Characterization and Degradation Analysis of Pigments in Paintings by Martiros Sarian: Attenuated Total Reflection Fourier Transform Infrared Spectroscopic Imaging and X-ray Fluorescence Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micro-Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopic Imaging
2.2. X-ray Fluorescence (XRF)
3. Results
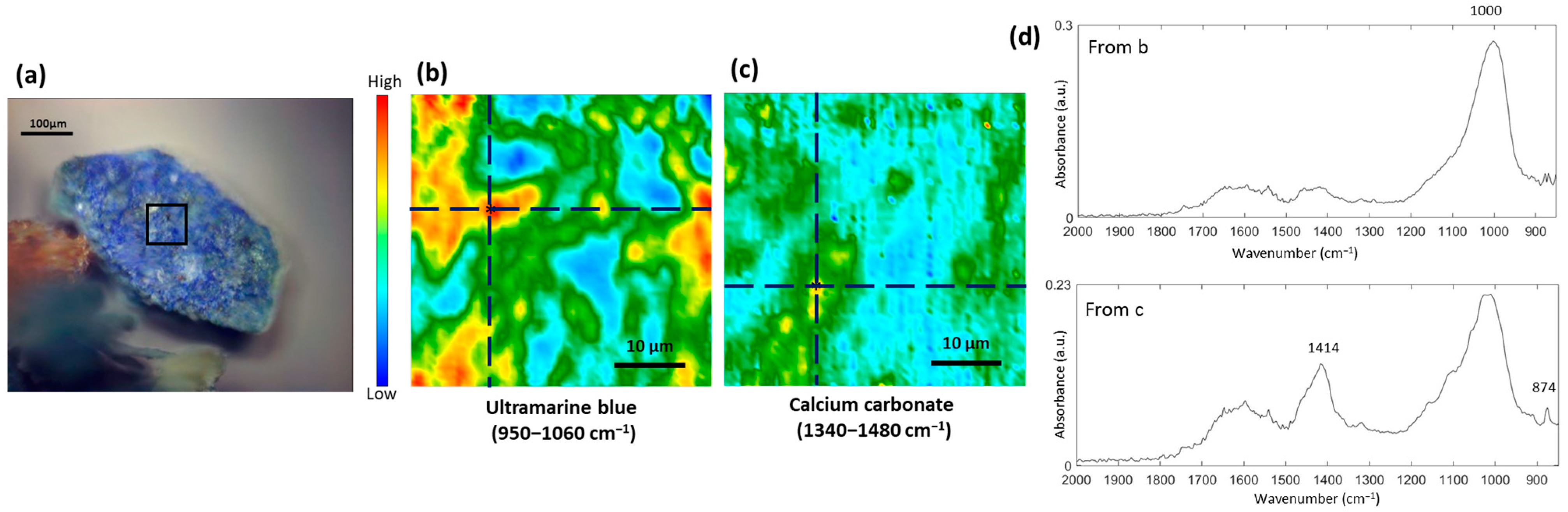
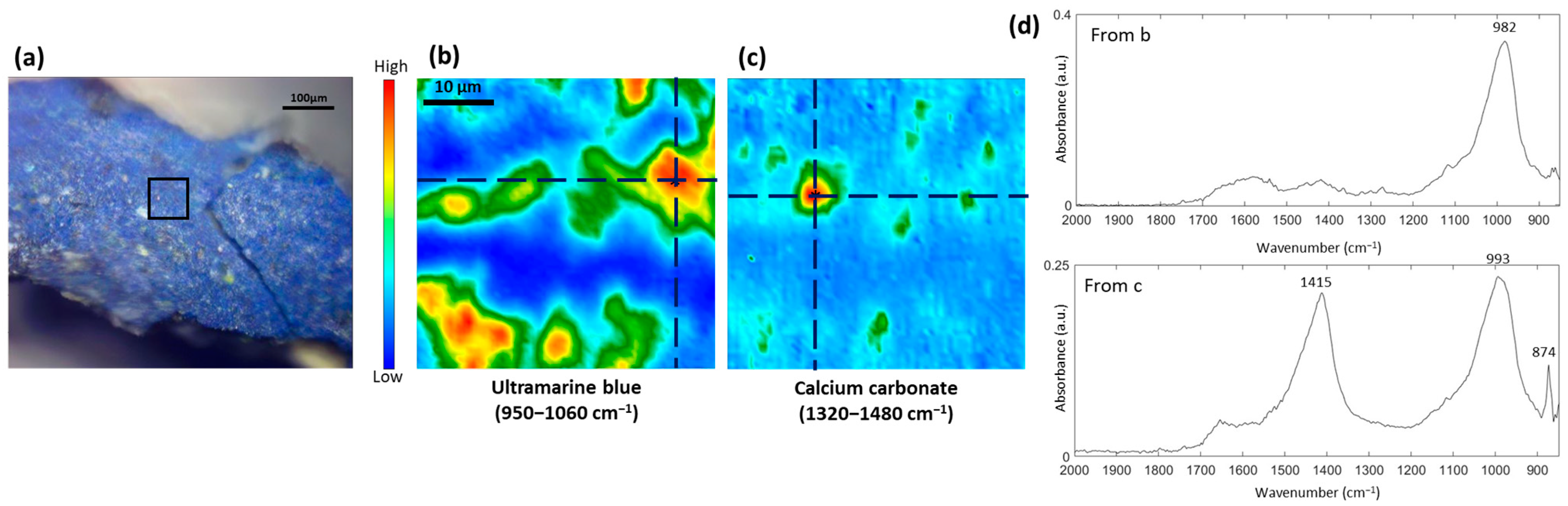
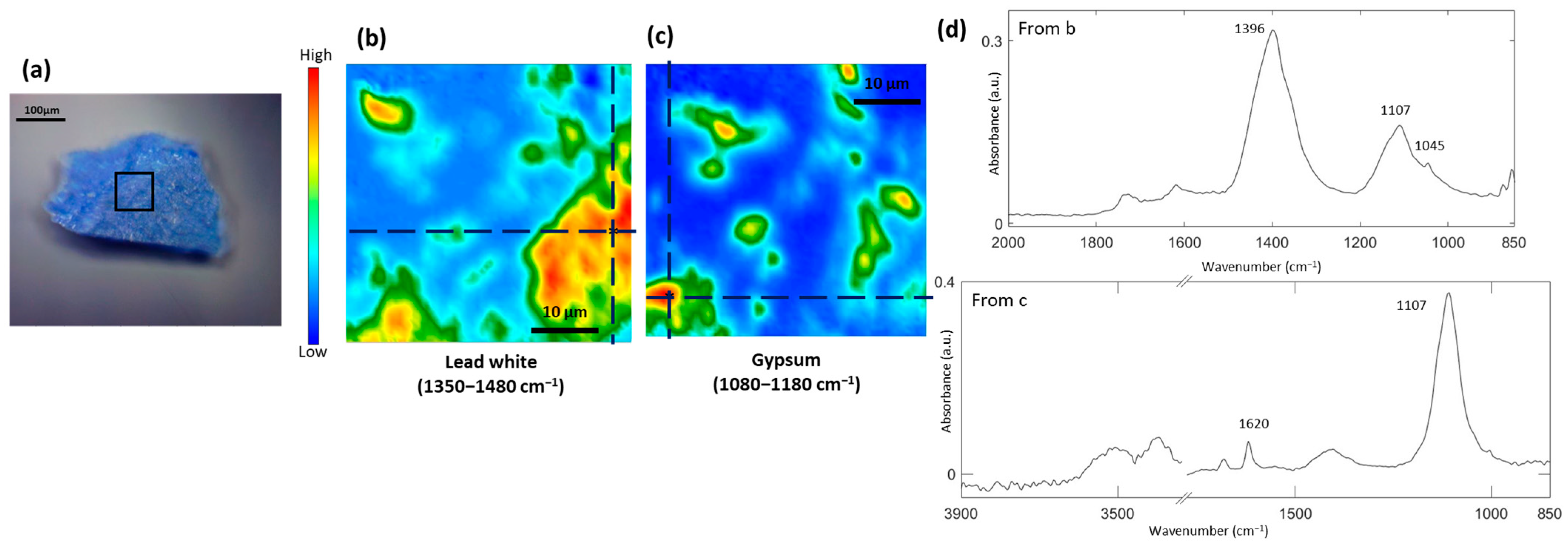
3.1. Study of the Blue Pigments
3.2. Study of the Green Pigment
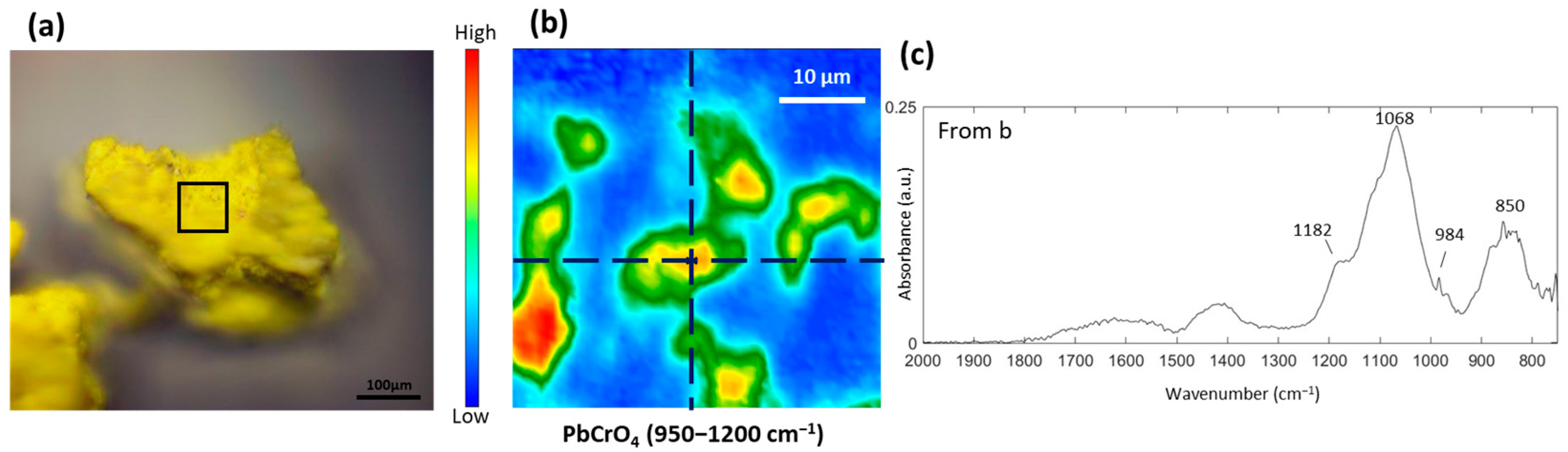
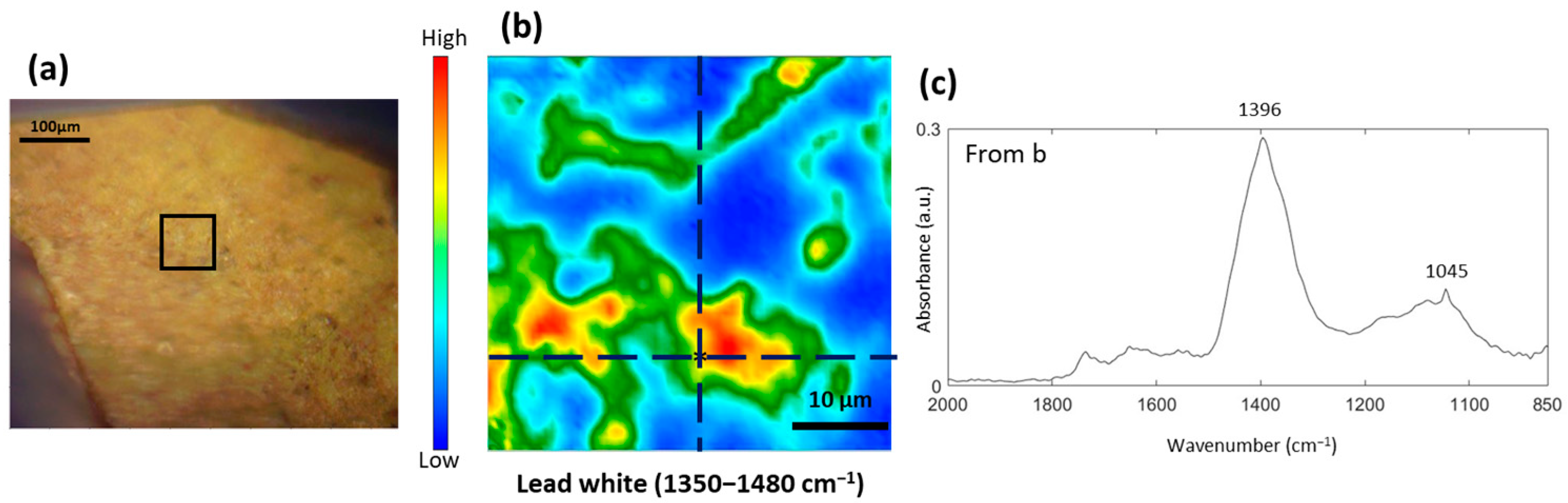
3.3. Study of the Yellow and Brown Pigments
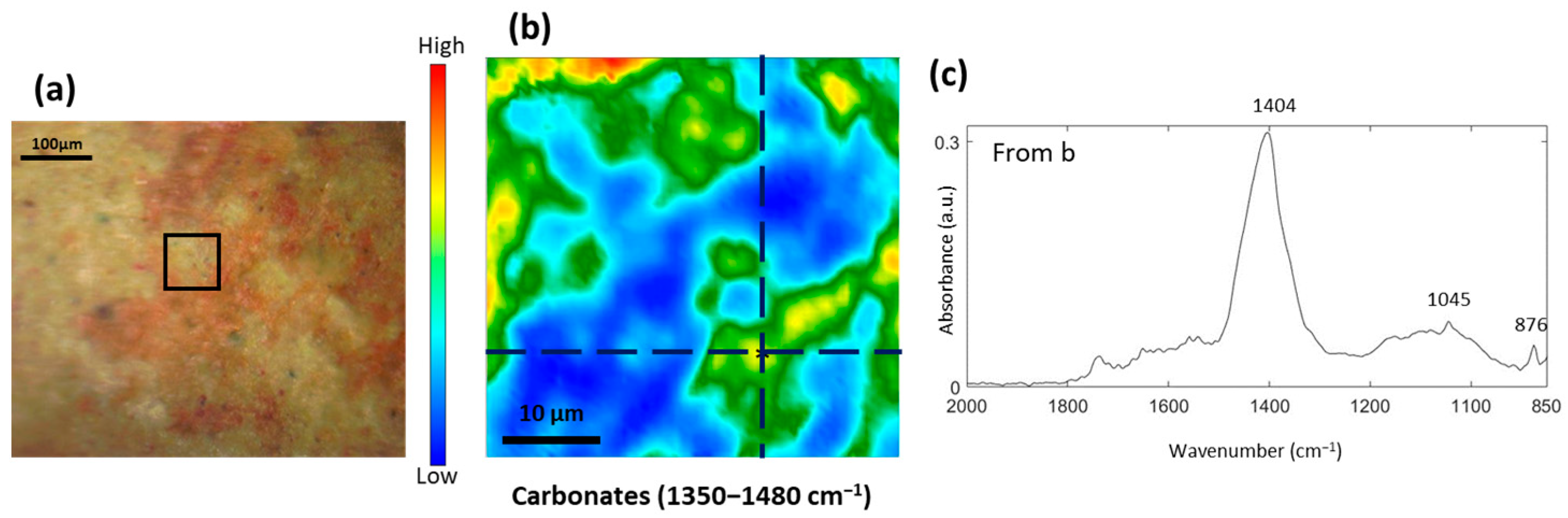
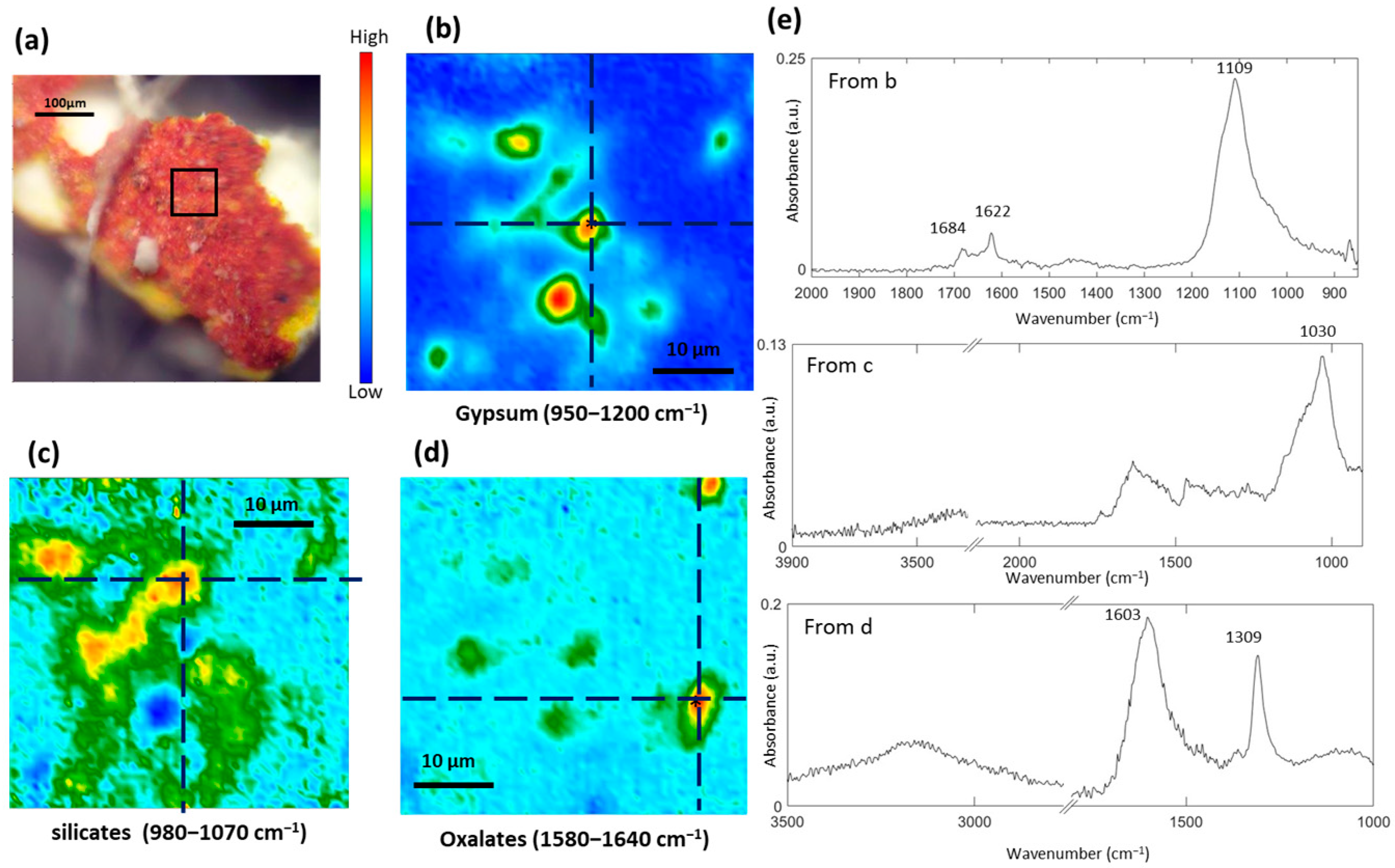
3.4. Study of the Red and Orange Pigments
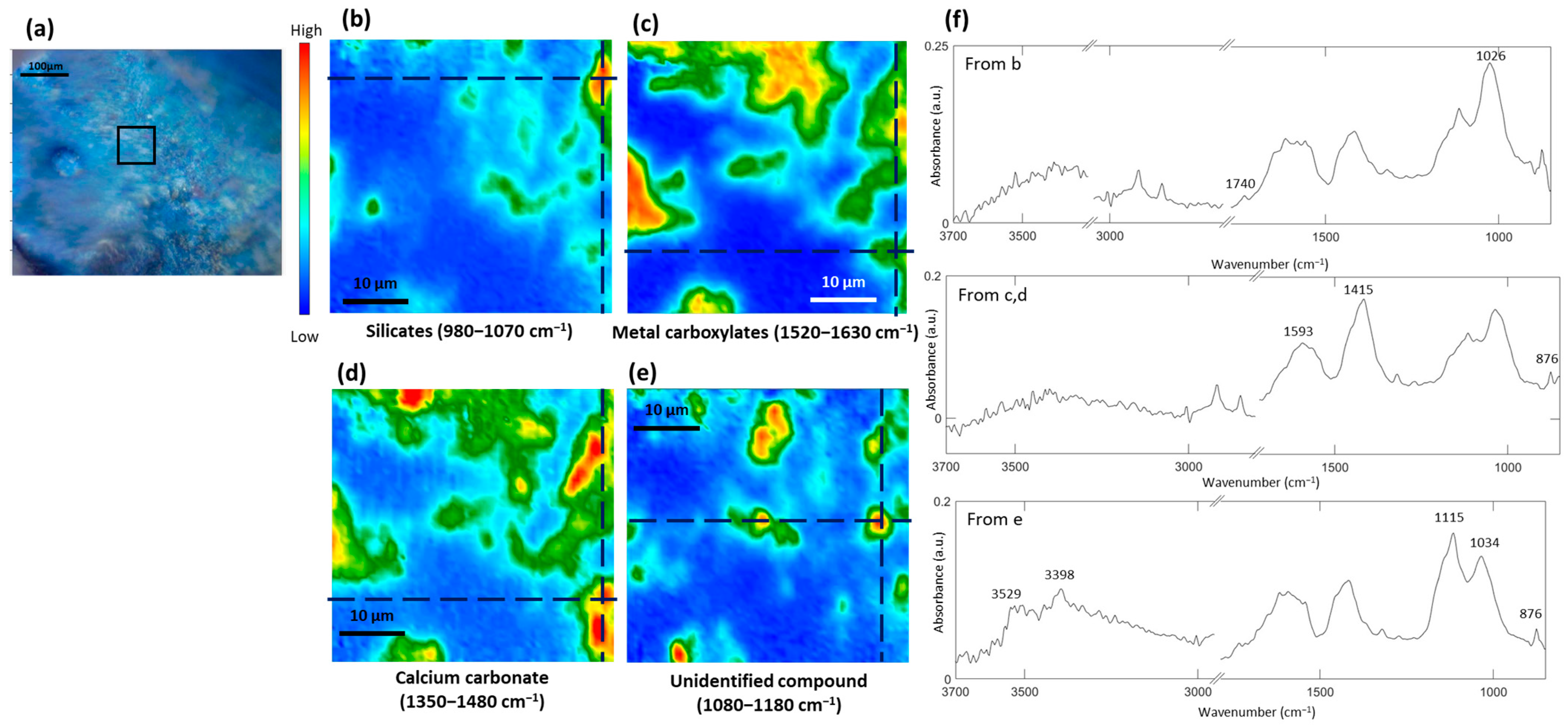
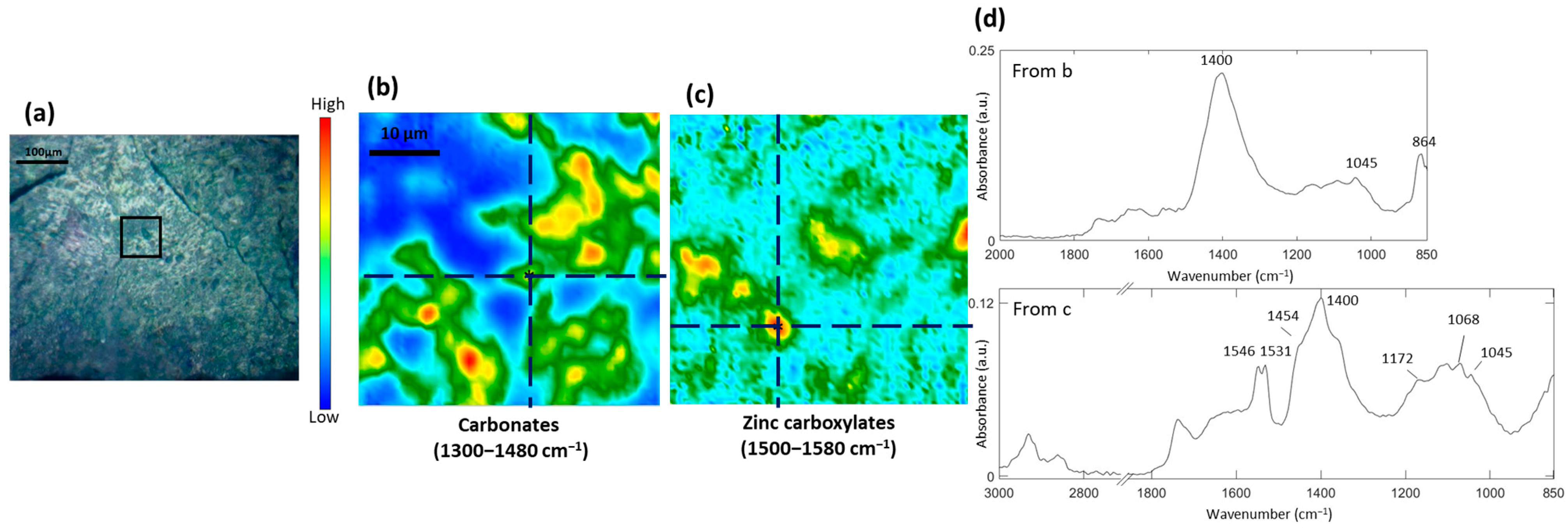
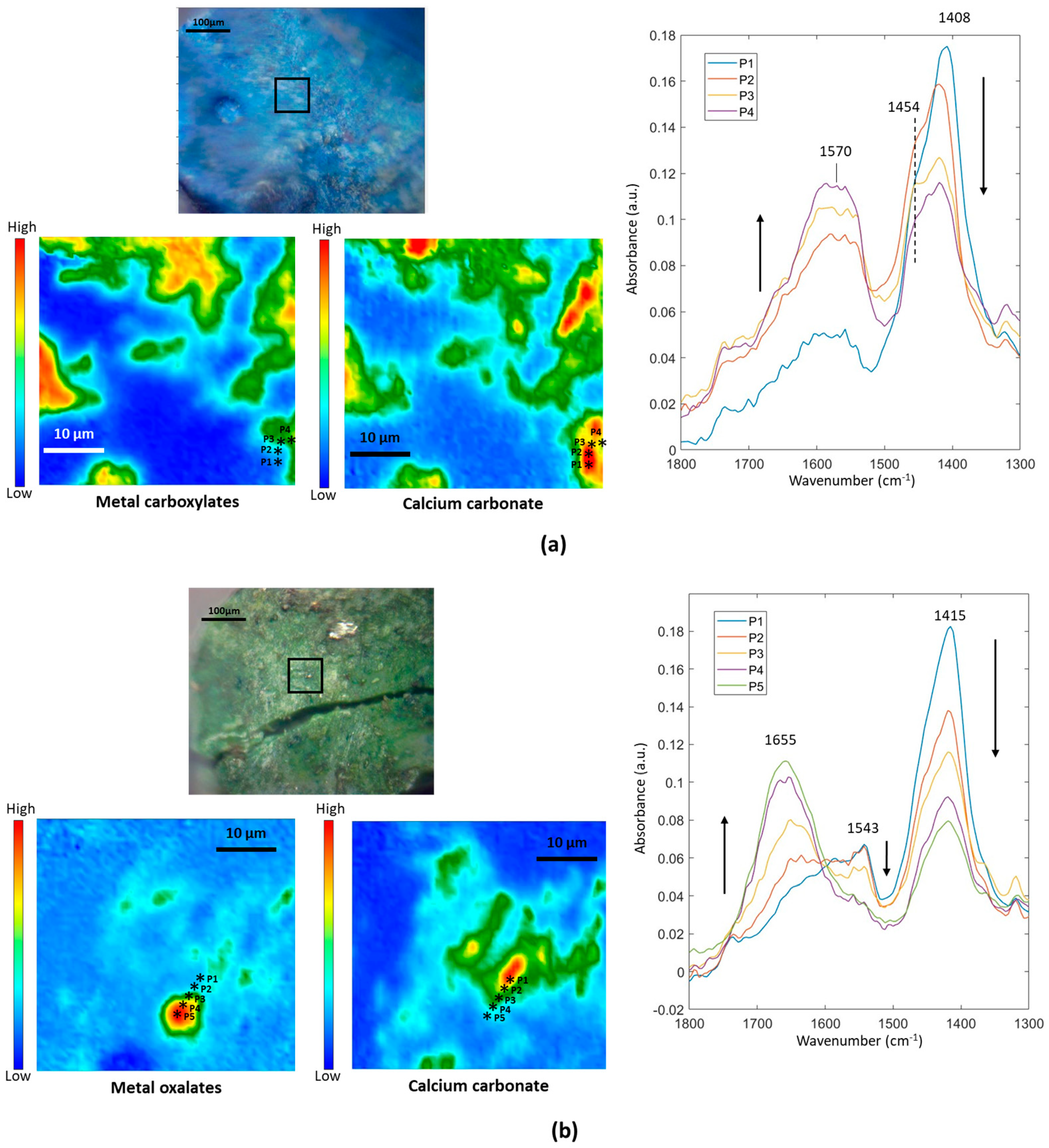
3.5. Formation of Metal Carboxylates and Oxalates in Oil Paints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azatyan, V. Disintegrating Progress: Bolshevism, National Modernism, and the Emergence of Contemporary Art Practices in Armenia. ARTMargins 2012, 1, 62–87. [Google Scholar] [CrossRef]
- Sahakyan, N. The Late Soviet Charm of Kantianism: Wilhelm Matevosyan’s Art History. Ph.D. Thesis, American University of Beirut, Beirut, Lebanon, 2021. [Google Scholar]
- The Editors of Encyclopaedia Britannica. Martiros Saryan. Available online: https://www.britannica.com/biography/Martiros-Saryan (accessed on 30 June 2023).
- Liu, G.-L.; Kazarian, S.G. Recent advances and applications to cultural heritage using ATR-FTIR spectroscopy and ATR-FTIR spectroscopic imaging. Analyst 2022, 147, 1777–1797. [Google Scholar] [CrossRef] [PubMed]
- Streli, C.; Wobrauschek, P.; Kregsamer, P. X-ray Fluorescence Spectroscopy, Applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 707–715. [Google Scholar]
- Spring, M.; Ricci, C.; Peggie, D.A.; Kazarian, S.G. ATR-FTIR imaging for the analysis of organic materials in paint cross sections: Case studies on paint samples from the National Gallery, London. Anal. Bioanal. Chem. 2008, 392, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, F.; Rosi, F.; Vichi, A.; Cartechini, L.; Pensabene Buemi, L.; Kazarian, S.G.; Miliani, C. Revealing the Nature and Distribution of Metal Carboxylates in Jackson Pollock’s Alchemy (1947) by Micro-Attenuated Total Reflection FT-IR Spectroscopic Imaging. Anal. Chem. 2017, 89, 1283–1289. [Google Scholar] [CrossRef]
