An Investigation of Electrochemical Dechlorination of Wrought Iron Specimens from the Marine Environment
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
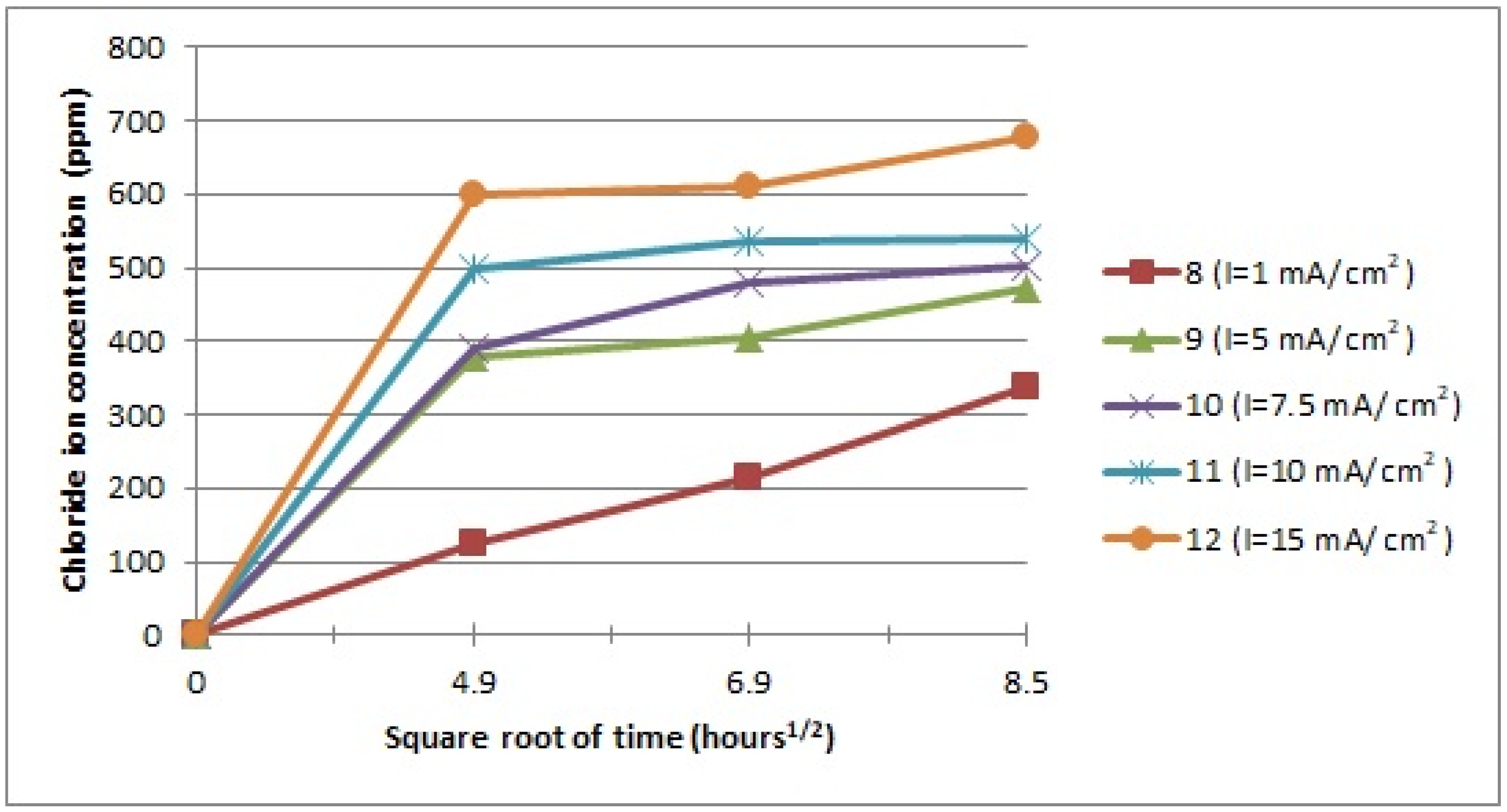
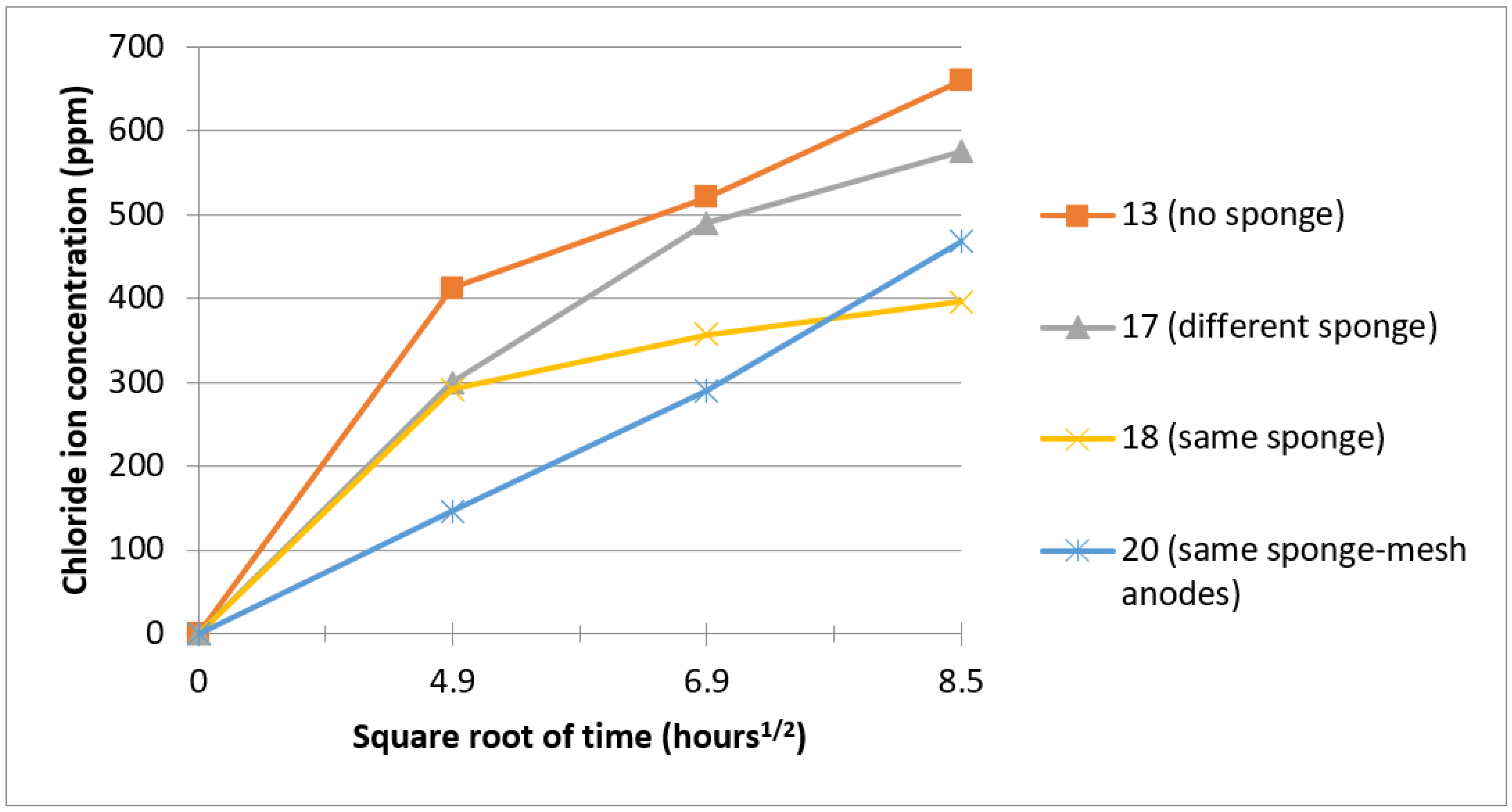
3.1. Electrochemical Treatment
3.2. Determination of Final Chloride Concentration with HNO3
3.3. Factors of Chloride Ion Diffusion
- I: current density;
- t: time;
- M: molecular weight of chloride;
- n: number of electrons on the chloride ion;
- F: Faraday constant.
