Deterioration Effects on Bricks Masonry in the Venice Lagoon Cultural Heritage: Study of the Main Façade of the Santa Maria dei Servi Church (14th Century)
Abstract
:1. Introduction
1.1. “Venice and Its Lagoon”
1.2. The Use of Bricks in Venice
1.3. The Santa Maria dei Servi Church
1.4. The Main Façade
2. Materials
3. Methods
4. Results and Discussion
4.1. Color Measurements
4.2. Texture and Mineralogy
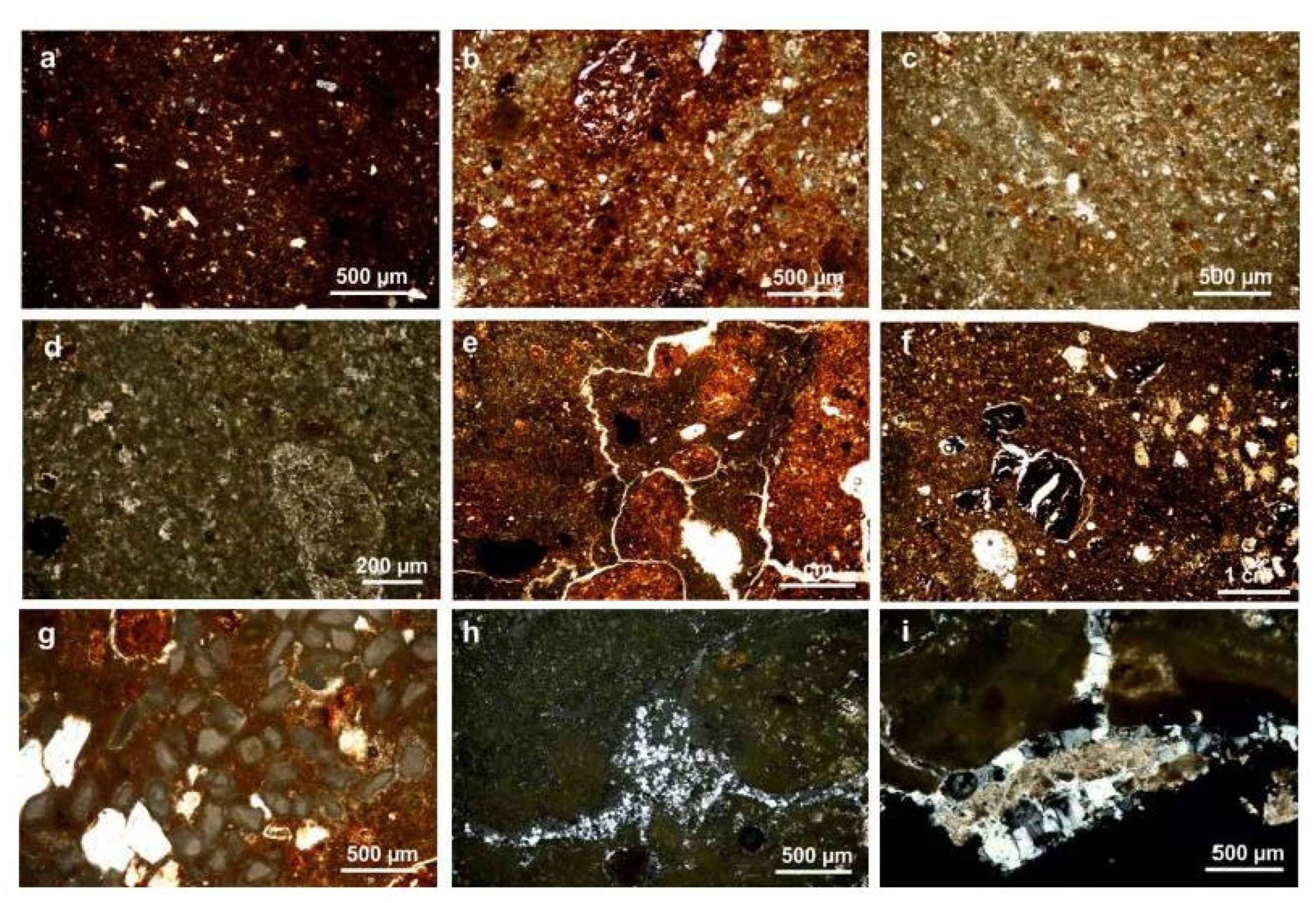
4.2.1. Petrographic Analysis
4.2.2. Micro-Textural and Micro-Chemical Analysis
4.2.3. Mineralogy and Decay Products
4.3. Mapping of Deterioration Products
5. Conclusions
- Petrographic and microstructural analysis by FESEM-EDS analysis was extremely useful in studying the microtextural and the mineralogical evolution of the collected bricks. Pore shape and dimensions, texture, and mineralogy detected suggested that temperatures reached during the firing process ranged between 700–1000 °C. The same samples displayed incomplete mineral reactions, while others had a high density and a wide presence of new silicates formed during firing. The presence of Ti-phases supported the theory that in the 14th century there was an initial phase of local bricks production using the local clay of the lagoon.