- Kaszowska, Z.; Malek, K.; Pańczyk, M.; Mikołajska, A. A joint application of ATR-FTIR and SEM imaging with high spatial resolution: Identification and distribution of painting materials and their degradation products in paint cross sections. Vib. Spectrosc. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Liu, G.-L.; Guerreiro, E.; Babington, C.; Kazarian, S.G. ATR-FTIR spectroscopic imaging of white crusts in cross sections from oil cartoons by Edward Poynter in the Heritage Collections at UK Parliament. J. Cult. Herit. 2023, 62, 251–267. [Google Scholar] [CrossRef]
- Pięta, E.; Proniewicz, E.; Szmelter-Fausek, B.; Olszewska-Świetlik, J.; Proniewicz, L.M. Pigment characterization of important golden age panel paintings of the 17th century. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 594–600. [Google Scholar] [CrossRef]
- Van der Snickt, G.; Janssens, K.; Dik, J.; De Nolf, W.; Vanmeert, F.; Jaroszewicz, J.; Cotte, M.; Falkenberg, G.; Van der Loeff, L. Combined use of Synchrotron Radiation Based Micro-X-ray Fluorescence, Micro-X-ray Diffraction, Micro-X-ray Absorption Near-Edge, and Micro-Fourier Transform Infrared Spectroscopies for Revealing an Alternative Degradation Pathway of the Pigment Cadmium Yellow in a Painting by Van Gogh. Anal. Chem. 2012, 84, 10221–10228. [Google Scholar] [CrossRef]
- Plesters, J. Ultramarine blue, natural and artificial. In Artist’s Pigments: A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications Ltd.: Washington, DC, USA; National Gallery of Art: New York, NY, USA, 1993; Volume 2. [Google Scholar]
- Silva, C.E.; Silva, L.P.; Edwards, H.G.M.; de Oliveira, L.F.C. Diffuse reflection FTIR spectral database of dyes and pigments. Anal. Bioanal. Chem. 2006, 386, 2183–2191. [Google Scholar] [CrossRef]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR spectral collection of conservation materials in the extended region of 4000-80 cm–1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef]
- Salter, M.A.; Perry, C.T.; Smith, A.M. Calcium carbonate production by fish in temperate marine environments. Limnol. Oceanogr. 2019, 64, 2755–2770. [Google Scholar] [CrossRef]
