Abstract
Painting materials used in Spanish American Colonial art comprised pigments and binders from European origin as well as those that were already known in pre-Hispanic times. In recent years, we have identified for the first time the mineral atacamite, a basic copper chloride (Cu2Cl(OH)3), in Andean Colonial art pieces (Viceroyalty of Peru, 16th–18th centuries). This work proposes a methodology based on a multitechnical approach to identify and establish the origin (natural or synthetic) of the atacamite pigment in Andean cultural heritage objects. Optical microscopy (OM), scanning electron microscopy with energy-dispersive spectroscopy (SEM-EDS), portable X-ray fluorescence spectroscopy (pXRF), attenuated total reflection infrared spectroscopy (FTIR-ATR), micro-Raman spectroscopy, and wide-angle X-ray scattering (WAXS) were applied to analyse green pigments from the altarpiece of the Church of Ancoraimes, atacamite mineral samples from Chile, and atacamite obtained as a secondary product from traditional recipes used to produce verdigris, a copper acetate. Viride salsum by Teófilo Presbítero (SXII) and the Spanish translation by Andrés de Laguna (1566) of “De Materia Médica” from Dioscorides are both texts that include recipes involving the use of metallic copper as a starting material. These studies will contribute to the history of Spanish American Colonial art and to the knowledge on technological capacities and skills in the Andean region during this period.
1. Introduction
1.1. Atacamite as a Pigment in Andean Colonial Art
Knowledge of the origin of the pigments used in a work of art can establish cultural and geographical relationships. On identification, the pigment may be a degradation product or an intentionally used compound. The latter can have a natural origin—mineral, vegetable, and animal—or be synthetic, produced in a workshop with recipes of the time.
During our investigations on the materials used in Andean Colonial art, some objects studied were shown not only to contain materials of European origin but also materials that were already known in pre-Hispanic times. Although European painting manuals circulated in America, our research work in the last decade has demonstrated the use of local minerals such as atacamite, cerussite, brochantite, antlerite, and lapis lazuli as pigments [1,2,3,4]. Atacamite, a basic copper chloride (Cu2Cl(OH)3), was identified and characterized for the first time in Spanish American Colonial art in a gilded and polychrome sculpture manufactured from maguey wood of Our Lady of Copacabana (1583) within the Viceroyalty of Peru [5,6], by applying Raman microspectroscopy and SEM-EDX as complementary techniques [1]. In this research, the pigment was characterized and its natural origin was proposed, as opposed to it being a degradation or synthetic product. The shape of the atacamite crystals, the identification of gypsum and hematite together with these crystals, and the absence of other compounds related to a degradation product point to a mineral origin of the pigment. This hypothesis is reinforced by the fact that the mineral is found in the Atacama region near the place of manufacture of the work and many of these minerals were known and used in pre-Hispanic times [5,7,8].
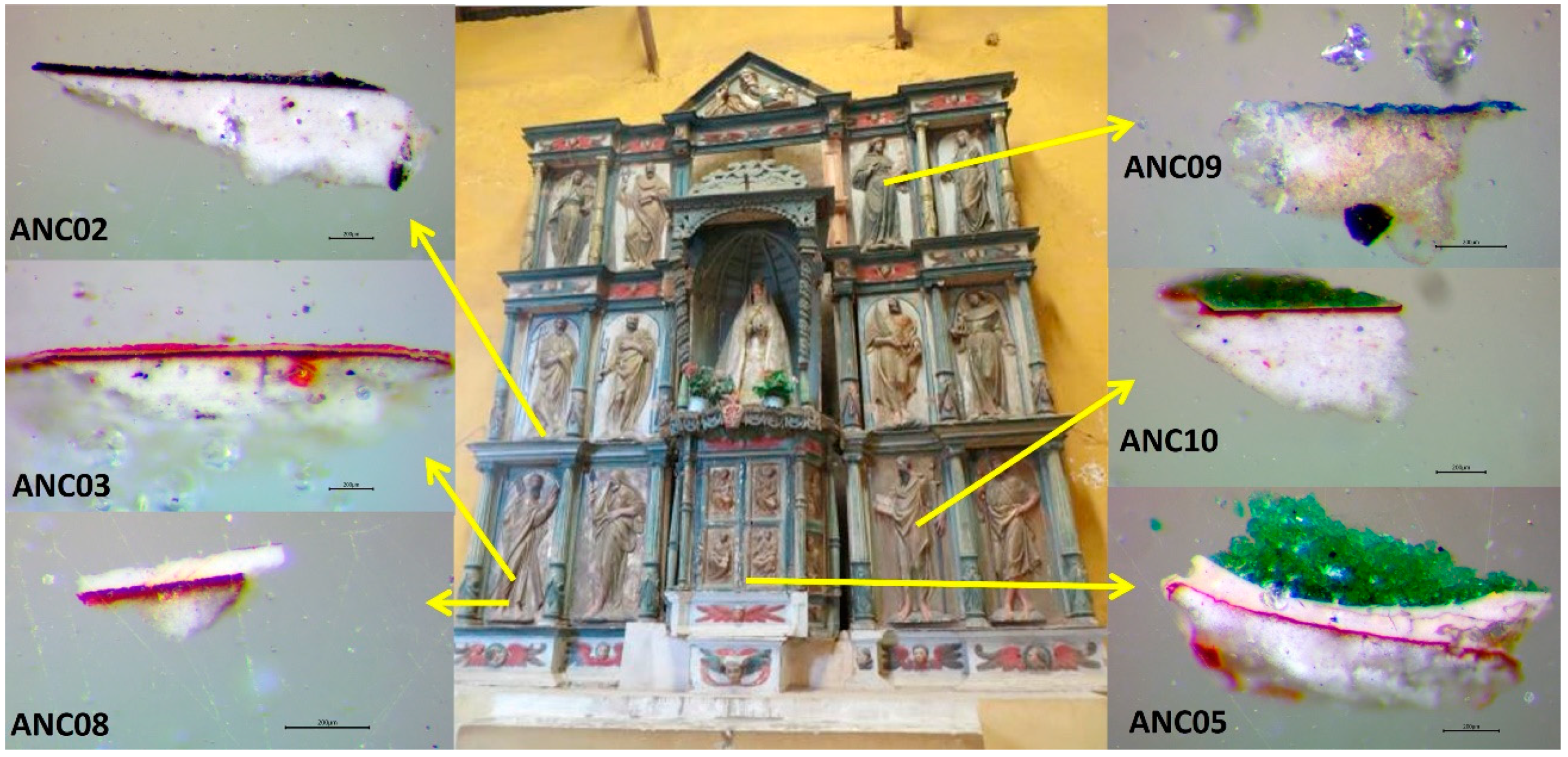
The image of Our Lady of Copacabana was made by a native sculptor named Francisco Tito Yupanqui, who carved and polychromed it in around 1583. In History of the Sanctuary of Our Lady of Copacabana written by Fr. Alonso de Ramos Gavilán, the priest shows how Tito Yupanqui worked on its manufacturing, and refers to Vargas, a Spanish sculptor active in La Paz, who taught him how to gild and colour the image while working on the gilding of the main altarpiece of the Church of San Francisco (La Paz, Bolivia) [9]. This altarpiece is the one that, according to some hypotheses, stands today in the Church of Ancoraimes (Figure 1), where atacamite has recently been identified in its polychrome [10]. This establishes a possible relationship, not only in the application of the manufacturing technique of sculpture and polychrome, but also in the acquisition and knowledge about the use of this pigment.
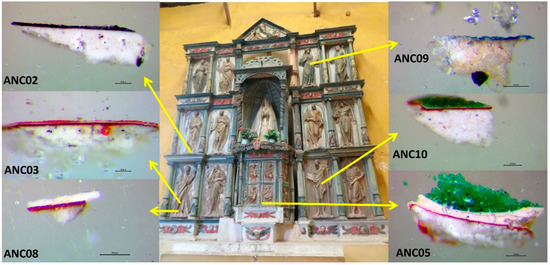
Figure 1.
Images of the altarpiece of Ancoraimes church and images of the cross-sections of the samples observed under the optical microscope (Scale reading 200 μm).
1.2. Atacamite as a Natural or Synthetic Mineral
To advance the development of a methodology to identify and characterize the origin of the atacamite pigment as a mineral of local use, this work aims to present an analysis with a multitechnical approach, where the strengths and weaknesses of the different techniques for this purpose are highlighted. For this reason, we worked with atacamite minerals from the Andean area such as from the Chuquicamata mine in Chile, and with synthetic compounds obtained from recipes of the time. This study also shows the application of this methodology in the study of the green samples of the polychromy of the Ancoraimes altarpiece.
The recipe for viride salsum from one of Theophilus Presbyter’s art treatises from the SXII [11] is one of the most used traditional recipes in Europe for the production of verdigris, copper (II) acetate that corresponds to the general formula Cux(C2H3O2)y(OH)z·nH2O. If this recipe is made under certain conditions, atacamite is obtained as the main product [12,13].