- XRPD analysis confirmed the presence of carbonates, illite, and new Ca-, Mg-, and Ti-silicate phases. The presence of these mineral phases and their abundances were important indicators not only for knowing the mineralogical composition of the raw materials used but also for understanding the temperature reached during the firing process. The raw materials used contained quartz, K-feldspar, plagioclase, as well as carbonates (calcite and dolomite). Gehlenite, diopside, and augite were secondary products of the firing reactions at T > 800 °C. The amorphous phase was present in a large number of samples (Clusters 1 and 2 in Figure 6). Gypsum was often found as a decay product. Meanwhile, zeolite (analcime) was found as a weathering product of samples that displayed more amorphous in XRPD patterns.
- The poor homogeneity in the mineralogical evolution among the collected bricks suggested the absence of good standardization in the firing process, probably in relation to a large number of materials to be fired in the kilns and the piling density. This result is perfectly in line with the technological possibility of that time, considering that the first public furnaces are dated back to 1327 [14], just three years before the beginning of the construction of the Santa Maria dei Servi Church.
- Regarding the weathering products, gypsum was found in many bricks’ diffraction patterns as well as in the hyperspectral SWIR maps in which sulfates resulted in covering most of the surface of the façade. Thus, the deterioration effects were not only caused by the capillary rise by also the sea spray, which is dominant on the external walls of the historical heritage of Venice.
- Biodeterioration was also mapped using hyperspectral VNIR ranges. Chlorophyll spectral bands were mapped in all the façade, in particular along mortar joints and exposed brick surfaces.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falchi, L.; Orio, E.; Balliana, E.; Izzo, F.C.; Zendri, E. Investigation on the relationship between the environment and Istria stone surfaces in Venice. Atmos. Environ. 2019, 210, 76–85. [Google Scholar] [CrossRef]
- Fassina, V. Basic chemical mechanism outdoors. In Basic Environmental Mechanisms Affecting Cultural Heritage, Understanding Deterioration Mechanisms for Conservation Purposes; Camuffo, D., Fassina, V., Havermans, J., Eds.; Nardini Editore: Florence, Italy, 2010; pp. 75–105. ISBN 978-88-404-4334-8. [Google Scholar]
- Coletti, C.; Cultrone, G.; Maritan, L.; Mazzoli, C. How to face the new industrial challenge of compatible and sustainable brick production: Study of various types of commercially available bricks. Appl. Clay Sci. 2016, 124, 219–226. [Google Scholar] [CrossRef]
- Foraboschi, P.; Vanin, A. Experimental investigation on bricks from historical Venetian buildings subjected to moisture and salt crystallization. Eng. Fail. Anal. 2014, 45, 185–203. [Google Scholar] [CrossRef]
