Experimental Study of Chalconatronite: From Its Identification to the Treatment of Copper Alloy Objects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corpus
2.1.1. Egyptian Objects
2.1.2. Synthesized Chalconatronite
2.2. Analytical Techniques
2.2.1. Scanning Electron Microscopy Coupled to Energy Dispersive X-ray Spectroscopy (SEM-EDS)
2.2.2. X-ray Fluorescence
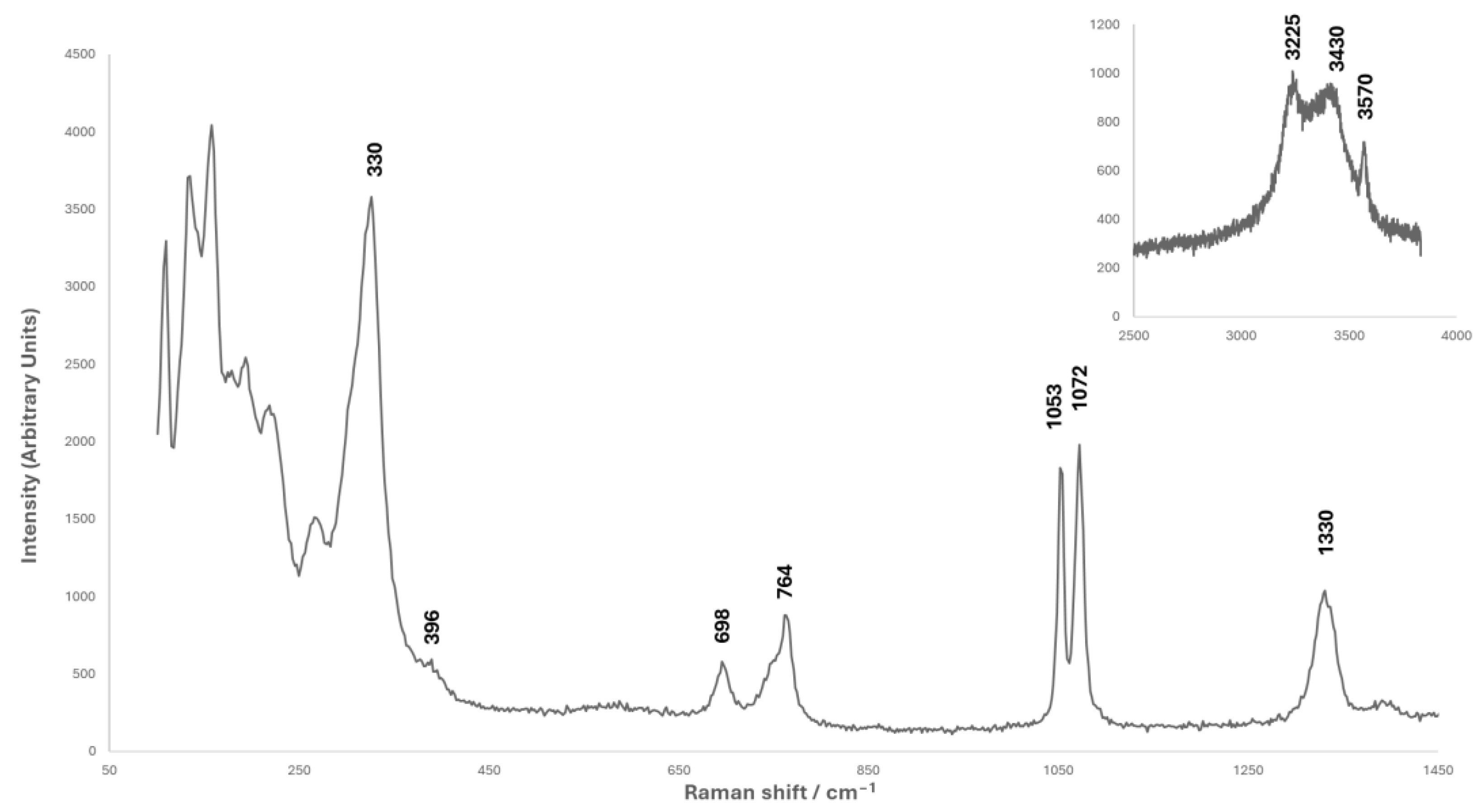
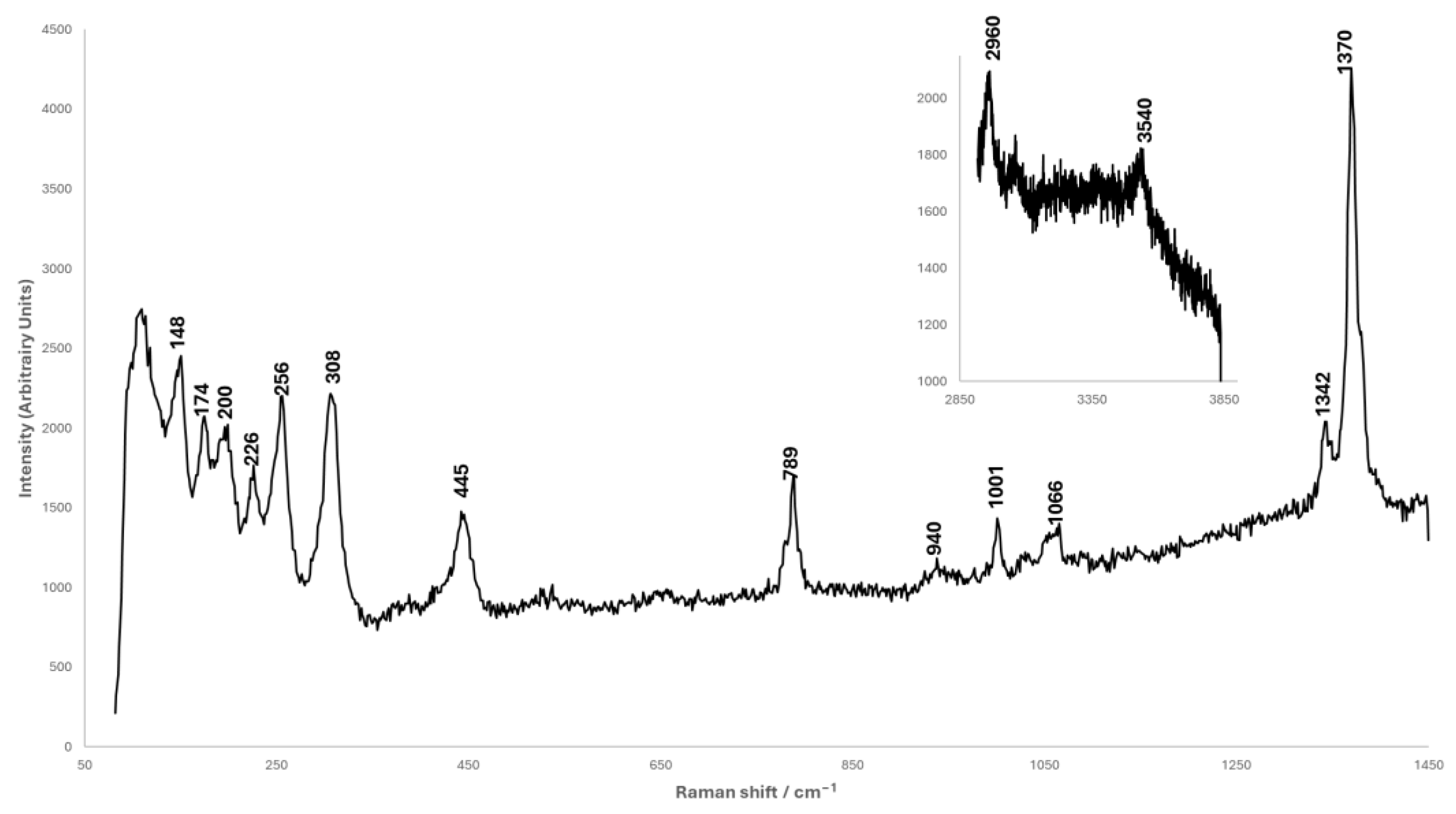
2.2.3. Raman Spectroscopy
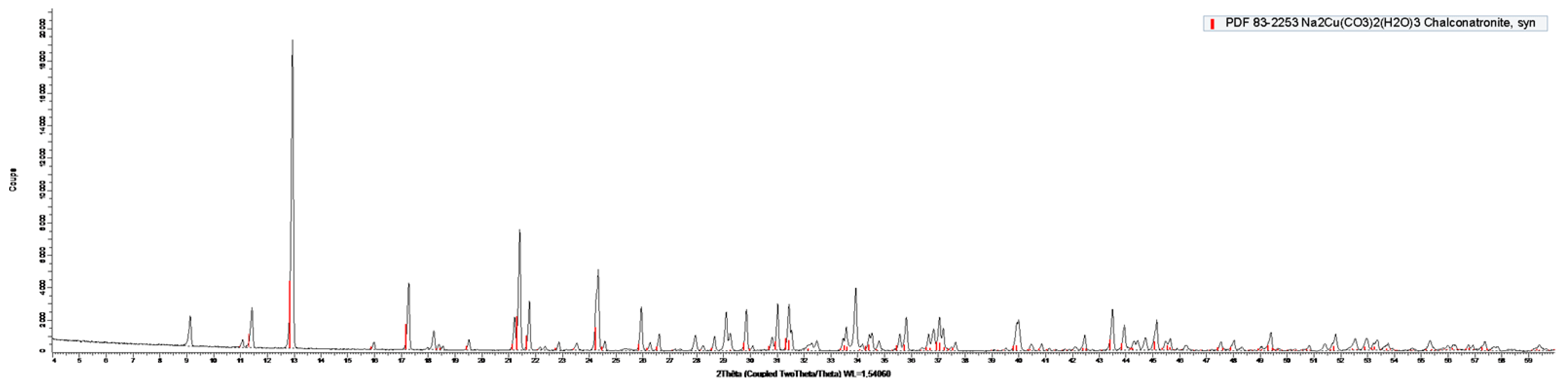
2.2.4. X-ray Diffraction
2.2.5. Infrared Spectroscopy
2.2.6. Conductivity, pH and Temperature Measurements
2.2.7. Sodium Titration by Atomic Absorption Spectrometry
3. Results
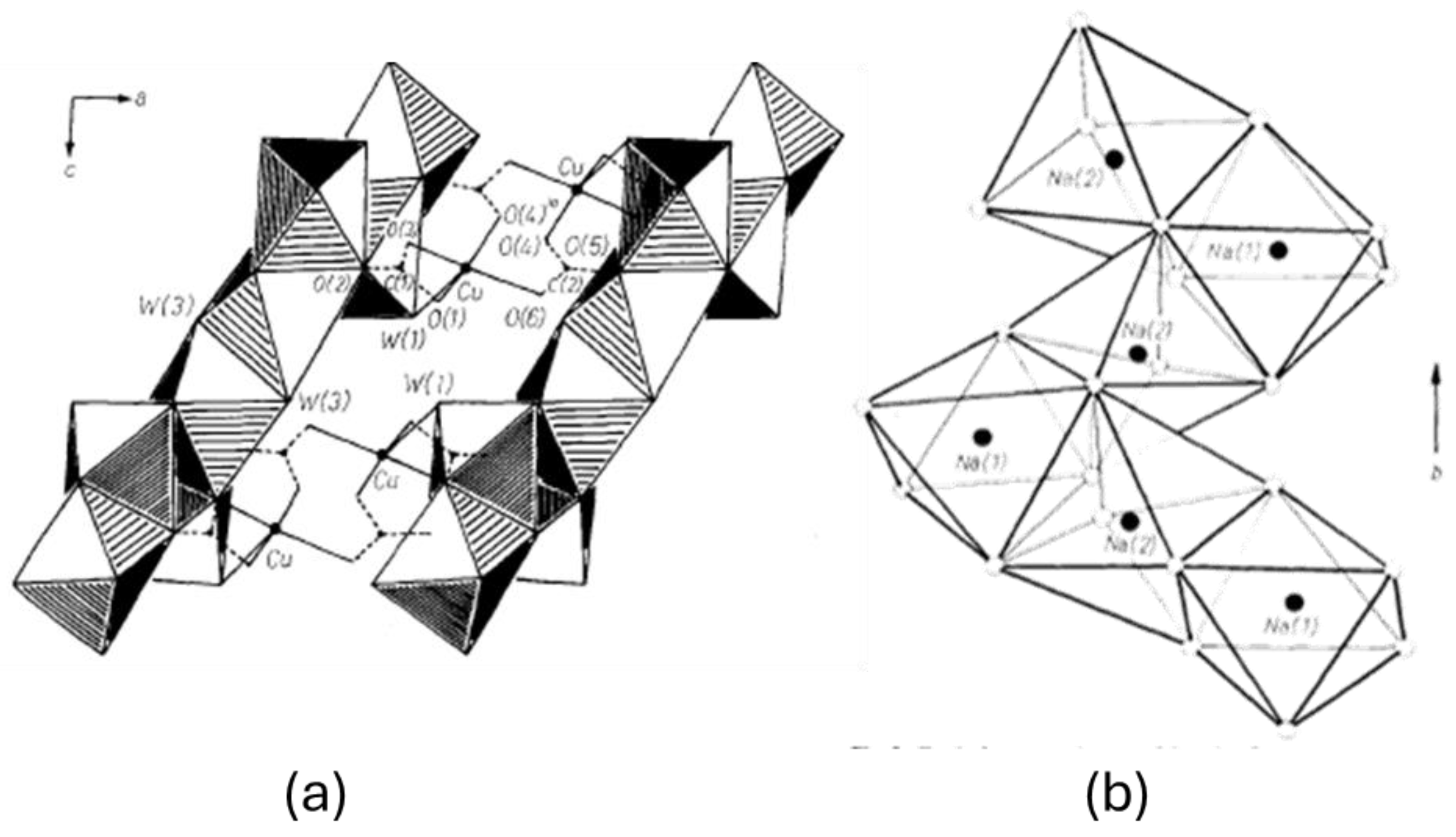
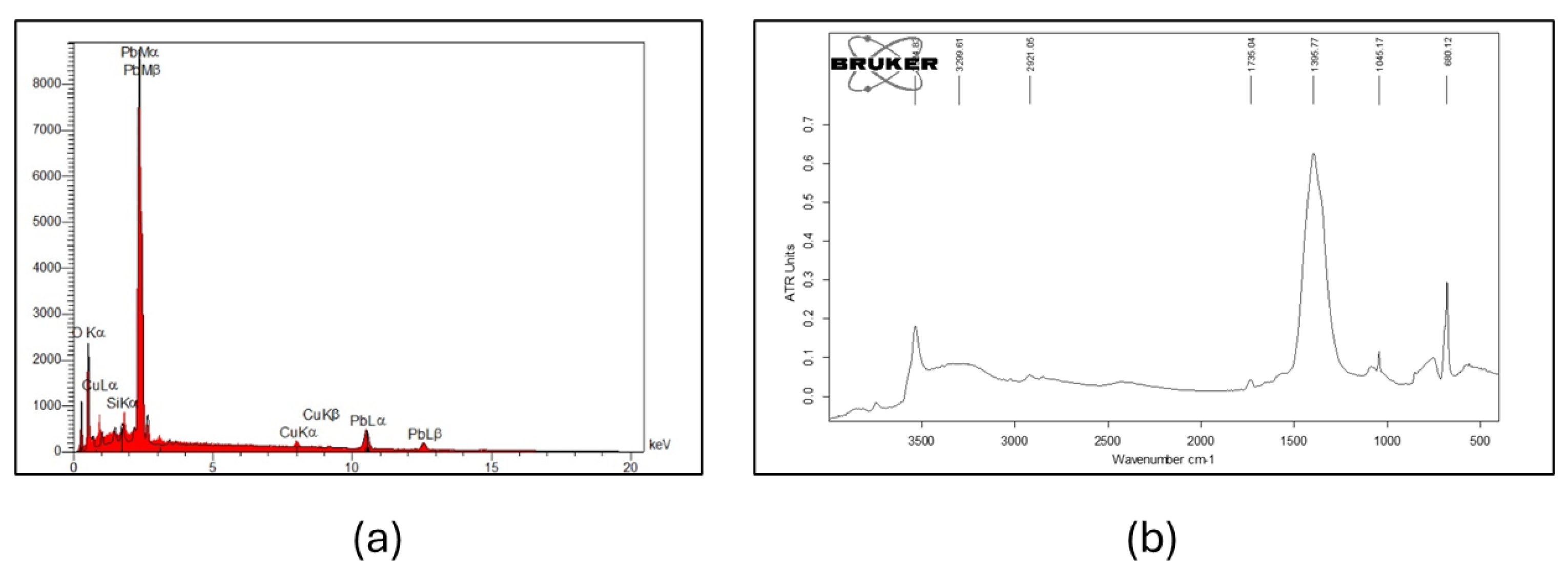
3.1. Analysis of Corrosion Products