In Spanish America, between the 16th and 18th centuries, various manuals and secret books circulated describing the process for making different pigments. Many were produced in artists’ workshops in the viceroyalties. This is the case of the production of cardenillo raído and cardenillo vermicular in the version of the recipes of Pedacio Dioscórides in De materia medica translated from Greek into Spanish, commented on and annotated by Andrés de Laguna in 1566 [14]. In both recipes of Teophilo and Dioscorides, translated by Laguna, metallic copper is used as a starting material together with other materials of daily use such as vinegar, salt, and honey. In this work, we carried out the syntheses of verdigris, trying to reproduce the conditions of the original recipe in the laboratory. These syntheses were observed over time, under different conditions and varying their ingredients to understand the processes occurring, as well as to find the optimal conditions to obtain atacamite as a main product.
The analytical techniques used in this work were optical and electron microscopy, with elemental analysis using SEM-EDS and pXRF and Raman and infrared spectroscopy in FTIR-ATR mode. The use of the WAXS technique carried out with equipment for small-angle X-ray scattering (SAXS) is highlighted. This nondestructive technique allows diffractograms to be obtained with a small size of sample, which makes it a particularly useful technique for use in the field of cultural heritage sciences.
This methodology is applied to the study of the pigments used in the work described, especially the green pigments, to establish relationships in their form of use, manufacturing processes, or source of supply, as well as to establish links with historical narratives.
2. Experimental
2.1. Samples and References
2.1.1. Altarpiece Painting Samples
Microsamples were extracted with a scalpel from coloured areas of the polychrome of the altarpiece of the Church of Ancoraimes (Figure 1). Some of them were fragmented to an area less than 1 mm2 and were embedded into an acrylic transparent resin Subiton® (Buenos Aires, Argentina) to prepare their cross-sections by polishing with a decreasing sandpaper particle size (down to 12,000 mesh). The description of their colours and location is given in Table 1. In Figure 1, the images of the cross-sections observed under the optical microscope are shown. In this work, we paid special attention to the green samples.

Table 1.
List of samples, colour, and location in the altarpiece.
2.1.2. Mineral Reference Samples
Three atacamite mineral samples were provided by the Museum of Mineralogy “Dra. Edelmira Mórtola” of the Departamento de Geología of the Facultad de Ciencias Exactas y Naturales (FCEN) of the Universidad de Buenos Aires (UBA). There were two from Chuqicamata, Chile (ATA S/N and ATA86) and one from an unspecified location in Chile (ATA84).
2.1.3. Synthetic References: Greproduction of Old Recipes
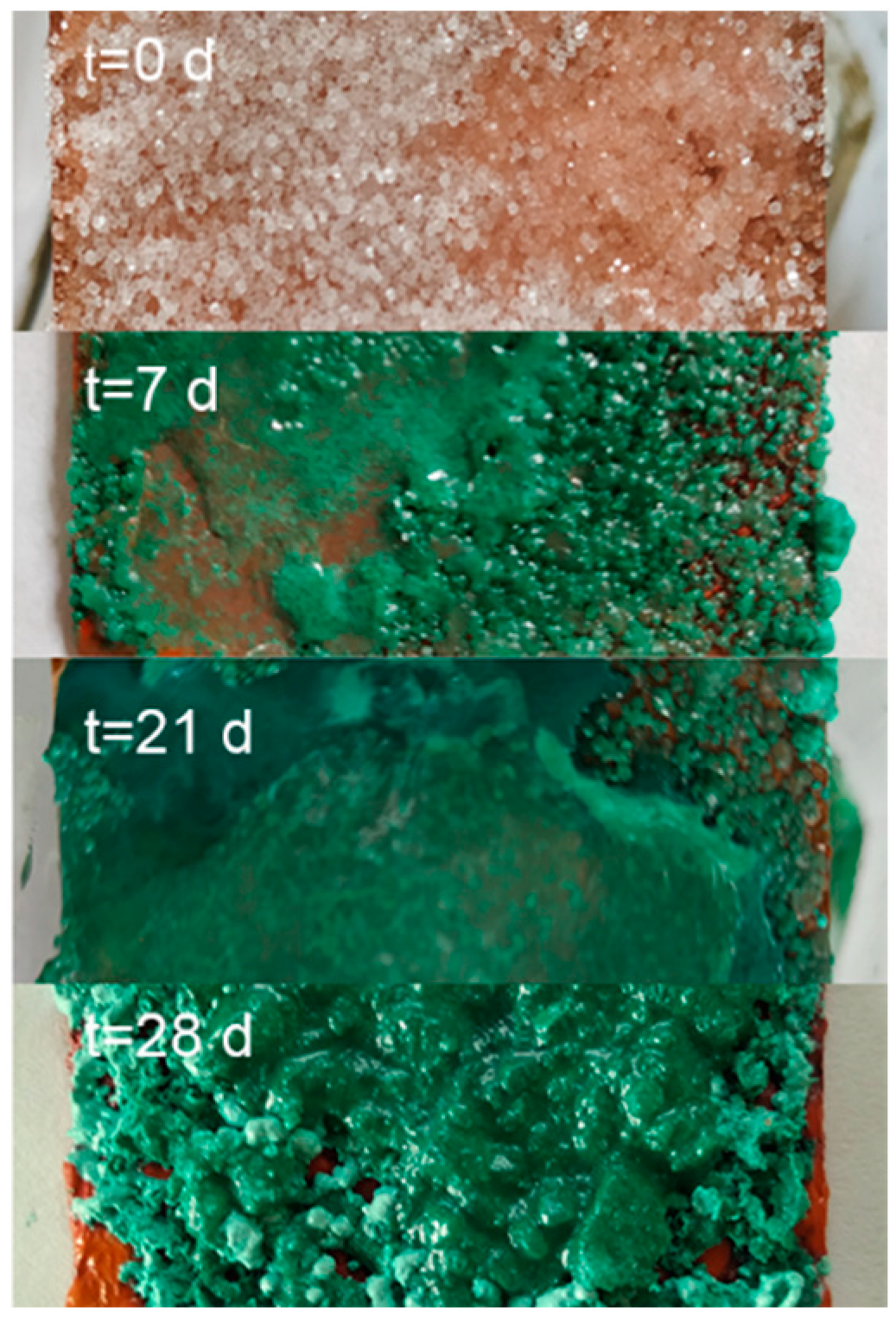
Recipe of Theophilus Presbyter (TEO): The recipe to produce verdigris viride salsum from the 12th century (Book I, Chapter XXXVII) describes the exposure of a copper plate covered with honey and salt to vinegar vapours in a closed environment [11]. Metallic copper plates and rods (Primalco S.A., Buenos Aires, Argentina) of 99.9% purity were used in contact with commercial wine vinegar of certified organic origin (so as not to introduce any additives), NaCl (Química Oeste S.A., Buenos Aires Argentina, analytical grade), and organic honey in an airtight glass container (Figure S1). Thirty replicates of the experiment were carried out, and we varied the exposure time to the vapours between 4 days and 3 months, the use of honey and the thickness of the deposited layer, the amount of sodium chloride, and the use of vinegar in the reaction medium.
Recipe of Dioscórides translated by Laguna (CR, CV): This recipe corresponds to the version translated into Spanish by Andres Laguna (1566) of the book De materia medica by Pedacio Dioscórides (SI) [14]. The production of verdigris is described in the forms cardenillo raído (CR) and cardenillo vermicular (CV) in Book V, Chapters L and LI.
In the CR recipe, the copper metal contacts vinegar vapours in the same configuration as for TEO. No production of atacamite is possible for this recipe, so it will be taken as a reference for obtaining only verdigris, but the results are not shown in this paper. For the CV, a copper mortar is needed and the addition of vinegar, alum, and salt. The quantities used were adapted to be used in a handmade mortar from the same copper plates. Considering that 1 drachm (dracma) equals 3.59 g and half a hemin (hemina) equals 140 cm3, 0.5 mL of commercial organic vinegar, 0.0124 g of alum (AlKSO4), and 0.0107 g of NaCl (Química Oeste S.A., Buenos Aires Argentina, analytical grade) were used. Then, the vinegar was placed in the mortar (Figure S2), and the bottom of the mortar was scraped for 7 min with the copper pestle. Finally, the product was left to stand for 3 weeks in the open air.
All the syntheses were followed during their development, made in different conditions, and varying their ingredients to understand all the processes that occurred, as well as to find the optimal conditions to obtain atacamite as the major product. From these experiments, several products were obtained; the presence of atacamite was monitored and separated by observing using MO and identified through FTIR-ATR analysis.