- Haddad, A.; Rogge, C.E.; Martins, A.; Dijkema, D. “Foundations of a great metaphysical style”: Unraveling Giorgio de Chirico’s early palette. Herit. Sci. 2022, 10, 70. [Google Scholar] [CrossRef]
- Brooker, M.H.; Sunder, S.; Taylor, P.; Lopata, V.J. Infrared and Raman spectra and X-ray diffraction studies of solid lead(II) carbonates. Can. J. Chem. 1983, 61, 494–502. [Google Scholar] [CrossRef]
- Bishop, J.L.; Lane, M.D.; Dyar, M.D.; King, S.J.; Brown, A.J.; Swayze, G.A. What Lurks in the Martian Rocks and Soil? Investigations of Sulfates, Phosphates, and Perchlorates. Spectral properties of Ca-sulfates: Gypsum, bassanite, and anhydrite. Am. Mineral. 2014, 99, 2105–2115. [Google Scholar] [CrossRef]
- Vichi, A.; Eliazyan, G.; Kazarian, S.G. Study of the Degradation and Conservation of Historical Leather Book Covers with Macro Attenuated Total Reflection–Fourier Transform Infrared Spectroscopic Imaging. ACS Omega 2018, 3, 7150–7157. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.; Banas, A.; Banas, K. Comparison of ATR-FTIR and O-PTIR Imaging Techniques for the Characterisation of Zinc-Type Degradation Products in a Paint Cross-Section. Molecules 2022, 27, 6301. [Google Scholar] [CrossRef]
- Helwig, K.; Poulin, J.; Corbeil, M.-C.; Moffatt, E.; Duguay, D. Conservation Issues in Several Twentieth-Century Canadian Oil Paintings: The Role of Zinc Carboxylate Reaction Products. In Issues in Contemporary Oil Paint; van den Berg, K.J., Burnstock, A., de Keijzer, M., Krueger, J., Learner, T., Tagle, D.A., Heydenreich, G., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 167–184. [Google Scholar]
- Zumbuehl, S.; Scherrer, N.C.; Berger, A.; Eggenberger, U. Early Viridian Pigment Composition CHARACTERIZATION OF A (HYDRATED) CHROMIUM OXIDE BORATE PIGMENT. Stud. Conserv. 2009, 54, 149–159. [Google Scholar] [CrossRef]
- Newman, R. Chromium Oxide Greens. In Artists’ Pigments, A Handbook of Their History and Characteristics; FitzHugh, E.W., Ed.; Archetype Publications Ltd.: Washington, DC, USA; National Gallery of Art: New York, NY, USA, 1997; Volume 3, pp. 273–286. [Google Scholar]
- Mineralogical Society of Great Britain and Ireland. The Infrared Spectra of Minerals; Mineralogical Society of Great Britain and Ireland: London, UK, 1974. [Google Scholar]