- [Cl−]th: the theoretical concentration of chloride ion extraction;
- [Cl−]t: the experimental concentration of chloride ions in the alkaline solution for 24, 48, and 72 h.
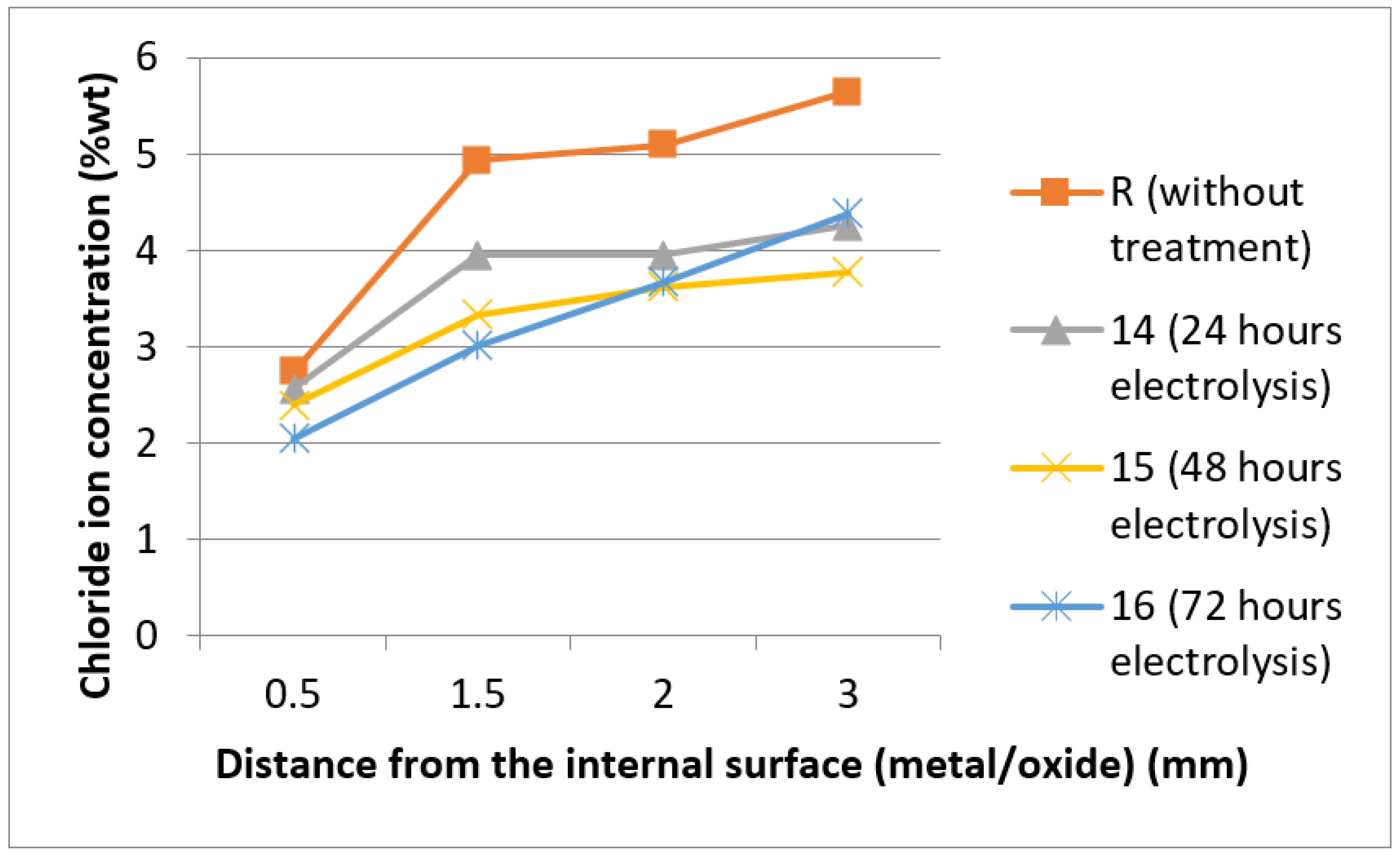
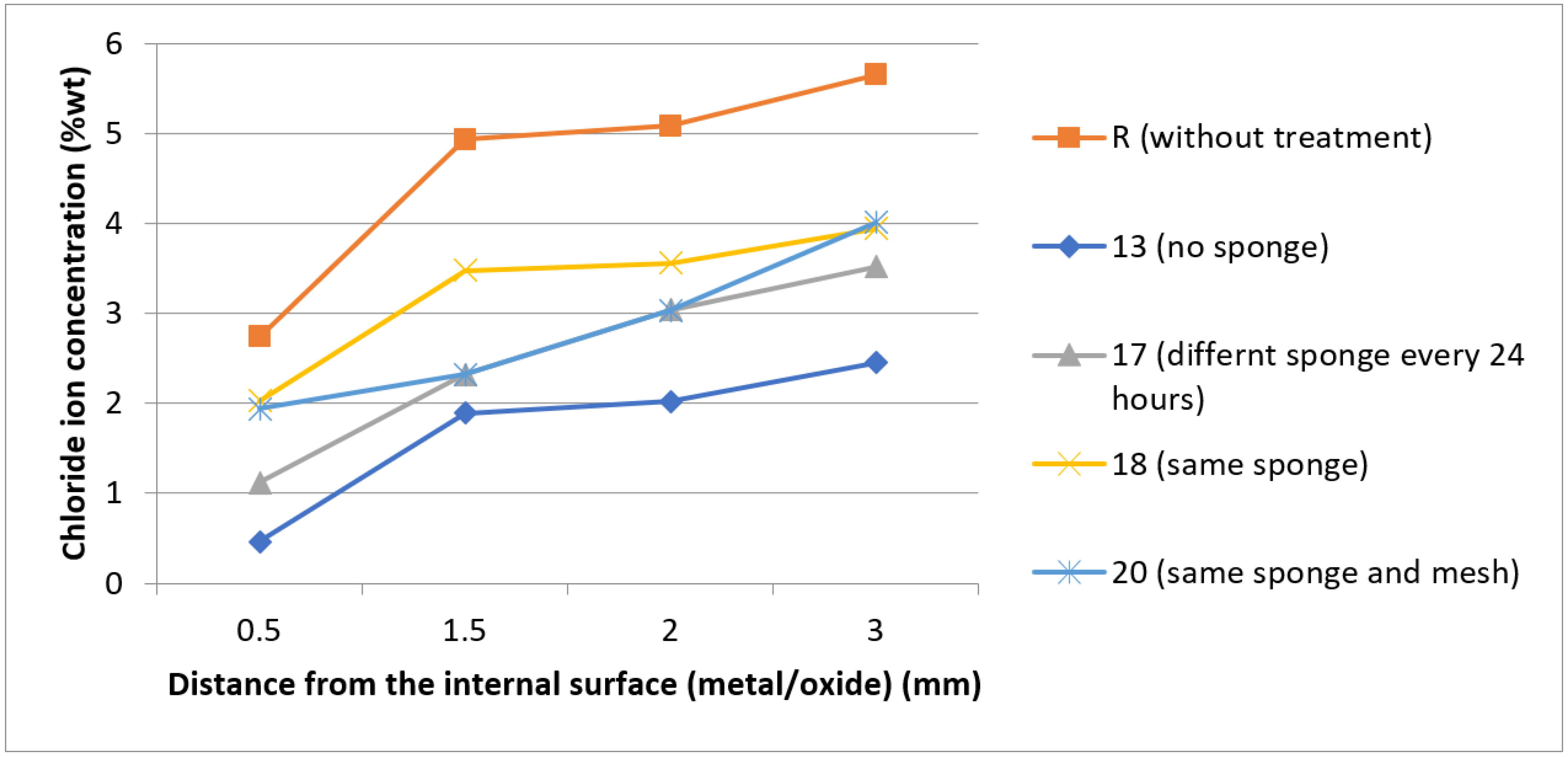
3.4. SEM-EDX Analysis
3.5. A Mathematical Model for Extraction Ratio of Chloride Ions
- Cf0: the initial concentration of the free chloride;
- Cb0: the initial concentration of the binding chloride;
- A: the area of the object;
- D: the length of the object.
