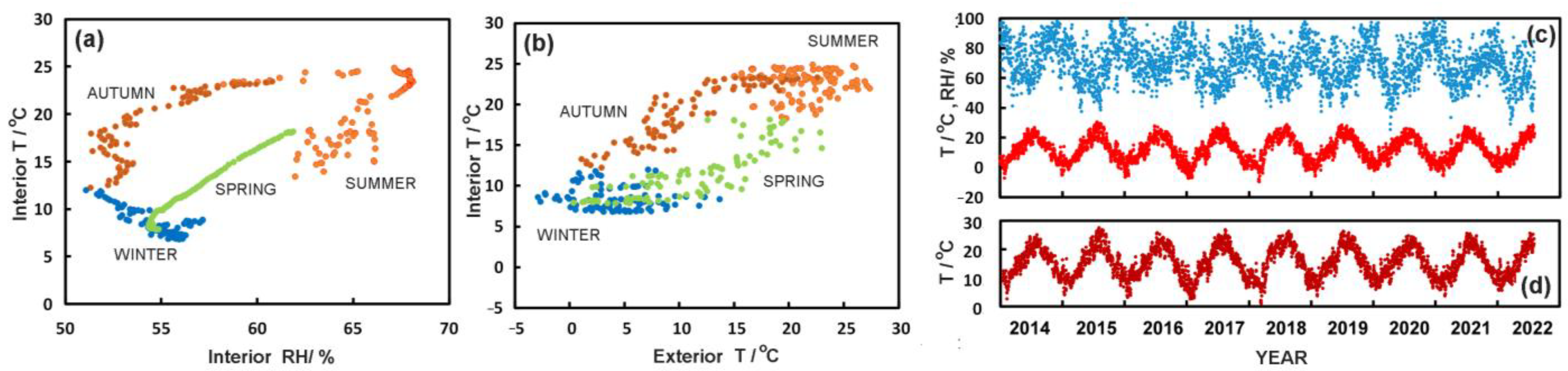
3.1. Library Climate
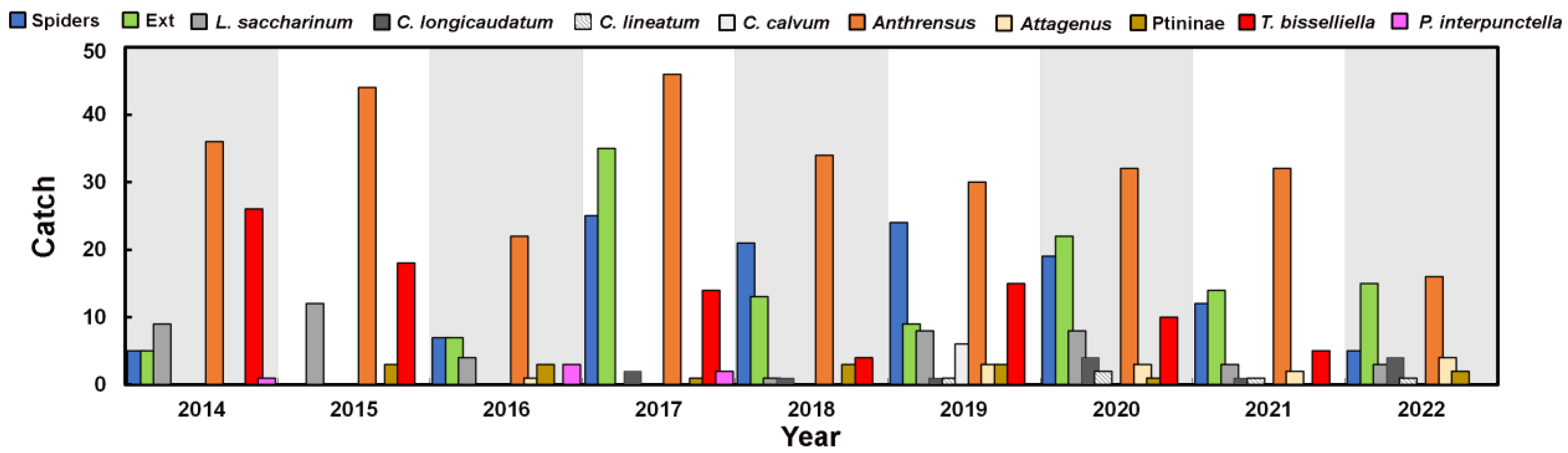
3.2. Insects Trapped
3.3. Insect Distribution
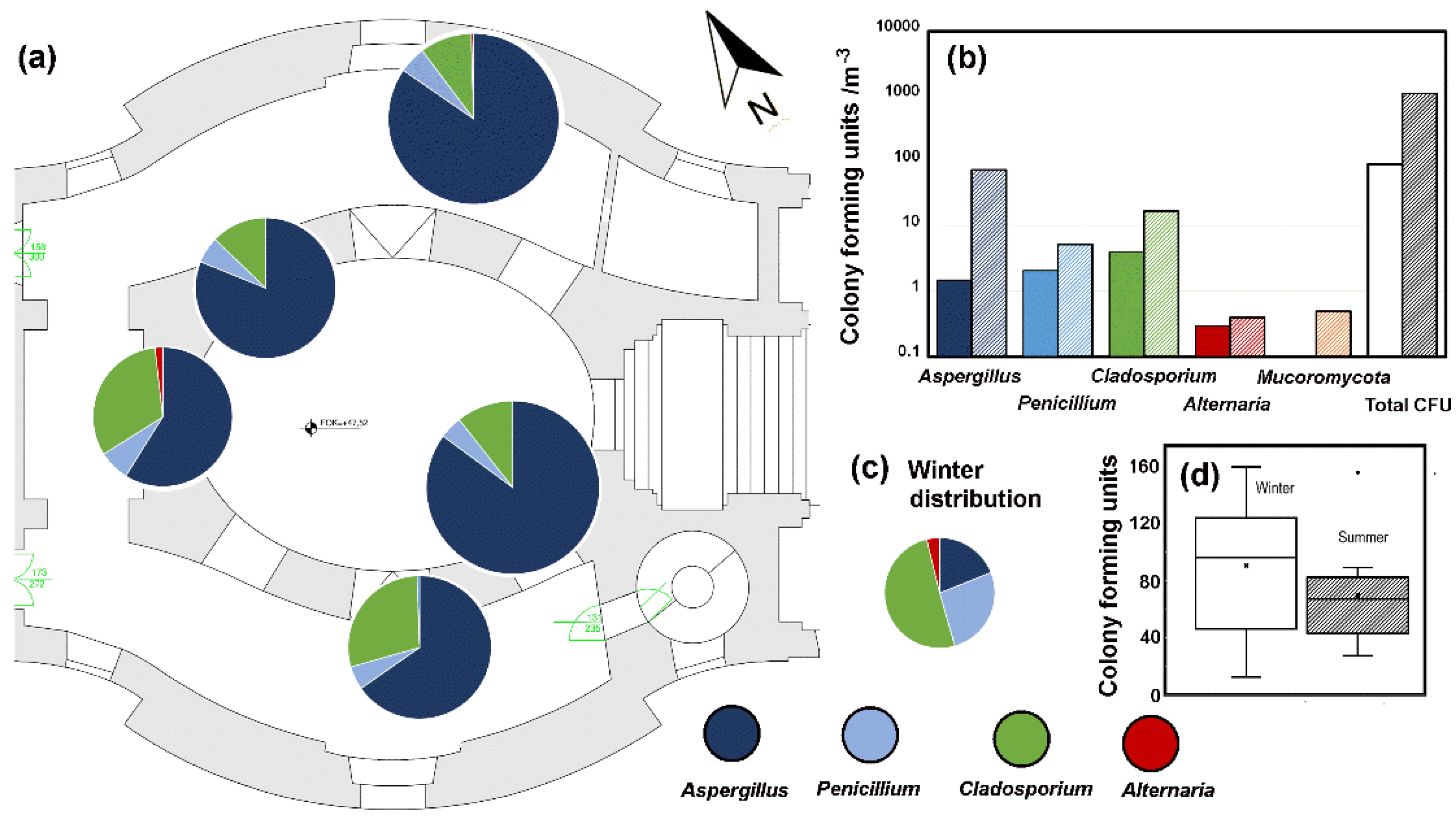
3.4. Fungal Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hansen, L.S.; Åkerlund, M.; Grøntoft, T.; Ryhl-Svendsen, M.; Schmidt, A.L.; Bergh, J.-E.; Jensen, K.-M.V. Future pest status of an insect pest in museums, Attagenus smirnovi: Distribution and food consumption in relation to climate change. J. Cult. Herit. 2012, 13, 22–27. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Lankester, P. Long-term changes in climate and insect damage in historic houses. Stud. Conserv. 2013, 58, 13–22. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Brimblecombe, C.T. Trends in insect catch at historic properties. J. Cult. Herit. 2015, 16, 127–133. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in the deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Querner, P.; Sterflinger, K.; Derksen, K.; Leissner, J.; Landsberger, B.; Hammer, A.; Brimblecombe, P. Climate Change and Its Effects on Indoor Pests (Insect and Fungi) in Museums. Climate 2022, 10, 103. [Google Scholar] [CrossRef]
- Blades, W. The Enemies of Books; Elliot Stock: London, UK, 1887. [Google Scholar]
- Waller, R. Conservation risk assessment: A strategy for managing resources for preventive conservation. Stud. Conserv. 1994, 39 (Suppl. 2), 12–16. [Google Scholar] [CrossRef]
- Hickin, N. Bookworms—The Insect Pests of Books, 2nd ed.; Edwards, R., Ed.; Richard Joseph Publishers LTD.: Nottingham, UK, 1992. [Google Scholar]
- Brokerhof, A.W.; Zanen, W.B.; Watering, K.; Porck, H. Buggy Biz, Integrated Pest Management in Collections; Netherlands Institute for Cultural Heritage (ICN) and IADA: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Trematerra, P.; Pinniger, D. Museum pests–Cultural heritage pests. In Recent Advances in Stored Product Protection; Springer: Berlin/Heidelberg, Germany, 2018; pp. 229–260. [Google Scholar]
- Pinniger, D. Integrated Pest Management in Cultural Heritage; Archetype Publications: London, UK, 2015. [Google Scholar]
- Pinniger, D.; Lauder, D. Pests in Houses Great and Small: Identification, Prevention and Eradication; English Heritage: Swindon, UK, 2018. [Google Scholar]