- Fiedler, I.; Bavard, M.A. Emerald Green and Scheele’s Green. In Artists’ Pigments, A Handbook of Their History and Characteristics; FitzHugh, E.W., Ed.; Archetype Publications Ltd.: Washington, DC, USA; National Gallery of Art: New York, NY, USA, 1997; Volume 3, pp. 219–271. [Google Scholar]
- Lipscher, J. ColourLex. Available online: https://colourlex.com/ (accessed on 10 July 2023).
- Vermeulen, M.; Miranda, A.S.O.; Tamburini, D.; Delgado, S.E.R.; Walton, M. A multi-analytical study of the palette of impressionist and post-impressionist Puerto Rican artists. Herit. Sci. 2022, 10, 44. [Google Scholar] [CrossRef]
- Klisińska-Kopacz, A.; Frączek, P.; Obarzanowski, M.; Czop, J. Non-Invasive Study of Pigment Palette Used by Olga Boznańska Investigated with Analytical Imaging, XRF, and FTIR Spectroscopy. Heritage 2023, 6, 1429–1443. [Google Scholar] [CrossRef]
- Moretto, L.M.; Orsega, E.F.; Mazzocchin, G.A. Spectroscopic methods for the analysis of celadonite and glauconite in Roman green wall paintings. J. Cult. Herit. 2011, 12, 384–391. [Google Scholar] [CrossRef]
- Lane, M.D. Mid-infrared emission spectroscopy of sulfate and sulfate-bearing minerals. Am. Mineral. 2007, 92, 1–18. [Google Scholar] [CrossRef]
- Monico, L.; Van der Snickt, G.; Janssens, K.; De Nolf, W.; Miliani, C.; Dik, J.; Radepont, M.; Hendriks, E.; Geldof, M.; Cotte, M. Degradation Process of Lead Chromate in Paintings by Vincent van Gogh Studied by Means of Synchrotron X-ray Spectromicroscopy and Related Methods. 2. Original Paint Layer Samples. Anal. Chem. 2011, 83, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Vahur, S.; Teearu, A.; Leito, I. ATR-FT-IR spectroscopy in the region of 550–230 cm−1 for identification of inorganic pigments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, I.; Bayard, M.A. Artist’s Pigments: A Handbook of Their History and Characteristics; Oxford University Press: New York, NY, USA, 1986; Volume 1. [Google Scholar]
- Topalova-Casadiego, B.; Plahter, U. Proceedings of the National Gallery Technical Bulletin 30th Anniversary Conference, London, UK, 16–18 September 2009; Archetype Publications Ltd.: Washington, DC, USA, 2011; pp. 244–252. [Google Scholar]
- Mass, J.L.; Opila, R.; Buckley, B.; Cotte, M.; Church, J.; Mehta, A. The photodegradation of cadmium yellow paints in Henri Matisse’s Le Bonheur de vivre (1905–1906). Appl. Phys. A 2013, 111, 59–68. [Google Scholar] [CrossRef]