- Camuffo, D. Four centuries of documentary sources concerning the sea level rise in Venice. Clim. Chang. 2021, 167, 54. [Google Scholar] [CrossRef]
- Camuffo, D. A Discussion on Sea Level Rise, Rate Ad Acceleration. Venice as a Case Study. Environ. Earth Sci. 2022, 81, 349. [Google Scholar] [CrossRef]
- Salvini, S.; Coletti, C.; Maritan, L.; Massironi, M.; Pieropan, A.; Spiess, R.; Mazzoli, C. Petrographic characterization and durability of carbonate stones used in UNESCO World Heritage sites in northeastern Italy. Environ. Earth Sci. 2023, 82, 49. [Google Scholar] [CrossRef]
- Camuffo, D.; Sturaro, G. Sixty-cm Submersion of Venice Discovered Thanks to Canaletto’s Paintings. Environ. Earth Sci. 2003, 58, 333–343. [Google Scholar]
- Benavente, D.; Linares-Fernandez, L.; Cultrone, G.; Sebastian, E. Influence of microstructure on the resistance to salt crystallisation damage in brick. Mater. Struct. 2006, 39, 105–113. [Google Scholar] [CrossRef]
- Coletti, C.; Cultrone, G.; Maritan, L.; Mazzoli, C. Combined multi-analytical approach for study of pore system in bricks: How much porosity is there? Mater. Charact. 2016, 121, 82–92. [Google Scholar] [CrossRef]
- Cracco, G. Venezia nel Medioevo dal Secolo XI al Secolo XIV; Utet: Torino, Italy, 1986. [Google Scholar]
- Doglioni, F.; Trovò, F. Mutamenti dei Laterizi e delle Murature Veneziane tra XII e XVI Secolo; Doglioni e Mirabella Roberti: Venice, Italy, 2011; pp. 33–66. [Google Scholar]
- Wolf, S. Estimation of the production parameters of very large medieval bricks from St. Urban, Switzerland. Archaeometry 2002, 44, 37–65. [Google Scholar] [CrossRef]
- Squassina, A. Murature di mattoni medievali a vista e resti di finiture a Venezia. Arqueol. Archit. 2011, 239–271. [Google Scholar] [CrossRef]
- Calliari, I.; Canal, E.; Cavazzoni, S.; Lazzarini, L. Roman bricks from the lagoon of Venice: A chemical characterization with methods of multivariate analysis. J. Cult. Herit. 2000, 2, 23–29. [Google Scholar] [CrossRef]
- Antonelli, F.; Cancelliere, S.; Lazzarini, L. Minero-petographic characterisation of historical bricks in the Arsenale, Venice. J. Cult. Herit. 2002, 3, 59–64. [Google Scholar] [CrossRef]
- Schiavon, N.; Mazzocchin, G.A.; Baudo, F. Chemical and mineralogical characterisation of weathered historical bricks from the Venice lagoonal environment. Environ. Geol. 2008, 56, 767–775. [Google Scholar] [CrossRef]
- Pavon, G.; Cauzzi, G. La memoria di un tempio. In Li Servi di San Marcilian ed il Canal Marovich in Venezia; Edizioni Helvetia: Venice, Italy, 1988. [Google Scholar]
- Vicentini, A.M. Santa Maria de’ Servi in Venezia; Messaggi Edizioni; Tipografica: Treviglio, Italy, 1920; pp. 44–45. [Google Scholar]
- Rossi, M. La Chiesa gotica scomparsa di Santa Maria dei Servi a Venezia. In Indagine Storico Artistica dalla sua Edificazione Trecentesca al XV Secolo; Università Ca’ Foscari Venezia: Venice, Italy, 2012. [Google Scholar]