- Pinniger, D. Managing Pests in Paper-Based Collections; Preservation Advisory Centre, British Library: London, UK, 2012; p. 15. [Google Scholar]
- Querner, P. Insect pests and integrated pest management in museums, libraries and historic buildings. Insects 2015, 6, 595–607. [Google Scholar] [CrossRef]
- Sterflinger, K.; Pinzari, F. The revenge of time: Fungal deterioration of cultural heritage with particular reference to books, paper and parchment. Environ. Microbiol. 2012, 14, 559–566. [Google Scholar] [CrossRef]
- Strlič, M.; Grossi, C.M.; Dillon, C.; Bell, N.; Fouseki, K.; Brimblecombe, P.; Menart, E.; Ntanos, K.; Lindsay, W.; Thickett, D.; et al. Damage function for historic paper. Part I: Fitness for use. Her. Sci. 2015, 3, 33. [Google Scholar] [CrossRef]
- Strlič, M.; Grossi, C.M.; Dillon, C.; Bell, N.; Fouseki, K.; Brimblecombe, P.; Menart, E.; Ntanos, K.; Lindsay, W.; Thickett, D.; et al. Damage function for historic paper. Part II: Wear and tear. Her. Sci. 2015, 3, 36. [Google Scholar] [CrossRef]
- Strlič, M.; Grossi, C.M.; Dillon, C.; Bell, N.; Fouseki, K.; Brimblecombe, P.; Menart, E.; Ntanos, K.; Lindsay, W.; Thickett, D.; et al. Damage function for historic paper. Part III: Isochrones and demography of collections. Her. Sci. 2015, 3, 40. [Google Scholar] [CrossRef]
- Aak, A.; Hage, M.; Magerøy, Ø.; Byrkjeland, R.; Lindstedt, H.H.; Ottesen, P.; Rukke, B.A. Introduction, dispersal, establishment and societal impact of the long-tailed silverfish Ctenolepisma longicaudata (Escherich, 1905) in Norway. BioInvasions Rec. 2021, 10, 483–498. [Google Scholar] [CrossRef]
- Querner, P.; Szucsich, N.; Landsberger, B.; Erlacher, S.; Trebicki, L.; Grabowski, M.; Brimblecombe, P. Identification and Spread of the Ghost Silverfish (Ctenolepisma calvum) among Museums and Homes in Europe. Insects 2022, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Hage, M.; Rukke, B.A.; Ottesen, P.S.; Widerøe, H.P.; Aak, A. First record of the four-lined silverfish, Ctenolepisma lineata (Fabricius, 1775) (Zygentoma, Lepismatidae), in Norway, with notes on other synanthropic lepismatids. Nor. J. Entomol. 2020, 67, 8–14. [Google Scholar]