4. Conclusions
- Marine iron objects can be electrochemically dechlorinated with the use of a porous medium with sodium hydroxide solutions;
- The use of a sponge is inferior for the removal of chloride ions versus the complete immersion in sodium hydroxide solutions;
- When a porous medium is used, a lower volume of alkaline solution is required than complete immersion;
- Changing the porous medium every 24 h increases chloride iron removal compared to using the same sponge during the electrochemical treatment;
- The performance of electrochemical method improves as the current density increases.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batis, G.; Zacharopoulou, A.; Zacharopoulou, E.; Siova, H.; Argyropoulos, V. Dechlorinationof Large Marine Iron Artifact Using a Novel Technique Involving Impressed Current. Anti-Corros. Methods Mater. 2015, 62, 259–269. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Lu, X.; Wang, Z. Effect of Temperature and Ultraviolet Radiation on Corrosion Behavior of Carbon Steel in High Humidity Tropical Marine Atmosphere. Mater. Chem. Phys. 2022, 277, 124962. [Google Scholar] [CrossRef]
- Tian, H.; Cui, Z.; Ma, H.; Zhao, P.; Yan, M.; Wang, X.; Cui, H. Corrosion Evolution and Stress Corrosion Cracking Behavior of Low Carbon Steel Bainite Steel in the Marine Environments: Effect of Marine Zones. Corros. Sci. 2022, 206, 110490. [Google Scholar] [CrossRef]
- De Baare, K.; Van Haelst, S.; Chaves, I.; Luyckx, D.; Van Den Bergh, K.; Verbeken, K.; De Meyer, E.; Verhasselt, K.; Meskens, R.; Potters, G.; et al. The Influence of Concretion on the Long-Term Corrosion Rate of Steel Shipwrecks in the Belgian North Sea. Corros. Sci. 2020, 56, 71–80. [Google Scholar] [CrossRef]
- Ashkenazi, D.; Nusbaum, I.; Shacham-Diamand, Y.; Cvikel, D.; Kahanov, Y.; Inberg, A. A Method of Conserving Ancient Iron Artefacts Retrieved from Shipwrecks Using a Combination of Silane Self-Assembled Monolayers and Wax Coating. Corros. Sci. 2017, 123, 88–102. [Google Scholar] [CrossRef]
- North, Ν.A.; Mac Leod, I.D. Corrosion of Metals. In Conservation of Marine Archaeological Objects, 2nd ed.; Series in Conservaion and Museology; Butterworth-Heinemann: Oxford, UK, 1987; pp. 68–98. [Google Scholar]
- Degrigny, C.; Spitery, L. Electrochemical Monitoring of Marine Iron Artefacts During Their Storage/Stabilisation in Alkaline Solution. In Proceedings of the Metal 2004, Canberra, Austrila, 4–8 October 2004; National Museum of Australia Camberra: Canberra, Austrila, 2004; pp. 315–331. [Google Scholar]
- Réguer, S.; Mirambet, F.; Rémazeilles, C.; Vantelon, D.; Kergourlay, F.; Neff, D.; Dillmann, P. Iron Corrosion in Archaelogical Context: Structural Refinement of the Ferrous Hydroxychloride β-Fe2(OH)Cl. Corros. Sci. 2015, 100, 589–598. [Google Scholar] [CrossRef]
- Turgoose, S. The Nature of Surviving Iron Objects, in Conservation of Iron. Stud. Conserv. 1982, 27, 97–101. [Google Scholar] [CrossRef]
- Bayle, M.; De Vivies, P.; Memet, J.-B.; Foy, E.; Dillmann, P.; Neff, D. Corrosion Product Transformation in Alkaline Baths Under Pressure and High Temperature: The Sub-Critical Stabilisation of Marine Iron Artefacts Stored Under Atmospheric Conditions. Mater. Corros. 2015, 167, 190–199. [Google Scholar] [CrossRef]