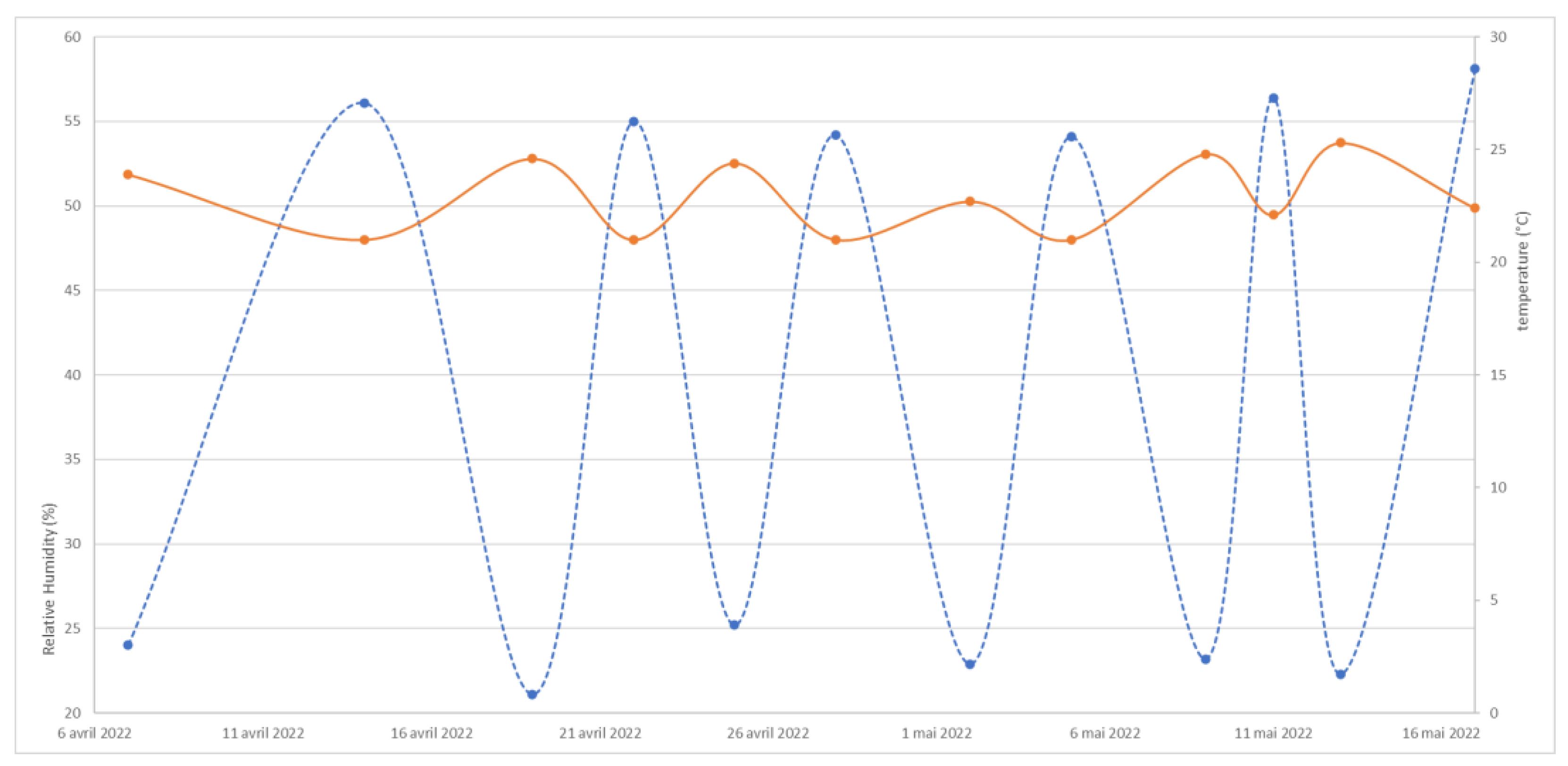
3.2. Stability of the Objects
3.3. Synthesis of the Chalconatronite
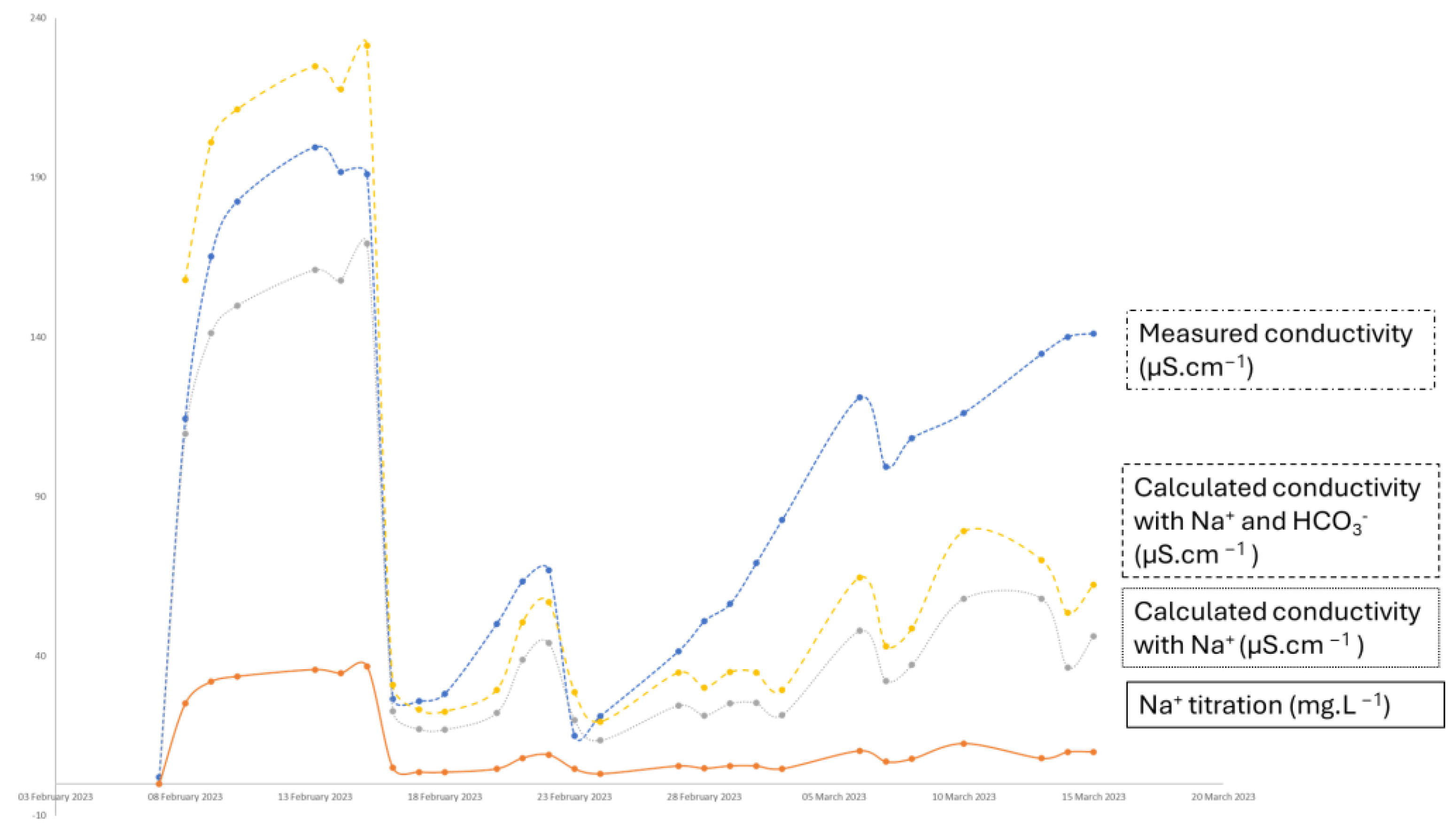
3.4. Dissolution of the Chalconatronite
3.5. Curative Treatment
3.5.1. Harpocrate Statuette
3.5.2. Isis Statuette
3.5.3. Preventive Conservation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- MacLeod, I.D. Conservation of Corroded Copper Alloys: A Comparison of New and Traditional Methods for Removing Chloride Ions. Stud. Conserv. 1987, 32, 25–40. [Google Scholar] [CrossRef]
- Pia, C.M. Sustainable Conservation of Bronze Artworks: Advanced Research in Materials Science. In the Artistry in Bronze. The Greeks and Their Legacy, 1st ed.; Daehner, J.-M., Lapatin, K., Spinelli, A., Eds.; J. Paul Getty Trust: Los Angeles, CA, USA, 2017; Chapter 35. [Google Scholar]