- Sterflinger, K.; Voitl, C.; Lopandic, K.; Piñar, G.; Tafer, H. Big sound and extreme fungi—Xerophilic, halotolerant Aspergilli and Penicillia with low optimal temperature as invaders of historic pipe organs. Life 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Leissner, J.; Kilian, R.; Kotova, L.; Jacob, D.; Mikolajewicz, U.; Brostrom, T.; Ashley-Smith, J.; Schellen, H.L.; Martens, M.; Van Schijndel, J.; et al. Climate for Culture: Assessing the impact of climate change on the future indoor climate in historic buildings using simulations. Herit. Sci. 2015, 3, 38. [Google Scholar] [CrossRef]
- Krus, M.; Kilian, R.; Sedlbauer, K. Mould growth prediction by computational simulation on historic buildings. In Museum Microclimates; Padfield, T., Borchersen, K., Eds.; National Museum of Denmark: Copenhagen, Denmark, 2007; ISBN 978-87-7602-080-4. [Google Scholar]
- Richards, J.; Brimblecombe, P. Moisture as a Driver of Long-Term Threats to Timber Heritage. Part I: Changing Heritage Climatology. Heritage 2022, 5, 1929–1946. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Richards, J. Moisture as a Driver of Long-Term Threats to Timber Heritage. Part II: Risks Imposed on Structures at Local Sites. Heritage 2022, 5, 2966–2986. [Google Scholar] [CrossRef]
- Lankester, P.; Brimblecombe, P. The Impact of Future Climate on Historic Interiors. Sci. Total Environ. 2012, 417–418, 248–254. [Google Scholar] [CrossRef]
- Sabbioni, C.; Brimblecombe, P.; Cassar, M. The Atlas Ofclimate Change Impact on European Cultural Heritage. In Scientific Analysis and Management Strategies; Anthem Press: London, UK, 2010. [Google Scholar]
- Alexander, K.N.A. Atlantopsocus adustus (Hagen) (Psoc.: Psocidae) new to Britain from East Cornwall. Entomol. Rec. 2007, 119, 76. [Google Scholar]
- Lankester, P.; Brimblecombe, P. Future thermohygrometric climate within historic houses. J. Cult. Herit. 2012, 13, 1–6. [Google Scholar] [CrossRef]
- Ryhl-Svendsen, M.; Padfield, T.; Smith, V.A.; De Santis, F. The indoor climate in historic buildings without mechanical ventilation systems. In Healthy Buildings 2003, Proceedings of ISIAQ 7th International Conference, Singapore, 7–11 December 2003; Healthy Buildings: Singapore, 2003; pp. 7–11. [Google Scholar]