- Shuai, S.; Zhang, Z.; Lv, X.; Hao, L. Assessment of new spectral indices and multi-seasonal ASTER data for gypsum mapping. Carbonates Evaporites 2022, 37, 34. [Google Scholar] [CrossRef]
- Benavente, D.; Martiñez-Verdú, F.; Bernabeu, A.; Viqueira, V.; Fort, R.; García del Cura, M.A.; Illueca, C.; Ordóñez, S. Influence of surface roughness on color changes in building stones. Color Res. Appl. 2002, 28, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Grossi, C.M.; Brimblecombe, P.; Esbert, R.; Alonso, F.J. Color changes in architectural limestones from pollution and cleaning. Color Res. Appl. 2007, 32, 320–331. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E.; de la Torre, M.J. Mineralogical and physical behaviours of solid bricks with additives. Constr. Build. Mater. 2005, 19, 39–48. [Google Scholar] [CrossRef]
- Maritan, L.; Nodari, L.; Mazzoli, C.; Milano, A.; Russo, U. Influence of firing conditions on ceramic products: Experimental study on clay rich in organic matter. Appl. Clay Sci. 2006, 31, 1–15. [Google Scholar] [CrossRef]
- Maritan, L. Ceramic abandonment. How to recognise post-depositional transformations. Archaeol. Anthropol. Sci. 2020, 12, 199. [Google Scholar] [CrossRef]
- Dominuco, P.; Messiga, B.; Riccardi, M. Firing process of natural clay. Some microtextures and related phase composition. Termochim. Acta 1998, 321, 185–190. [Google Scholar] [CrossRef]
- Pérez-Monserrat, E.M.; Causarano, M.A.; Maritan, L.; Chavarria, A.; Brogiolo, G.P.; Cultrone, G. Roman brick production technologies in Padua (Northern Italy) along the Late Antiquity and Medieval Times: Durable bricks on high humid environs. J. Cult. Herit. 2022, 54, 12–20. [Google Scholar] [CrossRef]
- Dondi, M.; Ercolani, G.; Fabbri, B.; Marsigli, M. An approach to the chemistry of pyroxenes formed during the firing of Ca-rich silicate ceramics. Clay Miner. 1998, 33, 443–452. [Google Scholar] [CrossRef]
- Busà, T. Valutazione del Fondo Naturale di Alcuni Metalli Pesanti (Cr e As) Presenti nei Sedimenti da Dragare Lungo il Canale Malamocco-Marghera ed Aree Adiacenti; Autorità portuale di Venezia: Venice, Italy, 2010. [Google Scholar]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Min. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Cultrone, G.; Rodriguez-Navarro, C.; Sebastián, E.; Cazalla, O.; de la Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Mineral. 2001, 13, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, B.; Gualtieri, S.; Shoval, S. The presence of calcite in archeological ceramics. J. Eur. Ceram. Soc. 2014, 34, 1899–1911. [Google Scholar] [CrossRef]
- Olszak-Humienik, M.; Jablonski, M. Thermal behavior of natural dolomite. J. Therm. Anal. Calorim. 2015, 119, 2239–2248. [Google Scholar] [CrossRef] [Green Version]