- Scott, D.-A. Bronze Disease: A Review of Some Chemical Problems and the Role of Relative Humidity. J. Am. Inst. Conserv. 1990, 29, 193–206. [Google Scholar] [CrossRef]
- Handbook of Mineralogy. Available online: https://www.handbookofmineralogy.org/pdfs/chalconatronite.pdf (accessed on 10 April 2024).
- Gettens, R.J.; Frondel, C. Chalconatronite: An alteration product on some ancient Egyptian bronzes. Stud. Conserv. 1955, 2, 64–75. [Google Scholar] [CrossRef]
- Chiavari, C.; Martini, C.; Montalbani, S.; Franzoni, E.; Bignozzi, M.C.; Passeri, M.C. The bronze panel (paliotto) of San Moise in Venice: Materials and causes of deterioration. Mater. Corros. 2016, 67, 141–151. [Google Scholar] [CrossRef]
- Liggins, F.; Vichi, A.; Liu, W.; Hogg, A.; Kogou, S.; Chen, J.; Liang, H. Hyperspectral imaging solutions for the non-invasive detection and automated mapping of copper trihydroxychlorides in ancient bronze. Herit. Sci. 2022, 10, 142. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Wu, N.; Liu, S.; Chen, J. A new application of Fiber optics reflection spectroscopy (FORS): Identification of “bronze disease” induced corrosion products on ancient bronzes. J. Cult. Herit. 2001, 49, 19–27. [Google Scholar] [CrossRef]
- Eggert, G.; Fischer, A.; Dinnebier, R. One heritage corrosion product less: Basic sodium copper carbonate. Herit. Sci. 2016, 4, 1–5. [Google Scholar]
- Wang, Q. Bronzes from the Sacred Animal Necropolis at Saqqara, Egypt: A study of the metals and corrosion. Br. Mus. Tech. Res. Bull. 2009, 3, 72–83. [Google Scholar]