2.2. Instrumental, Samples, and References
2.2.1. Microscopy and Microanalysis
Morphological and stratigraphic investigations were carried out by using optical microscopy (OM) and scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS). OM images were taken with a Leica MZ6 stereomicroscope and a Leica DM 750 microscope. SEM analysis by secondary electrons (SEs) and backscattered electrons (BSEs) were obtained by using a field environmental scanning electron microscope (FE-SEM) with a Zeiss:Supra 40 coupled with an energy-dispersive X-ray spectrometer (SEM-EDS) INCA X Sight (Oxford Instruments). The analysis was carried out under an accelerated potential of 20 KV. The cross-sections were coated by sputtering with a thin (less than 80 Ẳ) layer of platinum.
2.2.2. Portable X-ray Fluorescence Spectroscopy
A Bruker portable device, model Tracer III-SD, was used. The X-ray tube was equipped with a rhodium anode (Rh). The spectra were recorded with 40 KeV and 11 μA under vacuum conditions and the acquisition time was 120–240 s. Spectra were acquired and processed using S1PXRF and ARTAX7 programs.
2.2.3. Infrared Spectroscopy
Fourier-transform infrared spectroscopy (FTIR) spectra were obtained on a Nicolet iS50 FTIR spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) with a diamond single-bounce ATR accessory. For each sample, 64 scans were recorded in the 4000–400 cm−1 spectral range in the reflectance mode with a 4 cm−1 resolution. Spectral data were collected with the Omnic v9.2 (Thermo Fisher Scientific Inc., Waltham, MA, USA) software without post-run processing. The spectrum of air was used as background.
2.2.4. Micro-Raman Spectroscopy
The Raman spectra were obtained with a Renishaw Raman microscope (inVia), with a CCD detector and coupled with a Leica microscope (DM2500M model) and objectives N PLAN EPI (50×/0.75 and 20×/0.40). The spectra were excited at 785 nm and the laser power was always kept below 0.5 mW at the sample to avoid degradation. The samples were studied as received without any type of manipulation and the spectra were analysed using the Omnic v9.2 (Thermo Electron Corp)
2.2.5. Wide-Angle X-ray Scattering (WAXS)
Wide-angle X-ray scattering (WAXS) measurements were performed using a XEUSS 2.0 (from XENOCS, France) apparatus. This analysis was performed in transmission mode using a small beam size (ca 0.4 mm full width half maximum size) and this technique allows the use of small sample sizes and is microdestructive, which is especially useful for samples of cultural heritage. Patterns were registered with a 2D photon counting pixel X-ray detector Pilatus 200 k (DECTRIS, Baden, Switzerland) placed at 100 mm from the sample. The detector was placed in a motorized stage in order to cover a wide angle range from 10 to 60° in 2θ with an incident monochromatic beam of λ = 0.15419 nm, which is the weighted average of the X-ray wavelength of the Cu-Kα1,2 emission lines. The measurements were performed in transmission mode over a small piece of pigment. This was possible due to the small beam size pointed at the sample (<0.5 mm × 0.5 mm). Each WAXS frame was taken for 15 min.
3. Results and Discussion
3.1. Altarpiece Samples
3.1.1. Manufacturing Technique and Ground Layer
Light microscopy examination of the cross-sections of the samples (Figure 1) revealed different layers characteristic of a decorative technique called estofado. The layers from the bottom to the top are a white ground layer, a red layer, a gold layer, a white primer layer, and a layer with coloured paint. The application of a thin gold leaf on a white ground layer covered with an ochre layer of bole is characteristic of this technique, traditionally used for wood surfaces. The additional application of layers of paint on the gold leaf and the subsequent scraping of the paint to reveal the gold beneath with the formation of a certain pattern were used to imitate precious textiles such as damask and brocade [1,15]. Figure 2 shows the SEM and optical microscope image of the cross-section of the green sample ANC05. SEM-EDS analysis of the ground layer of all the samples revealed the presence of mainly calcium (Ca) and sulphur (S), and trace amounts of silicon (Si), aluminium (Al), and potassium (K). These elements may correspond to a compound of the CaSO4.XH2O system and to minor amounts of clays and feldspars, respectively. The presence of gypsum (CaSO4·2H2O) in the preparation layer was confirmed using Raman spectroscopy analysis (Figure S3) [16]. The red layer corresponding to the bole showed the content of iron (Fe), Ca, and Si, and minor amounts of Al and K, in all samples. This indicates hematite, which may contain naturally occurring clays and calcium carbonates, this mixture is commonly known as “Armenian bole”, onto which the thin sheet of gold was applied [5,15]. The next layer corresponds to pure gold leaf, and on top of this layer is the white primer layer containing lead, corresponding to cerussite (PbCO3). These compounds were confirmed by Raman spectroscopy (Figure S3) [17,18] and are consistent with those expected in the use of materials and techniques, as in the polychromy of Our Lady of Copacabana [1,2].
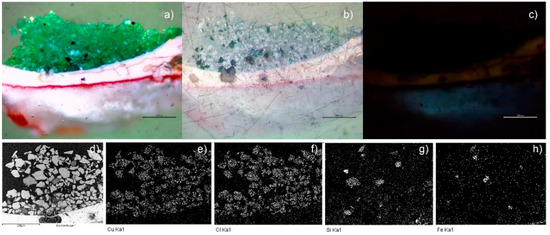
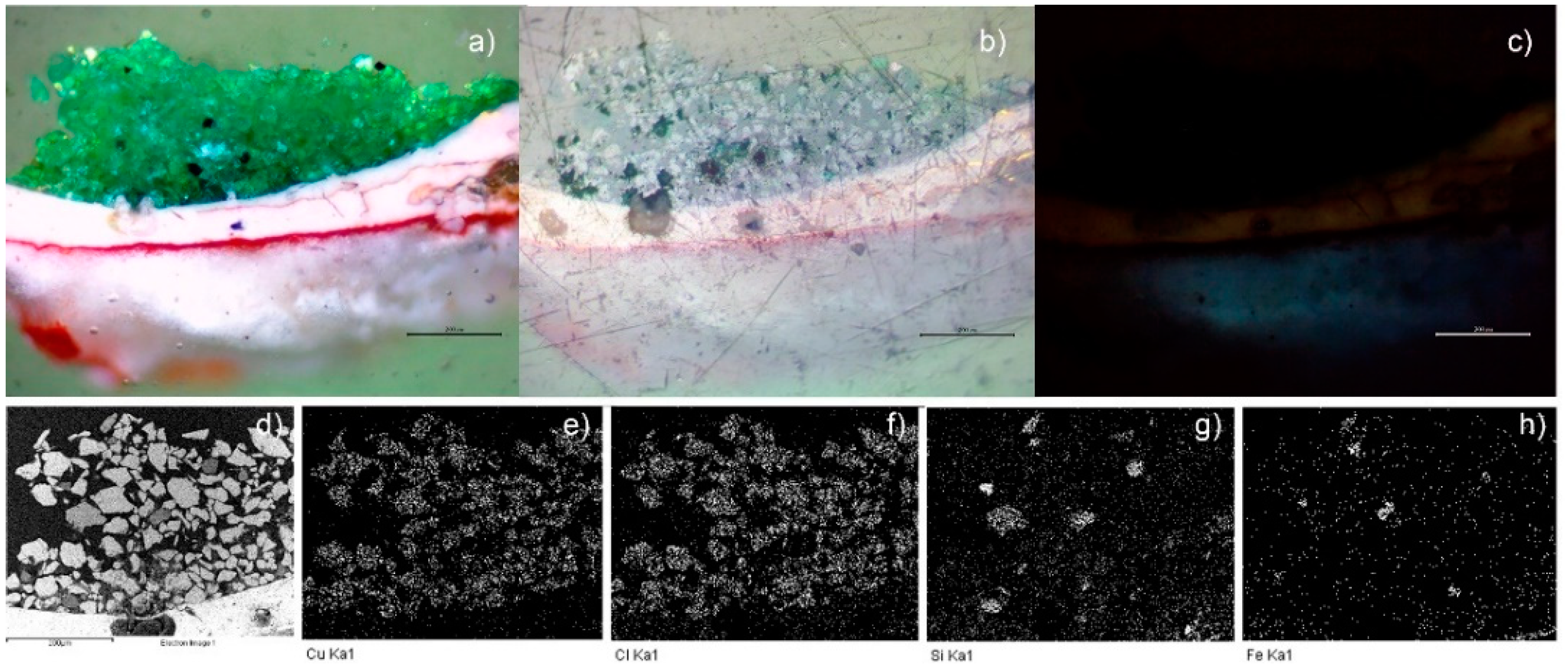
Figure 2.
Image of the cross-section of the green sample ANC05, optical image (50×): (a) visible, (b) polarized, and (c) UV (350 nm). SEM-EDS: (d) backscattered electrons (BSEs) micrography (400×), mapping of (e) Cu, (f) Cl, (g) Si, and (h) Fe. (Scale reading 200 μm.)