- Helwig, K. Iron oxide pigments: Natural and synthetic. In Artists’ Pigments, A Handbook of Their History and Characteristics; Berrie, B.H., Ed.; Archetype Publications Ltd.: Washington, DC, USA; National Gallery of Art: New York, NY, USA, 2007; Volume 4. [Google Scholar]
- Elias, M.; Chartier, C.; Prévot, G.; Garay, H.; Vignaud, C. The colour of ochres explained by their composition. Mater. Sci. Eng. B 2006, 127, 70–80. [Google Scholar] [CrossRef]
- Higgs, S.; Burnstock, A. An Investigation into Metal Ions in Varnish Coatings. In Metal Soaps in Art: Conservation and Research; Casadio, F., Keune, K., Noble, P., Van Loon, A., Hendriks, E., Centeno, S.A., Osmond, G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 123–140. [Google Scholar]
- Izzo, F.C.; Kratter, M.; Nevin, A.; Zendri, E. A Critical Review on the Analysis of Metal Soaps in Oil Paintings. ChemistryOpen 2021, 10, 904–921. [Google Scholar] [CrossRef]
- Rampazzi, L. Calcium oxalate films on works of art: A review. J. Cult. Herit. 2019, 40, 195–214. [Google Scholar] [CrossRef]
- Klaassen, L.; van der Snickt, G.; Legrand, S.; Higgitt, C.; Spring, M.; Vanmeert, F.; Rosi, F.; Brunetti, B.G.; Postec, M.; Janssens, K. Characterization and Removal of a Disfiguring Oxalate Crust on a Large Altarpiece by Hans Memling. In Metal Soaps in Art: Conservation and Research; Casadio, F., Keune, K., Noble, P., Van Loon, A., Hendriks, E., Centeno, S.A., Osmond, G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 263–282. [Google Scholar]
- Peer Reviewed: Clarifying the Haze: Efflorescence on Works of Art. Anal. Chem. 1997, 69, 416A–422A. [CrossRef]
- Noble, P. A Brief History of Metal Soaps in Paintings from a Conservation Perspective. In Metal Soaps in Art: Conservation and Research; Casadio, F., Keune, K., Noble, P., Van Loon, A., Hendriks, E., Centeno, S.A., Osmond, G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–22. [Google Scholar]
- Hermans, J.J.; Keune, K.; van Loon, A.; Corkery, R.W.; Iedema, P.D. Ionomer-like structure in mature oil paint binding media. RSC Adv. 2016, 6, 93363–93369. [Google Scholar] [CrossRef]
- Salvadó, N.; Butí, S.; Pradell, T.; Beltran, V.; Cinque, G.; Juanhuix, J. Identification and Distribution of Metal Soaps and Oxalates in Oil and Tempera Paint Layers in Fifteenth-Century Altarpieces Using Synchrotron Radiation Techniques. In Metal Soaps in Art: Conservation and Research; Casadio, F., Keune, K., Noble, P., Van Loon, A., Hendriks, E., Centeno, S.A., Osmond, G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 195–210. [Google Scholar]