- Rimmer, M.; Watkinson, D.; Wang, Q. The Efficiency of Chloride Extraction from Archaeological Iron Objects Using Deoxygenated Alkaline Solutions. Stud. Conserv. 2012, 57, 29–41. [Google Scholar] [CrossRef]
- Lv, G.C.; Wang, Z.S.; Wu, L.M.; Xu, C. Charactirization of Corrosion Products on Archaeological Iron Coins. Anti-Corros. Methods Mater. 2011, 58, 39–45. [Google Scholar] [CrossRef]
- Kergoulay, F.; Reguer, S.; Neff, D.; Foy, E.; Picca, F.E.; Saneb, M.; Hustache, S.; Mirambet, F.; Dillmann, P. Stabilization Treatment of Cultural Heritage Artefacts: In Situ Monitoing of Marine Iron Objects Dechlorinated in Alkaline Solution. Corros. Sci. 2018, 132, 21–38. [Google Scholar] [CrossRef]
- Coelho, J.C.; Oliveira, C.M.; Carvalho, M.D.; Fonseca, I.T.E. The Efficiency of Electochemical Methods for the Removal of Chloride Ions from Marine Archaeological Objects. Mater. Corros. 2014, 65, 38–44. [Google Scholar] [CrossRef]
- Argyropoulos, V.; Batis, G. Saving a Marine Iron Paddle Wheel Removed from the 1868 Steam Engine Shipwreck ‘Patris’ During an Economic Crises in Greece. In Big Stuff Heritage; Canada Science and Technology Museum: Ottawa, ON, Canada, 2013. [Google Scholar]
- Pedefferi, P. Cathodic Protection and Cathodic Prevention. Constraction Build. Mater. 1998, 10, 341–402. [Google Scholar] [CrossRef]
- Broomfield, J.P. The Application of Elecrochemical and Other Techniques to Our Aging Built Infrastructure for the Repair and Conservation of Buildings, Bridges and Structures of Historic and Architectural Importance; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Batis, G.; Rakanta, E. Corrosion of Steel Reinforcement Due to Atmospheric Pollution. Cem. Concr. Compos. 2005, 27, 269–275. [Google Scholar] [CrossRef]
- Kouloumbi, N.; Batis, G. Chloride Corrosion of Steel Rebars in Mortars with Fly Ash Admixtures. Cem. Concr. Compos. 1992, 14, 199–207. [Google Scholar] [CrossRef]
- Elsener, B.; Angst, U. Mechanism of Electrochemical Chloride Removal. Corros. Sci. 2007, 49, 4504–4522. [Google Scholar] [CrossRef]
- Feng, W.; Dong, Z.; Liu, W.; Cui, H.; Tang, W.; Xing, F. An Experimental Study on the Influence of Applied Voltage on Current Efficiency of Rebars with a Modified Accelerated Corrosion Test. Cem. Concr. Compos. 2021, 122, 10412. [Google Scholar] [CrossRef]
- Selwyn, L.S.; McKinnon, W.R.; Argyropoulos, V. Models of Chloride Ion Diffusion in Archaeological Iron. Stud. Conserv. 2001, 46, 109–121. [Google Scholar] [CrossRef]
- Kergoulay, F.; Remazeilles, C.; Neff, D.; Foy, E.; Conforto, E.; Guilminot, E.; Reguer, S.; Dillmann, P.; Nicot, F.; Mielcarek, F.; et al. Mechanisms of Dechlorinatio of Iron Archaeological Artifacts Extracted from Seawater. Corros. Sci. 2011, 53, 2474–2483. [Google Scholar] [CrossRef]
- Denbigh, K. The Principles of Chemical Equilibrium, 4th ed.; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Abbott, M.M.; Van Ness, H.C. Schaum’s Outline of Theory and Problems of Thermodynamics; McGraw-Hill: New York, NY, USA, 1972; Available online: https://snia.mop.gob.cl/repositoriodga/handle/20.500.13000/2577 (accessed on 12 December 2022).