3.1.2. Green Samples
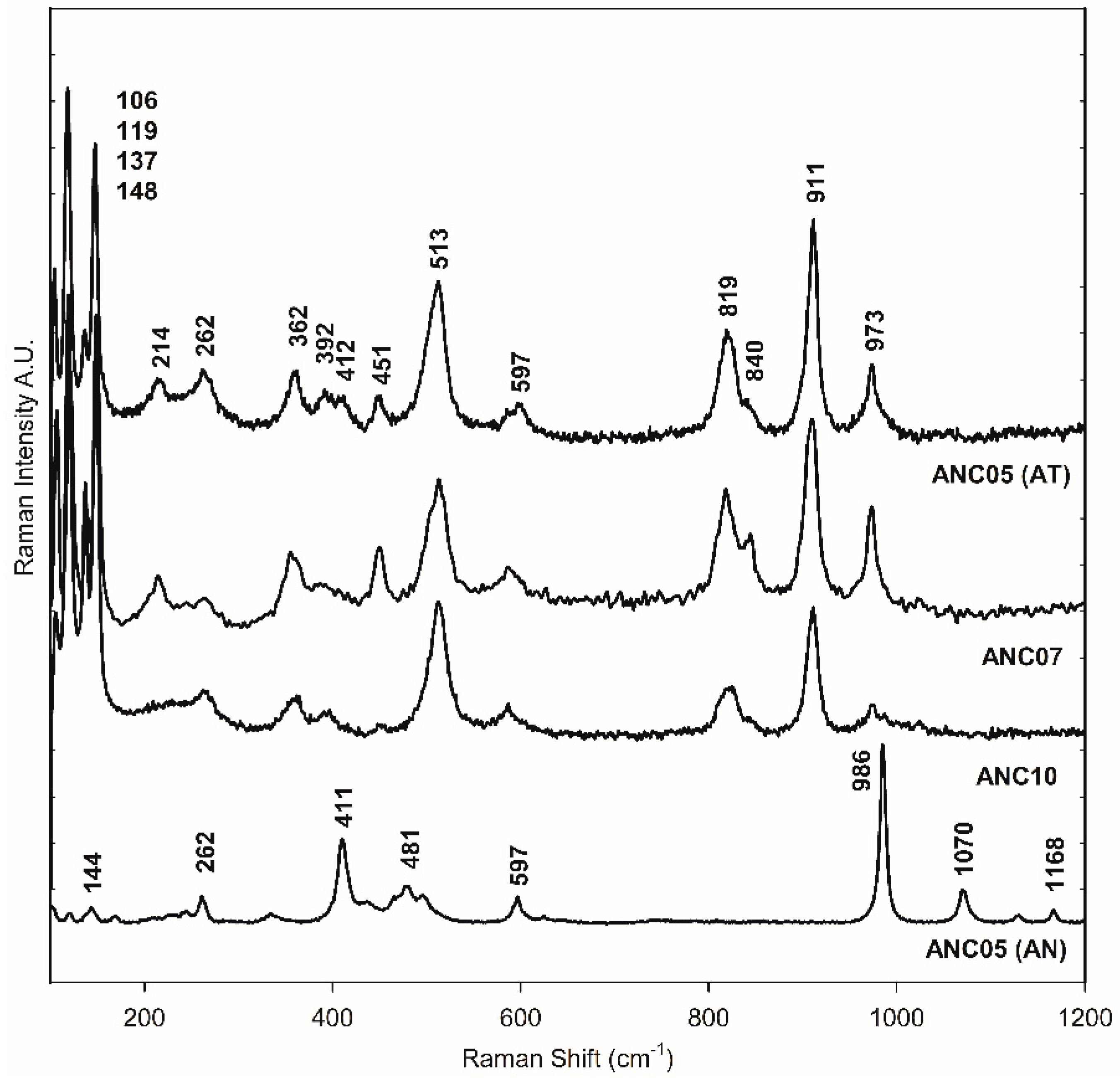
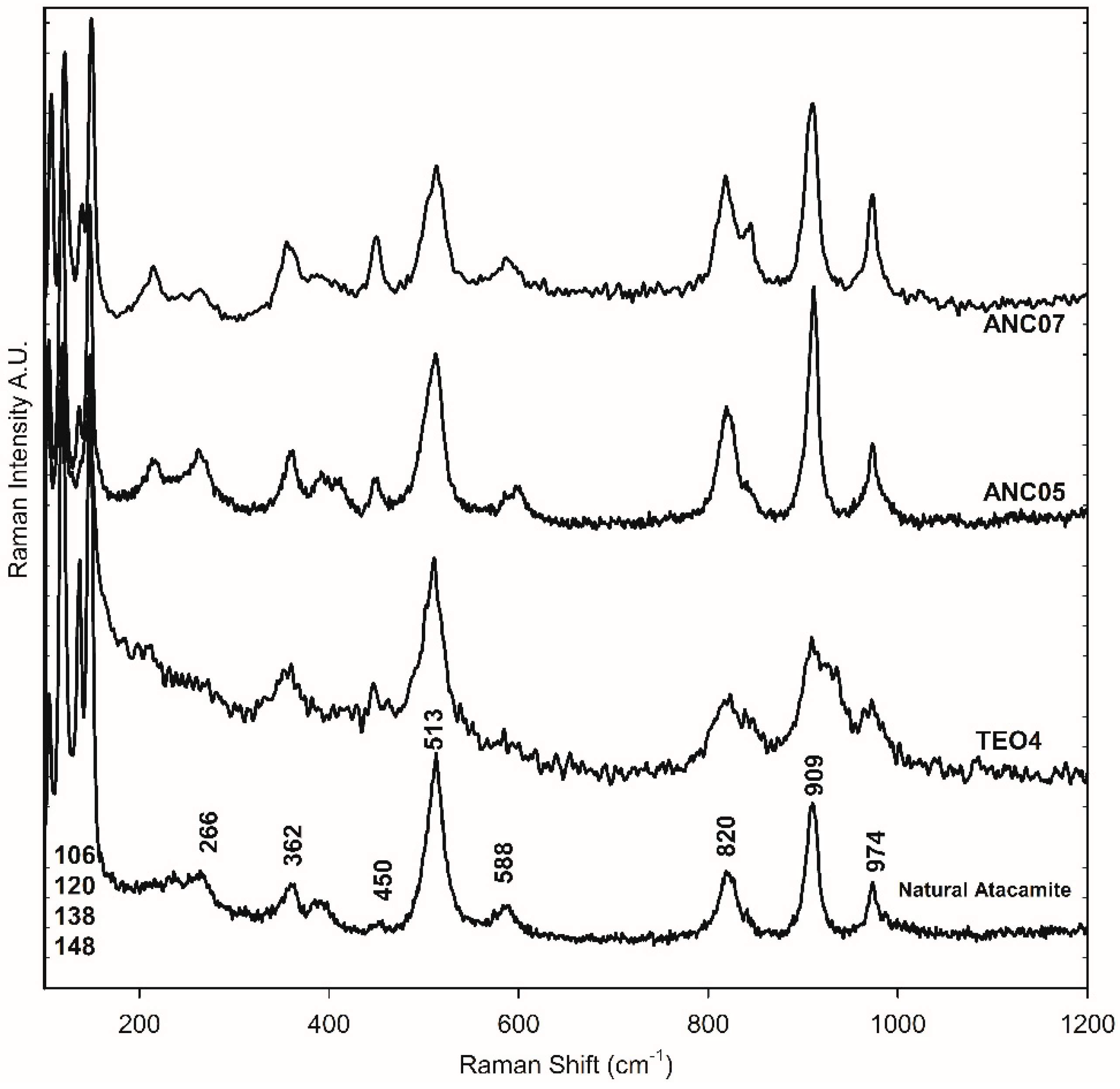
The green samples, ANC05, ANA07, and ANC10, were specially analysed as they showed atacamite as the main pigment in the different techniques used for pigment identification, as will be shown in the following. Figure 2d–h shows the SEM-EDS mapping of the cross-section of sample ANC05. The green layer of the sample shows mainly copper (Cu), chlorine (Cl), Si, and Fe content (see also Table S1). This observation, together with the distribution and size of crystals, is similar to that obtained for the green sample of Our Lady of Copacabana, where the presence of atacamite with traces of quartz (SiO4) and hematite (Fe2O3) was demonstrated, corresponding to the presence of the elements Si and Fe, respectively [1]. The samples were measured using Raman spectroscopy in the green layer of the cross-section as shown in Figure 3, showing the presence of atacamite in all the green samples. The main bands identifying this mineral are 148 and 119 cm−1, corresponding to O-Cu-O bending; 262 cm−1 to lattice modes; 362 cm−1 to Cu-Cl stretching; 513 cm−1 to CuO stretching; and 819, 911, and 973 cm−1 to O-H deformation [19,20]. Additionally, one of the samples (ANC05 (AN) in Figure 3) shows bands at 144, 262, 411, 481, 597, 986, 1070, and 1168 cm−1, corresponding to another copper compound, antlerite (Cu3SO4(OH)4) [21], which has already been identified in Andean mural paintings [3,4,22] and has recently been found together with atacamite [23].