- Cultrone, G.; Rodríguez-Navarro, C.; Sebastián, E. Limestone and brick decay in simulated polluted atmosphere: The role of particulate matter. In Air Pollution and Cultural Heritage; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Maritan, L. Archaeo-ceramic 2.0: Investigating ancient ceramics using modern technological approaches. Archaeol. Anthropol. Sci. 2019, 11, 5085–5093. [Google Scholar] [CrossRef]
- Joeckel, R.M.; Wally, K.D.; Ang Clement, B.J.; Hansan, P.R.; Dillon, J.S.; Wilso, S.K. Secondary minerals from extrapedogenic per latus acidic weathering environments at geomorphic edges, Eastern Nebraska, USA. Catena 2011, 85, 253–266. [Google Scholar] [CrossRef]







| Methods | Expected Results | N. Samples | |
|---|---|---|---|
| In lab | Spectrophotometry | Aesthetic aspect in dry and wet conditions | 23 |
| OM | Petrographic study, mineral content and texture/porosity | 23 | |
| FESEM-EDS | Texture, porosity, mineral boundary reactions | 5 | |
| XRD | Mineral composition | 25 | |
| HA | Mineralogy and decay products | 3 | |
| On-site | HA | Decay map of the overall main façade | |
| DRY | WET | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | L* | a* | b* | L* | a* | b* | ΔE | Group |
| A01 | 61.40 | 11.64 | 23.40 | 51.80 | 12.38 | 24.98 | 9.76 | group2_pink |
| A02 | 62.28 | 7.89 | 20.95 | 49.24 | 12.50 | 25.51 | 14.56 | group2_pink |
| A03 | 56.59 | 11.70 | 22.65 | 39.96 | 19.82 | 24.49 | 18.60 | group2_pink |
| A04 | 52.44 | 18.04 | 25.84 | 44.89 | 17.88 | 26.17 | 7.56 | group3_red |
| A05 | 59.00 | 13.35 | 24.33 | 57.69 | 11.48 | 26.97 | 3.50 | group3_red |
| A06 | 67.07 | 8.28 | 23.24 | 49.32 | 10.60 | 23.65 | 17.91 | group3_red |
| A07 | 60.95 | 8.64 | 20.76 | 55.78 | 12.51 | 27.10 | 9.05 | group2_pink |
| A08 | 66.25 | 8.33 | 23.07 | 58.62 | 7.81 | 27.33 | 8.76 | group2_pink |
| A09 | 66.33 | 5.21 | 23.16 | 49.97 | 13.95 | 24.94 | 18.63 | group2_pink |
| A10 | 70.42 | 5.24 | 18.96 | 60.72 | 11.15 | 28.20 | 14.64 | group2_pink |
| A11 | 67.25 | 9.63 | 19.89 | 56.60 | 6.20 | 27.59 | 13.59 | group2_pink |
| A13 | 45.23 | 12.60 | 25.05 | 51.41 | 15.97 | 26.80 | 7.26 | group2_pink |
| B02 | 59.67 | 4.06 | 21.10 | 51.04 | 14.49 | 35.47 | 19.74 | group1_yellow |
| B03 | 62.12 | 12.56 | 23.58 | 49.81 | 16.13 | 26.36 | 13.12 | group3_red |
| B05 | 58.68 | 12.54 | 22.27 | 43.47 | 17.34 | 24.66 | 16.12 | group3_red |
| B06 | 64.49 | 3.08 | 18.15 | 56.05 | 3.83 | 21.98 | 9.30 | group1_yellow |
| B07 | 50.20 | 18.17 | 23.96 | 36.81 | 23.45 | 24.78 | 14.42 | group3_red |
| B08 | 58.99 | 12.66 | 22.31 | 45.15 | 17.68 | 25.27 | 15.03 | group3_red |
| B09 | 64.79 | 6.89 | 19.06 | 55.42 | 10.88 | 24.31 | 11.46 | group2_pink |
| B10 | 60.38 | 8.17 | 20.34 | 51.95 | 10.26 | 23.29 | 9.17 | group2_pink |
| B11 | 57.65 | 5.13 | 19.13 | 40.69 | 9.37 | 18.46 | 17.49 | group1_yellow |
| C02 | 63.74 | 2.36 | 27.99 | 55.08 | 3.03 | 33.42 | 10.25 | group2_pink |