- Le Brun, H. Study of the Formation of Metal Oxalates and Soaps in Oil Paintings from the Southern Netherlands Using ToF-SIMS. Master’s Thesis, Université catholique de Louvain, Ottignies-Louvain-la-Neuve, Belgium, 2021. [Google Scholar]
- Haddad, A.; Pastorelli, G.; Ortiz Miranda, A.S.; Ludvigsen, L.; Centeno, S.A.; Duvernois, I.; Hoover, C.; Duffy, M.; Aviram, A.; Zycherman, L. Exploring the private universe of Henri Matisse in The Red Studio. Herit. Sci. 2022, 10, 168. [Google Scholar] [CrossRef]
- Pouyet, E.; Cotte, M.; Fayard, B.; Salomé, M.; Meirer, F.; Mehta, A.; Uffelman, E.S.; Hull, A.; Vanmeert, F.; Kieffer, J.; et al. 2D X-ray and FTIR micro-analysis of the degradation of cadmium yellow pigment in paintings of Henri Matisse. Appl. Phys. A 2015, 121, 967–980. [Google Scholar] [CrossRef]
- Mass, J.; Sedlmair, J.; Patterson, C.S.; Carson, D.; Buckley, B.; Hirschmugl, C. SR-FTIR imaging of the altered cadmium sulfide yellow paints in Henri Matisse’s Le Bonheur de vivre (1905–1906)—Examination of visually distinct degradation regions. Analyst 2013, 138, 6032–6043. [Google Scholar] [CrossRef] [PubMed]
- Defeyt, C.; Van Vyve, E.; Leen, F.; Vandepitte, F.; Gilbert, B.; Herens, E.; Strivay, D. Revealing Gauguin’s practice: Multi-analytical approach of the Portrait de Suzanne Bambridge. Herit. Sci. 2018, 6, 20. [Google Scholar] [CrossRef]
- Christensen, C. The Painting Materials and Technique of Paul Gauguin. Stud. Hist. Art 1993, 41, 62–103. [Google Scholar]
- Vermeulen, M.; Smith, K.; Eremin, K.; Rayner, G.; Walton, M. Application of Uniform Manifold Approximation and Projection (UMAP) in spectral imaging of artworks. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119547. [Google Scholar] [CrossRef]
- Januszczak, W. Techniques of the World’s Great Painters; Chartwell: Marlton, NJ, USA, 1980; pp. 132–135. [Google Scholar]
- Pollack, M. Odilon Redon, Paul Gauguin, and Primitivist Color. Art Bull. 2020, 102, 77–103. [Google Scholar] [CrossRef]
- Roy, A. Monet’s palette in the twentieth century: ‘Water-Lilies’ and ‘Irises’. Natl. Gallery Tech. Bull. 2007, 28, 58–68. [Google Scholar]
- Gutierrez, L.; Burnstock, A. Technical Examination of Works by Camille and Lucien Pissarro from the Courtauld Gallery. Art Matters 2013, 5, 13–16. [Google Scholar]