- Quyang, W.; Xia, C.; Wang, N. A Mathematical Model for Electrochemical Chloride Removal from Marine Cast Iron. Acta Metall. Sin. 2009, 22, 91–99. [Google Scholar] [CrossRef]




| Specimen | Dimensions (cm) (Length, Ø Diameter) |
|---|---|
| Reference (R) | 15, Ø 3.4 |
| 3 | 15, Ø 3.4 |
| 4 | 15, Ø 3.5 |
| 5 | 15, Ø 3.4 |
| 6 | 15, Ø 3.5 |
| 7 | 15, Ø 3.5 |
| 8 | 15, Ø 3.6 |
| 9 | 15, Ø 3.5 |
| 10 | 15, Ø 3.6 |
| 11 | 15, Ø 3.5 |
| 12 | 15, Ø 3.4 |
| 13 | 15, Ø 3.4 |
| 14 | 15, Ø 3.4 |
| 15 | 15, Ø 3.4 |
| 16 | 15, Ø 3.5 |
| 17 | 15, Ø 3.5 |
| 18 | 15, Ø 3.4 |
| 20 | 15, Ø 3.5 |
| Specimen | Porous Medium | Volume of Alkaline Solution 1N NaOH (mL) | Current Density (mA/cm2) | Voltage (mV) SSE | Time (h) |
|---|---|---|---|---|---|
| Reference R | - | - | - | - | - |
| 3 | Sponge (same) | 350 | 1 | −980 to −900 | 72 |
| 4 | Sponge (same) | 350 | 5 | −980 to −900 | 72 |
| 5 | Sponge(same) | 350 | 7.5 | −980 to −900 | 72 |
| 6 | Sponge (same) | 350 | 10 | −980 to −900 | 72 |
| 7 | Sponge (same) | 350 | 15 | −980 to −900 | 72 |
| 8 | No sponge | 1000 | 1 | −980 | 72 |
| 9 | No sponge | 1000 | 5 | −980 | 72 |
| 10 | No sponge | 1000 | 7.5 | −980 | 72 |
| 11 | No sponge | 1000 | 10 | −980 | 72 |
| 12 | No sponge | 1000 | 15 | −980 | 72 |
| 13 | No sponge | 1000 | 8 | −795 to −835 | 72 |
| 14 | Sponge | 180 | 4 | −880 to −840 | 24 |
| 15 | Sponge | 180 | 4 | −890 to −680 | 48 |
| 16 | Sponge | 180 | 4 | −820 to −660 | 72 |
| 17 | Different sponge every 24 h | 350 | 8 | −785 to −690 | 72 |
| 18 | Sponge | 350 | 8 | −760 to −700 | 72 |
| 20 | Sponge and mesh | 350 | 8 | −760 to −680 | 72 |
| Chloride Ion Concentration | |||
|---|---|---|---|
| Specimen | 24 h | 48 h | 72 h |
| 14 | 294.98 | - | - |
| 15 | - | 439.71 | - |
| 16 | - | - | 478.45 |
| Specimen | ||||||||
|---|---|---|---|---|---|---|---|---|
| R | 13 | 14 | 15 | 16 | 17 | 18 | 20 | |
| Chloride ion concentration (ppm) | 601 | 234 | 478 | 445 | 399 | 329 | 352 | 336 |
| Specimen 13 | Specimen 14 | Specimen 15 | Specimen 16 | Specimen 17 | Specimen 18 | Specimen 20 | |
|---|---|---|---|---|---|---|---|
| Chloride ion concentration (ppm) | 367 | 123 | 156 | 202 | 272 | 249 | 265 |
| Current Density (mA/cm2) | Theoretical Concentration of Chloride Ions Diffusion (g) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 1 | 0.032 | 0.064 | 0.095 |
| 4 | 0.127 | - | - |
| - | 0.254 | - | |
| - | - | 0.381 | |
| 5 | 0.159 | 0.317 | 0.476 |
| 7.5 | 0.238 | 0.476 | 0.715 |
| 8 | 0.264 | 0.508 | 0.762 |
| 10 | 0.317 | 0.634 | 0.953 |
| 15 | 0.476 | 0.952 | 1.429 |
| Specimen | Current Density (mA/cm2) | Porous Medium | 1 − α 24 h | 1 – α 48 h | 1 – α 72 h |
|---|---|---|---|---|---|
| 3 | 1 | Sponge | 0.102 | 0.387 | 0.534 |
| 4 | 5 | Sponge | 0.758 | 0.851 | 0.900 |
| 5 | 7.5 | Sponge | 0.849 | 0.899 | 0.929 |
| 6 | 10 | Sponge | 0.830 | 0.909 | 0.930 |