- Eggert, G. Copper and bronze in Art, and the search of rare corrosion products. Heritage 2023, 6, 1768–1784. [Google Scholar] [CrossRef]
- Mosset, A.; Bonnet, J.; Galy, J. Structure cristalline de la chalconatronite synthetique: Na2Cu(CO3)2·3H2O. Z. Für Krist.-Cryst. Mater. 1978, 148, 165–178. [Google Scholar] [CrossRef]
- Schlüter, J.; Hamburg, D.-P. Juangodoyite, Na2Cu(CO3)2, a new mineral from the Santa Rosa mine, Atacama desert, Chile. Neues Jahrb. Für Mineral.–Abh. Band 2005, 182, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Horis, C.V.; Vint, J.A. Chalconatronie: A by-product of conservation. Stud. Conserv. 1982, 27, 185–186. [Google Scholar]
- ASTM. X-ray Diffraction Powder Data; File nos. 10-442 and 22-1458; ASTM: West Conshohocken, PA, USA, 1989. [Google Scholar]
- Oddy, W.A.; Hughes, M.J. The stabilization of ‘active’ bronze and iron antiquities by the use of sodium sesquicarbonate. Stud. Conserv. 1970, 15, 183–189. [Google Scholar]
- Païn, S.; Bertholon, R.; Lacoudre, N. La dechloruration des alliages cuivreux par electrolyse à faible polarisation dans le sesquicarbonate de sodium. Stud. Conserv. 1991, 36, 33–43. [Google Scholar] [CrossRef]
- Scott, D. Chalconatronite: A Sodium-Copper Carbonate, Synthesis and use of chalconatronite. Chalconatronite as a corrosion product. In Copper and Bronze in Art: Corrosion, Colorants, Conservation; J. Paul Guetty Trust: Los Angeles, CA, USA, 2002; p. 120. [Google Scholar]
- Argyropoulos, V.; Mavroforaki, S.; Giannoulaki, M. New approaches in stabilizing chloride-contaminated ancient bronzes using corrosion inhibitors and/or electrochemical methods to