- Camuffo, D. Microclimate for Cultural Heritage: Measurement, Risk Assessment, Conservation, Restoration, and Maintenance of Indoor and Outdoor Monuments; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sahin, C.D.; Coşkunm, T.; Arsan, Z.D.; Akkurt, G.G. Investigation of indoor microclimate of historic libraries for preventive conservation of manuscripts. Case Study: Tire Necip Paşa Library, İzmir-Turkey. Sustain. Cities Soc. 2017, 30, 66–78. [Google Scholar] [CrossRef]
- Turhan, C.; Akkurt, G.G.; Durmus Arsan, Z. Impact of climate change on indoor environment of historic libraries in Mediterranean climate zone. Int. J. Global Warm. 2019, 18, 206. [Google Scholar] [CrossRef]
- Adam, G.; Pont, U.; Mahdavim, A. Evaluation of thermal environment and indoor air quality in university libraries in Vienna. Adv. Mat. Res. 2014, 899, 315–320. [Google Scholar]
- Frasca, F.; Siani, A.M.; Casale, G.R.; Pedone, M.; Bratasz, Ł.; Strojecki, M.; Mleczkowska, A. Assessment of indoor climate of Mogiła Abbey in Kraków (Poland) and the application of the analogues method to predict microclimate indoor conditions. Environ. Sci. Pollut. Res. 2017, 24, 13895–13907. [Google Scholar] [CrossRef]
- Haltrich, M. Die Stiftsbibliothek. In Das Stift Klosterneuburg; Ch, W., Ed.; Huber: Wettin-Löbejün, Germany, 2014; pp. 216–229. [Google Scholar]
- Ristory, H. Die Stiftsbibliothek. In Jahrbuch des Stiftes Klosterneuburg; Huber: Wettin-Löbejün, Germany, 2011; Volume 21, pp. 33–41. [Google Scholar]
- Opl, K. Klosterneuburg. In Handbuch der historischen Buchbestände in Österreich, Bd. 3; Hildesheim–Zürich: New York, NY, USA, 1996; pp. 130–136. [Google Scholar]
- Goebl, R. Kornhäusel, Joseph. Neue Dtsch. Biogr. 1980, 12, 593–594. [Google Scholar]
- Wexler, A. Vapor pressure formulation for water in range 0 to 100 C. A revision. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1976, 80, 775. [Google Scholar] [CrossRef]
- ZAMG Daten. Available online: https://data.hub.zamg.ac.at (accessed on 31 October 2022).
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute, Kew: Surrey, UK, 1971; p. 608. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Eching, Germany, 2007; ISBN 978-3-930167-69-2. [Google Scholar]