- Singer, B.; Aslaksby, T.E.; Topalova-Casadiego, B.; Tveit, E.S. Investigation of Materials Used by Edvard Munch. Stud. Conserv. 2010, 55, 274–292. [Google Scholar] [CrossRef]
- Berrie, B.H. Prussian Blue. In A Handbook of Their History and Characteristics; FitzHugh, E.W., Ed.; Archetype Publications Ltd.: Washington, DC, USA; National Gallery of Art: New York, NY, USA, 1997; Volume 3, pp. 191–217. [Google Scholar]
- Matisse, H.; Courthion, P. Chatting with Henri Matisse: The Lost 1941 Interview; Guilbaut, S., Ed.; Getty Trust Publications: Los Angeles, CA, USA, 2013; p. 368. [Google Scholar]
- Brouwer, P. Theory of XRF: Getting Acquainted with the Principles; PANalytical BV: Almelo, The Netherlands, 2010. [Google Scholar]
- Bordignon, F.; Postorino, P.; Dore, P.; Tabasso, M.L. The Formation of Metal Oxalates in the Painted Layers of a Medieval Polychrome on Stone, as Revealed by Micro-Raman Spectroscopy. Stud. Conserv. 2008, 53, 158–169. [Google Scholar] [CrossRef]
- Zoppi, A.; Lofrumento, C.; Mendes, N.F.C.; Castellucci, E.M. Metal oxalates in paints: A Raman investigation on the relative reactivities of different pigments to oxalic acid solutions. Anal. Bioanal. Chem. 2010, 397, 841–849. [Google Scholar] [CrossRef] [PubMed]

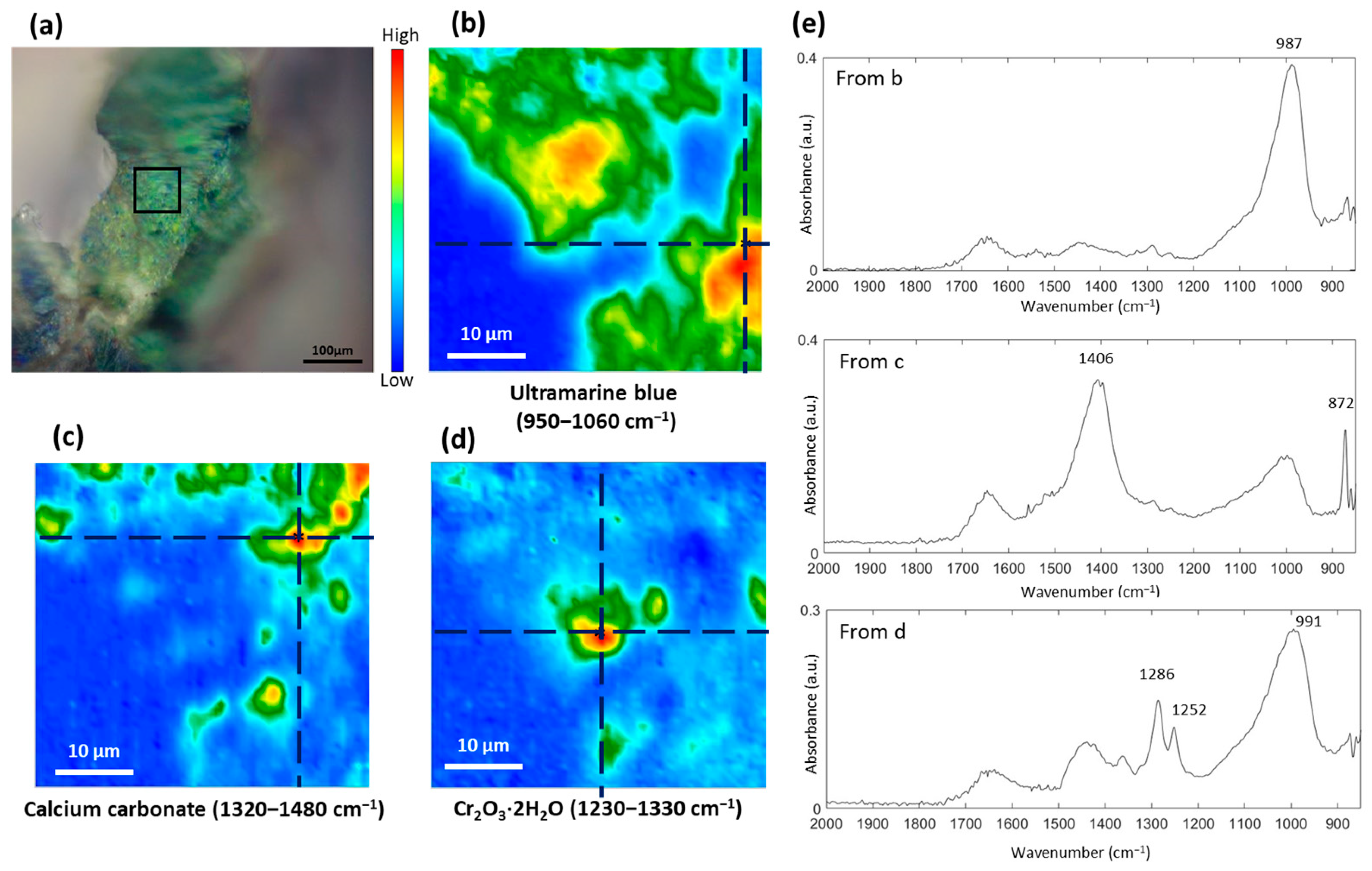
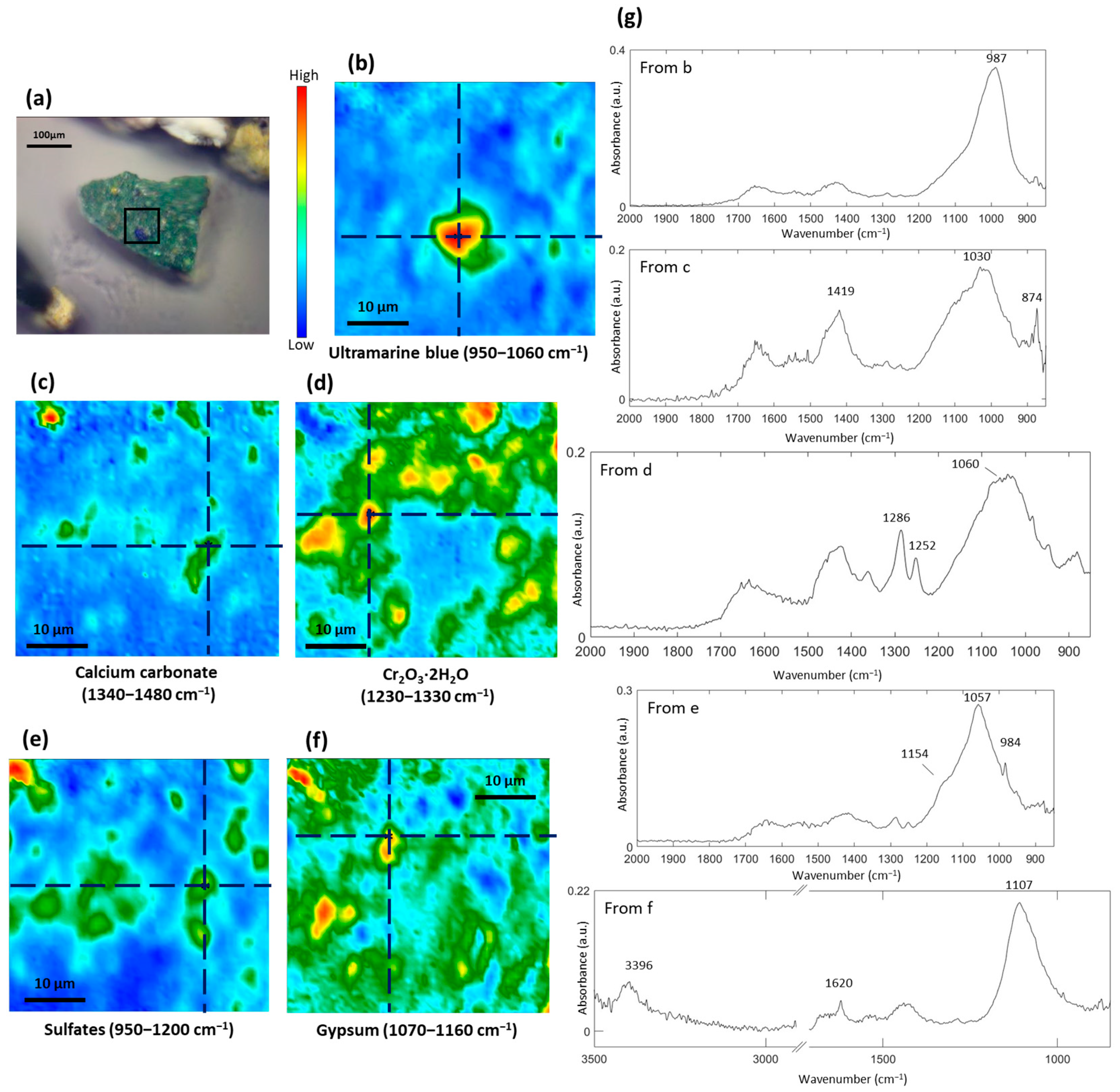
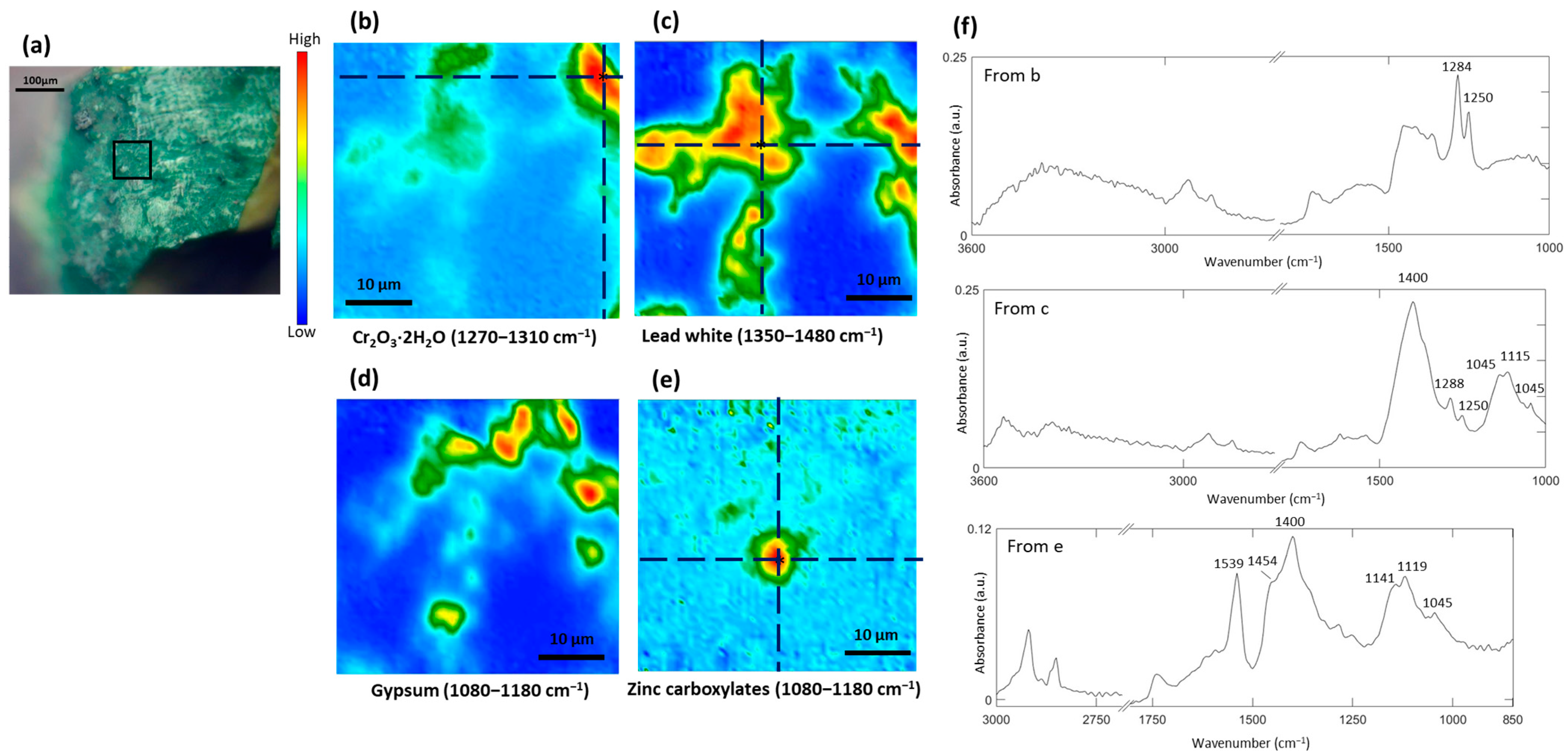
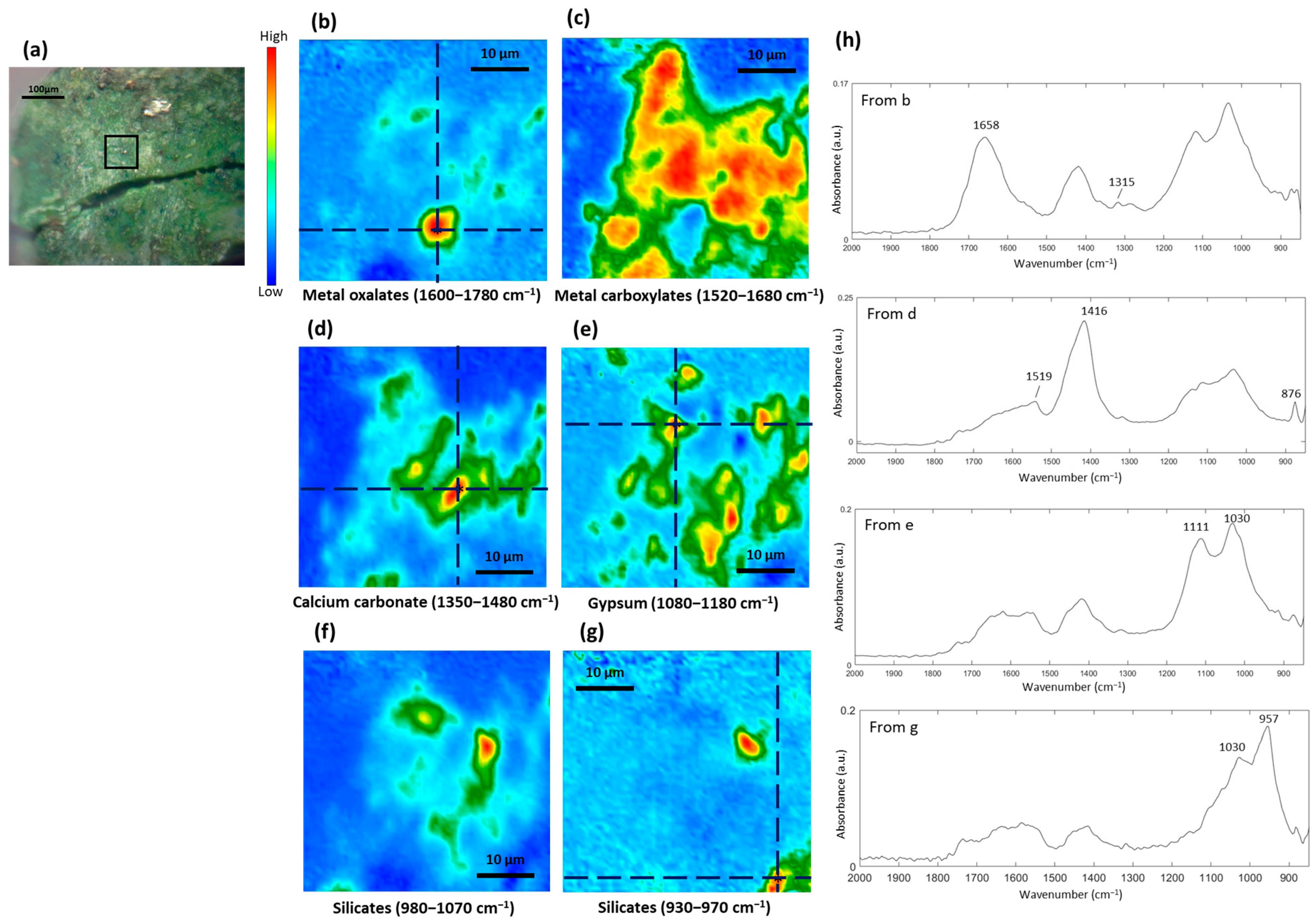



| Color | Painting | Detected Chemical Elements with XRF |
|---|---|---|
| Blue | Morning in Stavrino (1909) | Major: Zn, Ca, S, Si, Cr, Al, Pb, Cl, Fe Minor: Ti, Cu, As, P, Cd, Co Trace: Mn, Ga, Ni, Sr |
| Arabian Dancer (1913) | Major: Zn, Si, S, Al, Ca, Pb, Fe Minor: K, Cl, P, Cd, Cr, As, Ga, Co, Cu Trace: Ti, Mn, V | |
| Caravan (1926) | Major: Pb, Ca, Co, Al, Fe, Zn, S, Cd Minor: Cl, Cr, V Trace: Sr, Ga | |
| Yerevan Zangu River (1931) | Major: Zn, Sn, Co, Si, Ca, Fe, Cl, Cr Minor: As | |
| Light blue | Kirovakan (1948) | Major: Zn, Ca, Sn, Co Minor: Si, S, Cl, Fe, Trace: Cu, As, Cr, P |
| Color | Painting | Detected Chemical Elements with XRF |
|---|---|---|
| Green | Morning in Stavrino (1909) | Major: Ca, Cr, Si, S, Al, Fe, Zn, Cl, P Minor: Pb, Co, Ti, Cu Trace: As, Mn |
| Arabian Dancer (1913) | Major: Zn, Na, S, Si, Ca, Al, Cr, Fe, Pb, Ti Minor: P, Cl, Co, Cd Trace: Sn, As, Sr | |