| C03 | 61.13 | 8.16 | 15.05 | 41.31 | 16.04 | 20.55 | 22.03 | group1_yellow |
| Qz | K-fs | Pl | Cal | Dol | Ilt | Di | Geh | Aug | Fo | Gyp | Alu | Anl | Mir | Hal | Hem | Am | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A01 | 45 | 12 | 23 | 3 | 1 | 9 | 4 | 3 | |||||||||
| A02 | 23 | 16 | 33 | 23 | 2 | 3 | |||||||||||
| A03 | 22 | 33 | 22 | 5 | 4 | 12 | 2 | ||||||||||
| A04 | 13 | 25 | 5 | 19 | 22 | 2 | 10 | 1 | 1 | 2 | xx | ||||||
| A05 | 35 | 27 | 5 | 6 | 8 | 7 | 9 | 3 | x | ||||||||
| A06 | 27 | 16 | 17 | 10 | 12 | 9 | 3 | 3 | 1 | 2 | |||||||
| A07 | 6 | 21 | 33 | 15 | 3 | 13 | 4 | 3 | 1 | 1 | |||||||
| A08 | 9 | 12 | 13 | 6 | 28 | 15 | 3 | 11 | 1 | 2 | xx | ||||||
| A09 | 28 | 16 | 28 | 19 | 6 | 3 | |||||||||||
| A10 | 7 | 11 | 24 | 4 | 28 | 17 | 2 | 6 | 1 | ||||||||
| A11 | 5 | 22 | 29 | 28 | 4 | 11 | 1 | xx | |||||||||
| A13 | 25 | 18 | 25 | 4 | 13 | 14 | 1 | ||||||||||
| B02 | 3 | 8 | 33 | 16 | 21 | 6 | 11 | 2 | xx | ||||||||
| B03 | 27 | 16 | 33 | 11 | 12 | 1 | + | ||||||||||
| B05 | 31 | 19 | 12 | 11 | 12 | 12 | 2 | 1 | |||||||||
| B06 | 12 | 11 | 18 | 19 | 16 | 6 | 15 | 3 | xx | ||||||||
| B07 | 42 | 15 | 12 | 8 | 6 | 8 | 4 | 1 | 1 | 3 | |||||||
| B08 | 25 | 22 | 23 | 15 | 13 | 2 | |||||||||||
| B09 | 28 | 17 | 24 | 12 | 6 | 8 | 3 | 2 | x | ||||||||
| B10 | 21 | 22 | 15 | 15 | 7 | 14 | 3 | 1 | 1 | 1 | x | ||||||
| B11 | 19 | 15 | 18 | 11 | 11 | 22 | 3 | 1 | |||||||||
| C02 | 11 | 25 | 25 | 4 | 12 | 21 | 2 | xx | |||||||||
| C03 | 27 | 19 | 16 | 8 | 1 | 13 | 9 | 2 | 1 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coletti, C.; Cesareo, L.P.; Nava, J.; Germinario, L.; Maritan, L.; Massironi, M.; Mazzoli, C. Deterioration Effects on Bricks Masonry in the Venice Lagoon Cultural Heritage: Study of the Main Façade of the Santa Maria dei Servi Church (14th Century). Heritage 2023, 6, 1277-1292. https://doi.org/10.3390/heritage6020070
Coletti C, Cesareo LP, Nava J, Germinario L, Maritan L, Massironi M, Mazzoli C. Deterioration Effects on Bricks Masonry in the Venice Lagoon Cultural Heritage: Study of the Main Façade of the Santa Maria dei Servi Church (14th Century). Heritage. 2023; 6(2):1277-1292. https://doi.org/10.3390/heritage6020070
Chicago/Turabian StyleColetti, Chiara, Ludovica Pia Cesareo, Jacopo Nava, Luigi Germinario, Lara Maritan, Matteo Massironi, and Claudio Mazzoli. 2023. "Deterioration Effects on Bricks Masonry in the Venice Lagoon Cultural Heritage: Study of the Main Façade of the Santa Maria dei Servi Church (14th Century)" Heritage 6, no. 2: 1277-1292. https://doi.org/10.3390/heritage6020070
APA StyleColetti, C., Cesareo, L. P., Nava, J., Germinario, L., Maritan, L., Massironi, M., & Mazzoli, C. (2023). Deterioration Effects on Bricks Masonry in the Venice Lagoon Cultural Heritage: Study of the Main Façade of the Santa Maria dei Servi Church (14th Century). Heritage, 6(2), 1277-1292. https://doi.org/10.3390/heritage6020070