| 7 | 15 | Sponge | 0.850 | 0.921 | 0.943 |
| 8 | 1 | No sponge | 0.023 | 0.164 | 0.111 |
| 9 | 5 | No sponge | 0.403 | 0.680 | 0.752 |
| 10 | 7.5 | No sponge | 0.590 | 0.747 | 0.825 |
| 11 | 10 | No sponge | 0.609 | 0.789 | 0.858 |
| 12 | 15 | No sponge | 0.685 | 0.840 | 0.881 |
| 13 | 8 | No sponge | 0.610 | 0.744 | 0.784 |
| 14 | 4 | Sponge | 0.429 | - | - |
| 15 | 4 | Sponge | - | 0.567 | - |
| 16 | 4 | Sponge | - | - | 0.686 |
| 17 | 8 | Different sponge | 0.716 | 0.759 | 0.811 |
| 18 | 8 | Sponge | 0.723 | 0.824 | 0.870 |
| 20 | 8 | Sponge and mesh | 0.862 | 0.858 | 0.846 |
| Specimen | Average Chloride Ion Concentration % wt of the Points A, B, C, D | Time (h) |
|---|---|---|
| R (initial chloride ion concentration) | 4.61 | 0 |
| 13(I = 8 mA) no sponge | 1.71 | 72 |
| 14 (I = 4 mA) sponge | 3.69 | 24 |
| 15 (I = 4 mA) sponge | 3.38 | 48 |
| 16 (I = 4 mA) sponge | 3.20 | 72 |
| 17 (I = 8 mA) different sponge | 2.40 | 72 |
| 18 (I = 8 mA) same sponge | 3.07 | 72 |
| 20 (I = 8 mA) sponge and mesh | 2.67 | 72 |
| R (5 g) after dissolution with 5 N HNO3 | 0.13 | 48 |
| Specimen | Treatment | Factor (F) |
|---|---|---|
| 13 | No sponge | 0.630 |
| 14 | Sponge | 0.200 |
| 15 | Sponge | 0.266 |
| 16 | Sponge | 0.305 |
| 17 | Different sponge | 0.479 |
| 18 | Same sponge | 0.334 |
| 20 | Sponge with mesh | 0.419 |
| R reference after treatment with 5 N HNO3 | Treatment with 5 N HNO3 | 0.972 |
| Specimen | Current Density I (mA/cm2) | Volume of Alkaline Solution (mL) | Treatment | Extraction Ratio % |
|---|---|---|---|---|
| 13 | 8 | 1000 | No sponge (72 h) | 63.1 |
| 14 | 4 | 180 | Sponge (24 h) | 19.9 |
| 15 | 4 | 180 | Sponge (48 h) | 26.5 |
| 16 | 4 | 180 | Sponge (72 h) | 30.5 |
| 17 | 8 | 350 | Different sponge every 24 h | 47.1 |
| 18 | 8 | 350 | Sponge (72 h) | 33.4 |
| 20 | 8 | 350 | Sponge and mesh (72 h) | 41.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siova, E.; Argyropoulos, V.; Batis, G. An Investigation of Electrochemical Dechlorination of Wrought Iron Specimens from the Marine Environment. Heritage 2023, 6, 587-599. https://doi.org/10.3390/heritage6010031
Siova E, Argyropoulos V, Batis G. An Investigation of Electrochemical Dechlorination of Wrought Iron Specimens from the Marine Environment. Heritage. 2023; 6(1):587-599. https://doi.org/10.3390/heritage6010031
Chicago/Turabian StyleSiova, Eleni, Vasilike Argyropoulos, and George Batis. 2023. "An Investigation of Electrochemical Dechlorination of Wrought Iron Specimens from the Marine Environment" Heritage 6, no. 1: 587-599. https://doi.org/10.3390/heritage6010031
APA StyleSiova, E., Argyropoulos, V., & Batis, G. (2023). An Investigation of Electrochemical Dechlorination of Wrought Iron Specimens from the Marine Environment. Heritage, 6(1), 587-599. https://doi.org/10.3390/heritage6010031