| Yerevan Zangu River (1931) | Major: Zn, Pb, Si, Ca, Al, Fe, S, Cl, Cr, Co Minor: As, Er, V, Ga Trace: Mn | |
| Caravan (1926) | Major: Pb, Ca, Cr, Mg, Fe, Zn Minor: Cd, Cl, Ti Trace: Sr, Mn, Zr | |
| Blue-green | Caravan (1926) | Major: Pb, As, Cu, Ca, Co Minor: Zn, Fe, Cl, Sn Trace: Sr, Cr, Ti, Zr |
| Dark green | Yerevan Zangu River (1931) | N/A |
| Color | Painting | Detected Chemical Elements with XRF |
|---|---|---|
| Yellow | Morning in Stavrino (1909) | Major: Si, Ca, Zn, Cl, Pb, S, Fe, Cr, Sn, Ti Minor: As |
| Caravan (1926) | Major: Pb, Ca, Cd, Ti, Cl Minor: Fe, Zn, V, Cr Trace: Sr, Zr | |
| Brown | Caravan (1926) | Major: Pb, Ca, Cd, Zn Minor: Al, Fe, Cr, Co Trace: Sr, Ti, Cu, Zr, Rb |
| Color | Painting | Detected Chemical Elements with XRF |
|---|---|---|
| Red | Arabian Dancer (1913) | Major: Zn, Na, S, Ca, Al, Si, Cd, Fe, P Minor: Cl, K, As, Cr, Ti Trace: Co, Mn |
| Caravan (1926) | Major: Pb, Cl, Co, Ca, P, Fe, Zn, Er Minor: Cr Trace: Ga | |
| Orange | Kirovakan (1948) | Major: Zn, Si, Ca, Fe, Cl, S Minor: Ti, Cr, V, As, Co |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.-L.; Kazarian, S.G. Characterization and Degradation Analysis of Pigments in Paintings by Martiros Sarian: Attenuated Total Reflection Fourier Transform Infrared Spectroscopic Imaging and X-ray Fluorescence Approach. Heritage 2023, 6, 6777-6799. https://doi.org/10.3390/heritage6100354
Liu G-L, Kazarian SG. Characterization and Degradation Analysis of Pigments in Paintings by Martiros Sarian: Attenuated Total Reflection Fourier Transform Infrared Spectroscopic Imaging and X-ray Fluorescence Approach. Heritage. 2023; 6(10):6777-6799. https://doi.org/10.3390/heritage6100354
Chicago/Turabian StyleLiu, Guan-Lin, and Sergei G. Kazarian. 2023. "Characterization and Degradation Analysis of Pigments in Paintings by Martiros Sarian: Attenuated Total Reflection Fourier Transform Infrared Spectroscopic Imaging and X-ray Fluorescence Approach" Heritage 6, no. 10: 6777-6799. https://doi.org/10.3390/heritage6100354
APA StyleLiu, G.-L., & Kazarian, S. G. (2023). Characterization and Degradation Analysis of Pigments in Paintings by Martiros Sarian: Attenuated Total Reflection Fourier Transform Infrared Spectroscopic Imaging and X-ray Fluorescence Approach. Heritage, 6(10), 6777-6799. https://doi.org/10.3390/heritage6100354