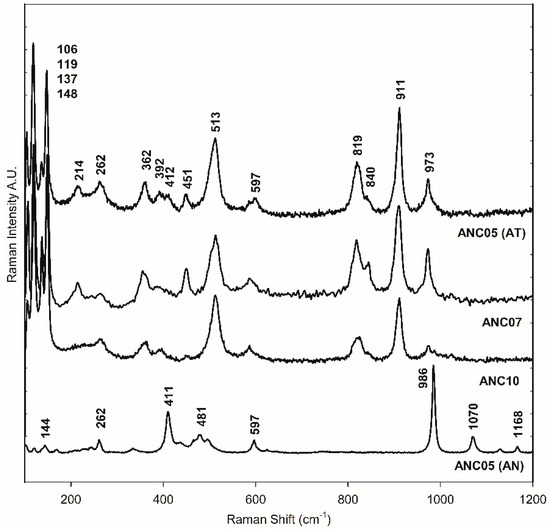
Figure 3.
Raman spectra of green layer in the cross-section of samples ANC05, ANC07, and ANC10. ANC05 (AT) and ANC05 (AN) show bands of atacamite (AT) and antlerite (AN), respectively.
3.1.3. Other Colour Samples
SEM-EDS and Raman spectroscopy were used to identify the different pigments in the rest of the altarpiece samples. The black sample (ANC02)’s spectrum (Figure S4) showed the typical 1342 and 1595 cm−1 bands of a carbon-based black pigment [24], which correspond to a high carbon and oxygen content, and then traces of Si, Al, Cl, and Ca obtained from elemental analysis. For sample ANC03, the high content of mercury (Hg) and S indicate the presence of vermilion or cinnabar (HgS), which is manifested by the bands at 252, 284, and 343 cm−1 in the Raman spectrum of Figure S4 [25]. The spectrum of the white sample, ANC08, showed the cerussite (PbCO3) bands at 149, 171, 220, 681, 839, and 1053 cm−1, consistent with the results of the SEM-EDS elemental analysis, which showed only the presence of lead (Pb) in addition to carbon (C) and oxygen (O) [18]. The blue sample, ANC09, showed Fe, S, Ca, barium (Ba), and traces of K, Si, and Al, and the stratigraphy did not show the layered composition corresponding to the estofado technique. The Raman spectrum showed bands at 277, 531, and 2153 cm−1, corresponding to Prussian blue [26], and 1007 cm−1, corresponding of gypsum; this composition for blue results in a mixture probably used in an old restoration. Although Prussian Blue was used from the end of the 18th century in South America [27], the presence of barium (Ba) suggests the use of barium sulphate (BaSO4), which started to be used later as an alternative to lead white, and then as a filler or extender in paints; moreover, it was not found in any other sample.
3.2. Mineral Samples of Atacamite
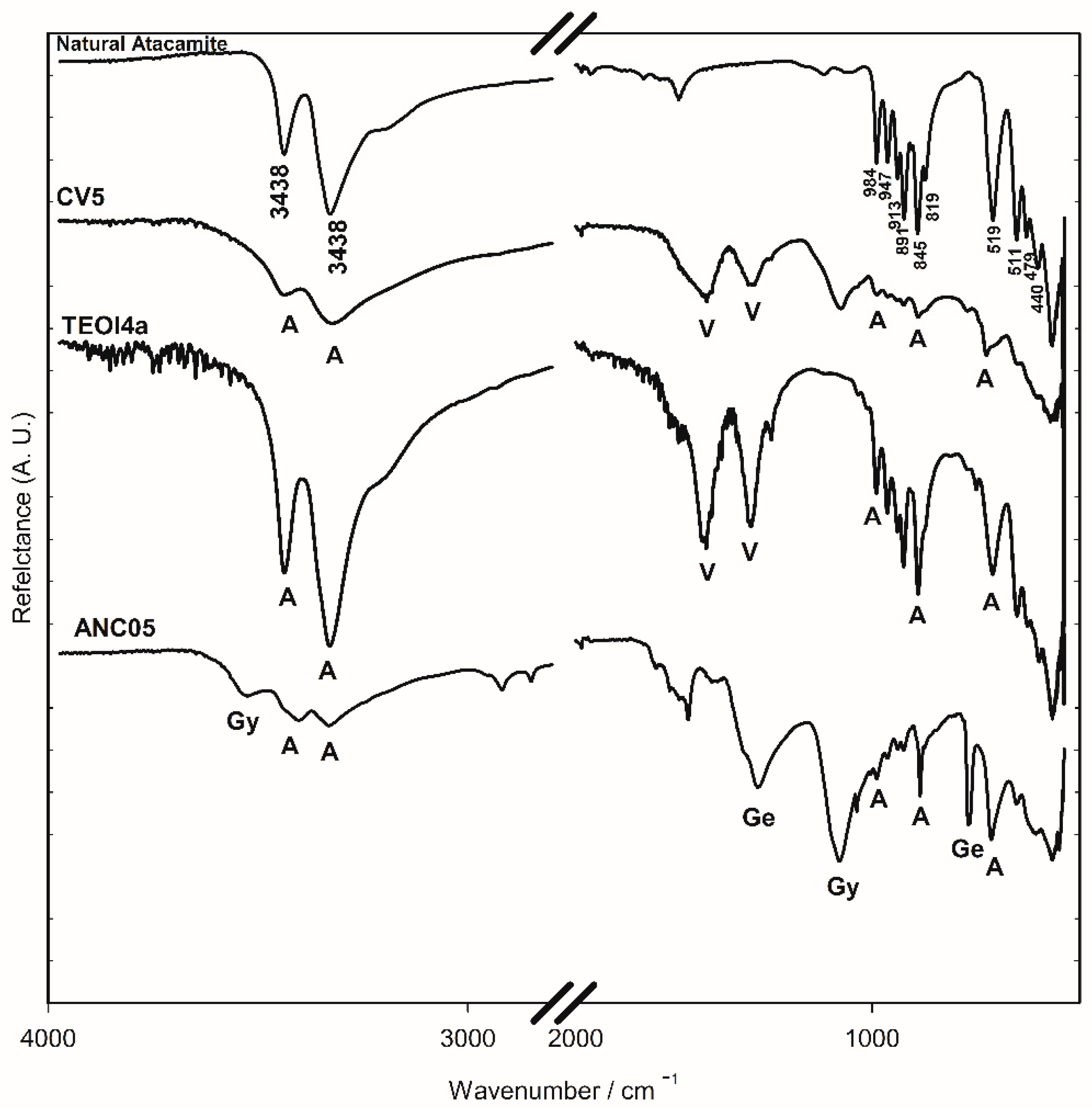
Minerals samples from Chuquicamata Mine in Chile were observed under the optical microscope and using SEM-EDS electron microscopy. Despite the morphological differences that were observed between the three samples, in all cases, they were principally composed of atacamite. From the portable XRF spectroscopy analysis, the elemental composition was obtained. Using ATR-FTIR and Raman spectroscopy, atacamite was identified [19,20] (Figure 4 and Figure 5). The bands at 3438 and 3328 cm−1 in the FTIR-ATR spectra are associated with the stretching of the O-H bond of the hydroxy group, those between 984 and 819 cm−1 are associated with the deformation vibration of the same bond, and those between 591 and 440 cm−1 are assigned to the stretching of the Cu-Cl and Cu-O bonds in atacamite [19]. The Raman bands of the mineral samples—106, 120, 138, 148, 266, 362, 450, 513, 588, 820, 909, and 974 cm−1—were also the references to identify atacamite in the other samples as shown in Figure 5.
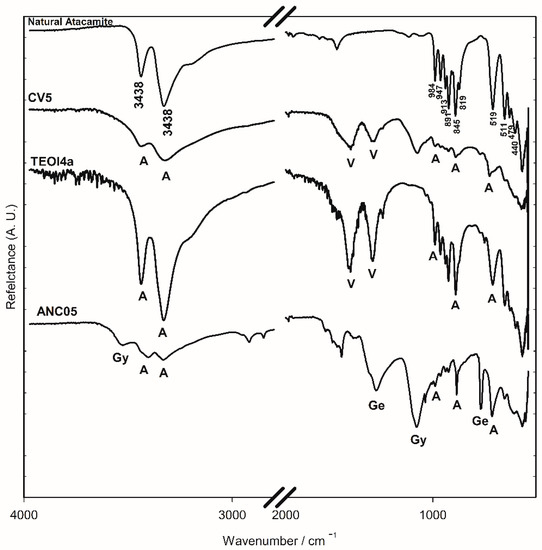
Figure 4.
FTIR-ATR spectra of the surface of altarpiece (ANC05), synthesised using recipes (TEO4a) and (CV5) and natural atacamite (ATA84) samples. V: verdigris, A: atacamite, Gy: gypsum, and Ge: gerhardtite.