- de Hoog, G.S.; Guarro, J. (Eds.) Atlas of Clinical Fungi; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 1995. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species, 1st ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; ISBN 90-70351-46-3. [Google Scholar]
- Pitt, J.I. A Laboratory Guide to Common Penicillium Species, 3rd ed.; Food Science Australia: North Ryde, Australia, 2000; ISBN 0643048375. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; Westerdijk Laboratory Manual Series: Utrecht, The Netherlands, 2019; ISBN 978-94-91751-18-9. [Google Scholar]
- Piñar, G.; Poyntner, C.; Lopandic, K.; Tafer, H.; Sterflinger, K. First rapid diagnosis of biological infection in cultural artefacts using the MinION Nanopore sequencing technology. Int. Biodeterior. Biodegrad. 2020, 148, 104908. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Innis. In PCR Protocols; Gelfand, M.A.D., Sninsky, H., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List; Bolchacova, E.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Querner, P.; Oberthaler, E.; Strolz, M. Biological pest control of a biscuit beetle (Stegobium paniceum) infestation in an old masters paintings storage area. Stud. Conserv. 2019, 64, 373–380. [Google Scholar] [CrossRef]
- Querner, P.; Biebl, S. Using parasitoid wasps in Integrated Pest Management in museums against biscuit beetle (Stegobium paniceum) and webbing clothes moths (Tineola bisselliella). J. Entomol. Acarol. Res. 2011, 43, 169–175. [Google Scholar] [CrossRef]
- Polo, A.; Cappitelli, F.; Villa, F.; Pinzari, F. Biological invasion in the indoor environment: The spread of Eurotium halophilicum on library materials. Int. Biodeterior. Biodegrad. 2017, 118, 34–44. [Google Scholar] [CrossRef]
- EN. Conservation of Cultural Property-Specifications for Temperature and Relative Humidity to Limit Climate-Induced Mechanical Damage in Organic Hygroscopic Materials; European Committee for Standardisation (CEN): Brussels, Belgium, 2010; p. EN15757. [Google Scholar]



| Location | Site | Height/cm | Logger Number |
|---|---|---|---|
| 1: shelf 5 | in cupboard | 107 | SK001 |
| 2: shelf 5 | shelf | 228 | SK002 |
| 3: shelf 76 | in cupboard | 104 | SK003 |
| 4: shelf 76 | shelf | 184 | SK004 |
| 5: shelf 51 | top of shelf | 490 | SK005 |