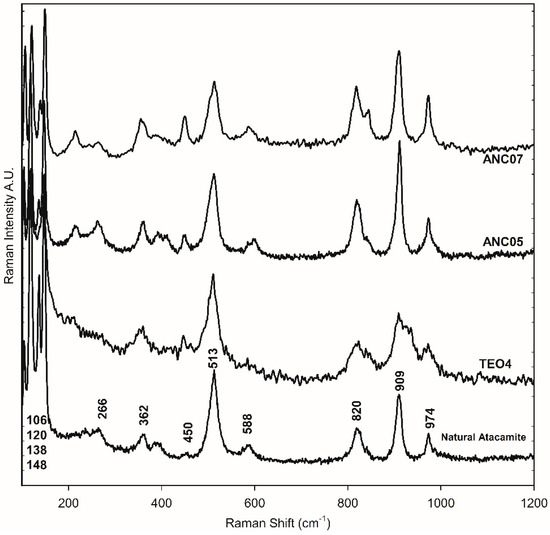
Figure 5.
Raman spectra of the green layer in the cross-section of samples ANC05, ANC07, mineral atacamite (86), and synthetic atacamite (TEO).
3.3. Synthetic Copper Green Pigments Using Old Recipes
3.3.1. Viride salsum by Theophilus (TEO)
Samples were prepared following the recipe of the viride salsum. It consists of putting honey and salt on a metallic copper surface in an environment of vapours of vinegar. The reaction time was varied from three days to three months. The identification of the compounds present in the synthesis was carried out using FTIR-ATR spectroscopy, due to the characteristics of the spectrum and the simplicity of its analysis. From these spectra, the presence of atacamite and/or verdigris as a product of the synthesis was determined. In Figure 4, the bands in the region 800–900 cm−1 are associated with the deformation vibrations of the atacamite (A) hydroxy group, and their characterisation resulted in an easy and fast method to identify the presence of the compound. The bands at 1565 and 1410 cm−1 for the synthetic samples are characteristic of the pigment verdigris (V). The results so far indicate that the production of atacamite as a major product with respect to verdigris was favoured in the replicates with a high amount of salt and absence or very thin layers of honey. The reaction time showed no appreciable influence on the composition from the first days of the reaction (Figure 6). Although the literature is very varied, the results and influences of the reaction conditions are neither precise nor conclusive; however, what has been observed seems to indicate that the process is largely influenced by the availability of chloride anions on the metal surface. This is consistent with the observation made by Scott [12,13], who states that the ions involved in the corrosion process have a greater influence on the course of the process than other environmental factors, and proposes that high salt promotes the formation of nantokite (CuCl), and in a humid environment, results in atacamite. Other authors observed similar results [28,29]. No differences were observed under exposure to sunlight or not. Figure 5 shows the Raman spectrum of a synthesised product sample, compared to the other atacamite, mineral, and altarpiece samples.
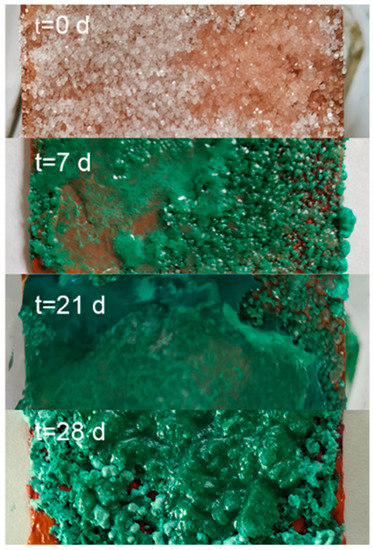
Figure 6.
Image of the progress of the verdigris synthesis experiment with the viride salsum recipe (TEO).
3.3.2. Cardenillo vermicular by Laguna (CV)
For the reproduction of cardenillo vermicular (CV), a mixture of sodium chloride, alum, and vinegar was placed in a copper bowl. It was mixed with the help of a copper pestle and left to evaporate in the sun, as the recipe specifies, resulting in mixtures of acetates and atacamite (Figure 7). The product of the cardenillo vermicular synthesis turned out to be composed of different types of crystals with characteristics dependent on the reaction conditions and availability of anions (Figure S5). In accordance with this hypothesis, towards the centre of the bowl, a generally higher concentration of atacamite was obtained according to the solvent evaporation process. SEM-EDS analysis showed similarities in the elemental composition of the different crystals (Table S2). In all cases, traces of elements related to the used reagents appeared. To identify atacamite and verdigris, each sample was analysed with FTIR-ATR, and all showed similar spectra (Figure 4). The results show that the composition was similar, but what differed was the concentration of these compounds.

Figure 7.
Picture of copper mortar after cardenillo vermicular synthesis (CV).
3.4. Global Comparative Analysis: Tools for Characterization Method Development
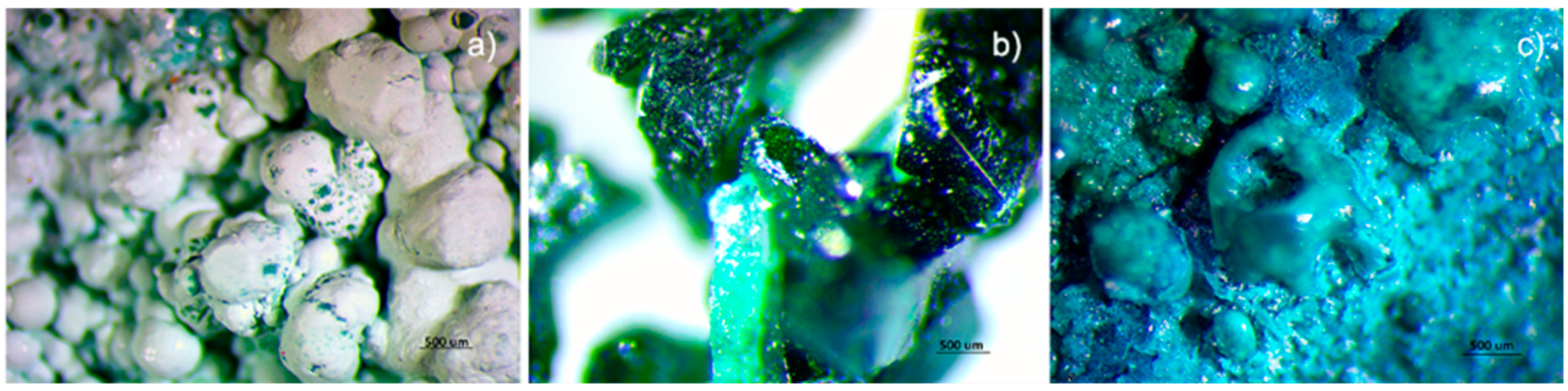
Figure 8a,c show OM images of the results obtained in one of the viride salsum (TEO) and cardenillo vermicular (CV) experiments, respectively, together with one of the natural atacamite samples in Figure 8b. Using FTIR-ATR and SEM-EDS, it was confirmed that these were atacamite particles. By observing the samples under the MO optical microscope, we found there was a notable difference between the crystallization form of the synthetic compound and the natural one. However, if these observations are accompanied by molecular analysis, each compound can be identified, and hypotheses of a natural origin can be formulated.

Figure 8.
Stereoscopic images of atacamite obtained from the viride salsum recipe (a), natural atacamite (b), and verdigris obtained from the viride salsum recipe (c) (stereoscopic magnification at 10×).
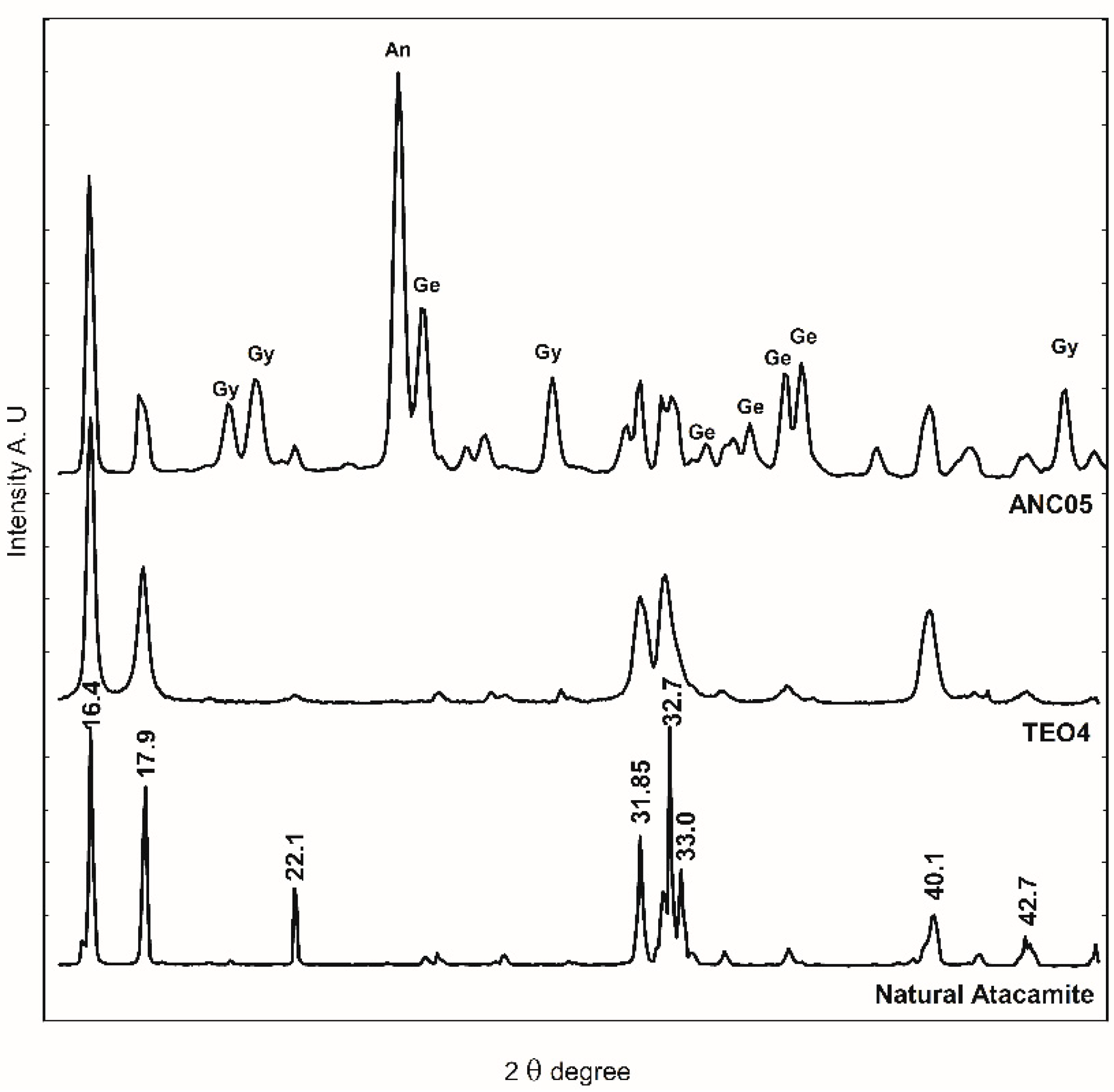
FTIR-ATR spectroscopy was used for identification due to characteristic spectra and the ease of analysis. In Figure 4, the bands at 800–900 cm−1 region are associated with the deformation of the O-H bond of the hydroxy group of atacamite (A) and provide a rapid method to identify the presence of the compound. Until now, the conditions for reproducing the recipe and obtaining atacamite as the only product have not been achieved, so the presence of acetate bands (1565 and 1410 cm−1) characteristic of verdigris (V), together with those of atacamite, provides elements to consider a synthetic origin of the pigment in a sample that presents them. The presence of a copper nitrate in the altarpiece of Ancoraimes sample, ANC05, was evident (1385 and 675 cm−1) and was coincident with gerhardtite (Ge) Cu2NO3(OH)3 [30], a mineral that typically accompanies atacamite as well as gypsum (Gy) (3526 and 116 cm−1), which was observed in the green sample of the Virgin of Copacabana [1]. However, with respect to the characteristics of the atacamite spectrum of the sample, natural or synthetic, no significant differences were observed. The presence of this mineral gerhardtite was confirmed with WAXS, and this is the first time that it has been identified in a sample of Andean Colonial painting (Figure 9) [31]. In the same sample, the presence of antlerite (peak at 2θ = 24.80 degrees) [32] and gypsum (peaks at 2θ = 20.2, 20.9, and 29.4 degrees) was also determined, in addition to atacamite (peaks at 2θ = 16.4, 17.9, 22.1, 31.8, 32.7, 33.0, 40.1, and 42.7 degrees) [33]. The samples of the altarpiece and the synthetic atacamite analysed with this technique showed no differences, except for the presence of other minerals in the sample (gerhardtite, antlerite, and gypsum).
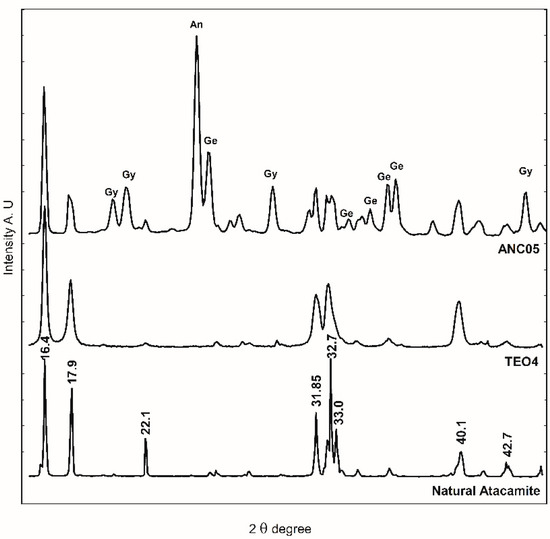
Figure 9.
WAXS diffractogram of sample ANC05, natural atacamite, and synthetic atacamite (TEO). An: antlerite, Gy: gypsum, and Ge: gerhardtite.
Raman spectroscopy is sensitive to the state of structural order and disorder and the degree of crystallinity of minerals, causing changes in the spectra. These can be the broadening or disappearance of a series of bands and even variation in the frequencies of characteristic bands. As an approximation of the determination of the degree of crystallinity of the samples studied here, it can be said that the degree of crystallinity of the natural atacamite can be considered from the sharp peaks observed. The Raman spectra showed the presence of atacamite with different degrees of crystallinity. The synthetic samples showed a spectrum of broader bands—the natural ones more defined due to their higher crystallinity—and this was observed in the altarpiece sample as well. Similar observations have been reported by other authors [19,34,35].
The mixture with other natural minerals, the absence of acetates, the degree of crystallinity, and the morphology of the pigment suggest a natural origin for the atacamite present in the green paint of the sample from the altarpiece of the Church of Ancoraimes.
4. Conclusions
Through the synthesis based on recipes and the study of minerals and samples of the Altarpiece of the Church of Ancoraimes, it was observed that the recipes usually produced mixtures and that the mineral atacamite was usually found together with minerals of natural origin (antlerite, gerhardtite, quartz, and others) and without other compounds of the recipes. These observations must be considered to determine the natural origin of the pigment. The different techniques used here allowed obtaining detailed and complementary information of the composition of the different materials. This methodology will be useful to establish relationships between reference samples and microsamples extracted from Andean Colonial art objects and paintings of the 17th and 18th centuries. At the same time, it will give us information on manufacturing processes and the sources of supply of atacamite. These studies will contribute to the history of Spanish Colonial art and to the knowledge on technological capacities in the Andean region during this period. Furthermore, this is the first time that the mineral gerhardtite has been identified in a sample of Andean Colonial painting, and therefore, the information obtained will contribute to the database of local pigments of mineral origin used in Andean Colonial art.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/heritage6070272/s1. Figure S1: Image of the experiment of synthesis of antiquity viride salsum recipe (TEO); Figure S2: Image of the experiment the reproduction of cardenillo vermicular recipe (CV). Left: Mixture of sodium chloride, alum, and vinegar in a copper bowl. Right: first crystals of the reaction in the copper bowl. Figure S3: Raman spectra of the ground layer, bole, and priming layer in the cross-section of the green sample, ANC05. Figure S4: Raman spectra of samples of other colours. Figure S5: Image of the scratched crystals of the product of the cardenillo vermicular recipe (CV) on the copper plate. Table S1: elemental composition of the ANC07 and ANC10 green layer samples. Table S2: elemental composition (atomic %) of the different crystals of the product of the cardenillo vermicular recipe (CV).
Author Contributions
Conceptualization, G.S. and E.T.; methodology, E.T.; validation, E.T., G.S. and M.S.M.; formal analysis, A.D.H., M.C., C.H.-I. and E.T.; investigation, A.D.H., C.R.L., G.S. and E.T.; resources, M.C., C.R.L., C.H.-I., G.S., M.S.M. and E.T.; data curation, A.D.H. and E.T.; writing—original draft preparation, A.D.H. and E.T.; writing—review and editing, E.T.; supervision, E.T., G.S. and M.S.M.; project administration, E.T. and G.S.; funding acquisition, E.T., G.S. and M.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de Buenos Aires (UBACyT 20020130300010BA and 20020170100340BA) and by Agencia Nacional de Promoción Científica y Tecnológica (PICT 2017-1716) and CONICET (PIP 11220130100288).
Data Availability Statement
Not applicable.