| 5: shelf 51 | behind shelf | 390 | SK005e |
| 6: shelf 40 | top of shelf | 490 | SK006 |
| 6: shelf 40 | behind shelf | 390 | SK006e |
| 7: shelf 30 | top of shelf | 490 | SK007 |
| 7: shelf 30 | behind shelf | 390 | SK007e |
| 8: shelf 3 | top of shelf | 411 | SK008 |
| 8: shelf 3 | behind shelf | 310 | SK008e |
| 9: shelf 6 | top of shelf | 411 | SK009 |
| 9: shelf 6 | behind shelf | 310 | SK009e |
| 10: shelf 3 | shelf | 201 | SK010 |
| 11: shelf 51 | shelf | 184 | SK011 |
| 14 shelf 35 | floor | 0 | SK014 |
| 15: shelf 3 | floor | 0 | SK015 |
| 16: shelf 55 | floor | 0 | SK016 |
| Species of Fungi Identified in Air Samples | |
|---|---|
| 2017 | 2022 |
| Alternaria sp. Aspergillus candidus Aspergillus ochraceus Aspergillus versicolor Cladosporium spp. Epicoccum sp. Exophiala sp. Fusarium sp. Oidodendron sp. Penicillium brevicompactum Penicillium commune Penicillium chrysogenum Paecilomyces sp. Wallemia sp. | Alternaria spp. Aspergillus fumigatus Aspergillus sect. nigri spp. Aspergillus spp. Aspergillus versicolor Cladosporium spp. Eurotium spp. Fusarium sp. Mucor sp. Paecilomyces sp. Penicillium chrysogenum Penicillium cf. rubrum Penicillium spp. Rhizopus sp. Syncephalastrum sp. Walllemia sp. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brimblecombe, P.; Sterflinger, K.; Derksen, K.; Haltrich, M.; Querner, P. Thermohygrometric Climate, Insects and Fungi in the Klosterneuburg Monastic Library. Heritage 2022, 5, 4228-4244. https://doi.org/10.3390/heritage5040218
Brimblecombe P, Sterflinger K, Derksen K, Haltrich M, Querner P. Thermohygrometric Climate, Insects and Fungi in the Klosterneuburg Monastic Library. Heritage. 2022; 5(4):4228-4244. https://doi.org/10.3390/heritage5040218
Chicago/Turabian StyleBrimblecombe, Peter, Katja Sterflinger, Katharina Derksen, Martin Haltrich, and Pascal Querner. 2022. "Thermohygrometric Climate, Insects and Fungi in the Klosterneuburg Monastic Library" Heritage 5, no. 4: 4228-4244. https://doi.org/10.3390/heritage5040218
APA StyleBrimblecombe, P., Sterflinger, K., Derksen, K., Haltrich, M., & Querner, P. (2022). Thermohygrometric Climate, Insects and Fungi in the Klosterneuburg Monastic Library. Heritage, 5(4), 4228-4244. https://doi.org/10.3390/heritage5040218