Acknowledgments
The authors are indebted to the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Universidad de Buenos Aires (UBA), and Universidad Nacional de Tres de Febrero (UNTREF). The authors would like to thank Teresita Montenegro and Pablo Leal for the mineral samples (Museum of Mineralogy “Dra. Edelmira Mórtola” Departamento de Geología of the Facultad de Ciencias Exactas y Naturales, UBA). A.D.H. thanks the University of Buenos Aires for the stimulus scholarship. C.H.-I., G.S., M.S.M. and E.T. are Research Members of CONICET.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tomasini, E.P.; Landa, C.R.; Siracusano, G.; Maier, M.S. Atacamite as a natural pigment in a South American colonial polychrome sculpture from the late XVI century. J. Raman Spectrosc. 2013, 44, 637–642. [Google Scholar] [CrossRef]
- Tomasini, E.P.; Marte, F.; Careaga, V.P.; Landa, C.R.; Siracusano, G.; Maier, M.S. Virtuous colours for Mary. Identification of lapis lazuli, smalt and cochineal in the Andean colonial image of Our Lady of Copacabana (Bolivia). Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20160047. [Google Scholar] [CrossRef]
- Tomasini, E.; Castellanos Rodríguez, D.; Gómez, B.A.; de Faria, D.L.A.; Landa, C.R.; Siracusano, G.; Maier, M.S. A multi-analytical investigation of the materials and painting technique of a wall painting from the church of Copacabana de Andamarca (Bolivia). Microchem. J. 2016, 128, 172–180. [Google Scholar] [CrossRef]
- Tomasini, E.; Costantini, I.; Rúa Landa, C.; Guzmán, F.; Pereira, M.; Castro, K.; Siracusano, G.; Madariaga, J.M.; Maier, M. Identification and characterization of basic copper sulfates as mineral green pigments in Andean colonial mural paintings: Use of temperature-controlled stage for the study of thermal induced antlerite degradation. J. Raman Spectrosc. 2021, 52, 2204–2217. [Google Scholar] [CrossRef]
- Siracusano, G. Pigments and Powers in the Andes: From the Material to the Symbolic in Andean Cultural Practices 1500–1800; Archetype Publications: London, UK, 2011. [Google Scholar]
- Siracusano, G.A. Mary’s Green Brilliance: The Case of the Virgin of Copacabana. J. Interdiscip. Hist. 2014, 45, 389–406. [Google Scholar] [CrossRef]
- Sepúlveda, M. Pinturas rupestres y tecnología del color en el extremo sur de Chile. Magallania 2011, 39, 193–210. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Figueroa, V. Pigmentos y pinturas de mineral de cobre en la región de Tarapacá, norte de Chile: Nuevos datos para una tecnología pigmentaria prehispánica. Estud. Atacameños 2014, 48, 23–37. [Google Scholar] [CrossRef]
- Ramos Gavilán, A. Historia del Santuario de Nuestra Señora de Copacabana [1621]; Pastor, I.P., Ed.; Talleres Gráficos PL Villanueva: Lima, Peru, 1988. [Google Scholar]
- Siracusano, G.; Maier, M.; Tomasini, E.; Rua Landa, C. “If you want to be a painter, paint the mother monkey with her baby”. New Studies on the Image of Our Lady of Copacabana. In Materia Americana in the body of Spanish American Images from 16th to Mid 19th Centuries; Siracusano, G., Rodriguez Romero, A., Eds.; Eduntref: Saenz Peña, Argentina, 2020; pp. 385–398. [Google Scholar]
- Dodwell, C.R. Theophilus: De Diversis Artibus; Thomas Nelson & Sons: Oxford, UK, 1961. [Google Scholar]
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants and Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 2002. [Google Scholar]
- Scott, D.A.; Taniguchi, Y. The verisimilitude of verdigris: A review of the copper carboxylates. Stud. Cons. 2001, 46, 73–91. [Google Scholar] [CrossRef]
- De Laguna, A. Pedacio Dioscórides Anazarbeo, Acerca de la Materia Médica Medicinal y de Los Venenos Mortíferos (S. I d. C), Trad. del Griego al Castellano y Comentarios de A. de Laguna (1566); Biblioteca de Clásicos de la Medicina y de la Farmacia Española: Madrid, Spain, 1999. [Google Scholar]
- Sandu, I.C.A.; Afonso, L.U.; Murta, E.; De Sa, M.H. Gilding techniques in religious art between East and West, 14th–18th centuries. Int. J. Conserv. Sci. 2010, 1, 28–31. [Google Scholar]
- Prieto-Taboada, N.; Larrañaga, A.; Gómez-Laserna, O.; Martínez-Arkarazo, A. Raman spectroscopy for the characterization of the CaSO4–H2O system compounds. Microchem. J. 2015, 122, 102–109. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Brooker, M.H.; Sunder, S.; Taylor, P.; Lopata, V.J. Infrared and Raman spectra and X-ray diffraction studies of solid lead(II) carbonates. Can. J. Chem. 1983, 61, 494–502. [Google Scholar] [CrossRef]
- Martens, W.; Frost, R.L.; Williams, P.A. Raman and infrared spectroscopic study of the basic copper chloride minerals—Implications for the study of the copper and brass corrosion and ‘bronze disease’. Neues Jahrb. Fur Mineral. Abhandlungen. 2002, 178, 197–215. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.N.; Kloprogge, J.T.; Williams, P.A. Raman spectroscopy of the basic copper chloride minerals atacamite and paratacamite—Implications for the study of copper, brass and bronze objects of archeological significanc. J. Raman Spectrosc. 2002, 33, 801–806. [Google Scholar] [CrossRef]
- Frost, R. Raman spectroscopy of selected copper minerals of significance in corrosion. Spectrochim. Acta A 2003, 59, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Tomasini, E.; Cárcamo, J.; Castellanos Rodríguez, D.M.; Careaga, V.; Gutiérrez, S.; Rúa Landa, C.; Sepúlveda, M.; Guzman, F.; Pereira, M.; Siracusano, G.; et al. Characterization of pigments and binders in a mural painting from the Andean church of San Andrés de Pachama (northernmost of Chile). Herit. Sci. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Tomasini, E.; Costantini, I.; Careaga, V.; Rua Landa, C.; Castro, K.; Madariaga, J.M.; Maier, M.S.; Siracusano, G. Identification of pigments in a 17th century mural painting (Bolivia) using spectroscopic techniques with imaging methods. J. Cult. Herit. 2023. submitted. [Google Scholar] [CrossRef]
- Tomasini, E.P.; Halac, E.B.; Reinoso, M.; Di Liscia, E.J.; Maier, M.S. Micro-Raman spectroscopy of carbon-based black pigments. J. Raman Spectrosc. 2012, 43, 1671–1675. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H.; Firth, S. Raman spectroscopy as a means for the identification of plattnerite (PbO2), of lead pigments and of their degradation products. Analyst 2001, 126, 222–227. [Google Scholar] [CrossRef]
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J. Raman spectroscopic library of natural and synthetic pigments (pre-∼ 1850 AD), Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- Seldes, A.M.; Burucúa, J.E.; Maier, M.S.; Gonzalo, A.; Jáuregui, A.; Siracusano, G. Blue Pigments in South American Painting (1610-1780). J. Am. Inst. Conserv. 1999, 38, 100–123. [Google Scholar]
- de La Roja, J.M.; Baonza, V.G. Application of Raman microscopy to the characterization of different verdigris variants obtained using recipes from old treatises. Spectrochim. Acta A 2017, 68, 1120–1125. [Google Scholar] [CrossRef]
- Buse, J.; Otero, V. New Insights into Synthetic Copper Greens: The Search for Specific Signatures by Raman and Infrared Spectroscopy for Their Characterization in Medieval Artworks. Herit. Sci. 2019, 2, 1614–1629. [Google Scholar] [CrossRef]
- Aguirre, J.M.; Adamo, G.; Giraldo, O. Simple Route for the Synthesis of Copper Hydroxy Salts. J. Braz. Chem. Soc. 2011, 22, 546–551. [Google Scholar] [CrossRef]
- Yoder, C.H.; Bushong, E.; Liu, X.; Weidner, V.; McWilliams, P.; Martin, K.; Lorgunpai, J.; Haller, J.; Schaeffer, R.W. The synthesis and solubility of the copper hydroxyl nitrates: Gerhardtite, rouaite and likasite. Mineral. Mag. 2010, 74, 433–440. [Google Scholar] [CrossRef]
- Pollard, A.M.; Thomas, R.G.; Willams, P.A. The stabilities of antlefite and Cu3SO4(OH)4.2H2O: Their formation and relationships to other copper (II)sulfate minerals. Mineral. Mag. 1992, 56, 359–365. [Google Scholar] [CrossRef]
- Scott, D.A. A Review of Copper Chlorides and Related Salts in Bronze Corrosion and as Painting Pigments. Stud. Conserv. 2000, 45, 39–53. [Google Scholar] [CrossRef]
- Yong, L. Copper trihydroxychlorides as pigments in China. Stud. Conserv. 2012, 57, 106–111. [Google Scholar] [CrossRef]
- Coccato, A.; Moens, L. On the stability of mediaeval inorganic pigments: A literature review of the effect of climate, material selection, biological activity, analysis and conservation treatments. Herit. Sci. 2017, 5, 1–25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).