preserve information in the patinas. In Artistry in Bronze the Greeks and Their Legacy: Acta of the XIX International Congress on Ancient Bronzes, 1st ed.; Daehner, J., Lapatin, K., Spinelli, A., Eds.; J. Paul Getty Trust: Los Angeles, CA, USA, 2017. [Google Scholar]
- Organ, R.M. Corrosion, use it or lose it? In Proceedings of the Archeometallurgia, Ricerche e Prospettive, Atti del Colloquio Internazionale di Archeometallurgia, Bologna, Italy, 18–21 October 1988; pp. 423–437. [Google Scholar]
- Berthelon, R. La Limite de la Surface D’origine des Objets Métalliques Archéologiques. Caractérisation, Localisation et Approche des Mécanismes de Conservation. Ph.D. Thesis, Panthéon-Sorbonne, Paris, France, 20 December 2000. [Google Scholar]
- Fischer, A.; Eggert, G.; Dinnebier, R.; Runcevski, T. When Glass and Metal Corrode Together, V: Sodium copper Formate. Stud. Conserv. 2017, 63, 342–355. [Google Scholar] [CrossRef]
- Scott, D.; Dobb, L.S. Examination, conservation and analysis of a gilded Egyptian Bronze Osiris. J. Cult. Herit. 2002, 3, 333–345. [Google Scholar] [CrossRef]
- El Baz, F. The Geology of Egypt: An Annotated Bibliography, 1st ed.; Brill: Leiden, The Netherlands, 1984; p. 337. [Google Scholar]
- Fischer, A.; Eggert, G.; Stelzner, J. When Glass and Metal Corrode Together, V: Chalconatronite. Stud. Conserv. 2019, 65, 152–159. [Google Scholar] [CrossRef]
- Pollard, A.M.; Thomas, R.G.; Williams, P.A. Mineralogical changes arising from the use of aqueous sodium carbonate solutions for the treatment of archaeological copper objects. Stud. Conserv. 1990, 35, 148–152. [Google Scholar] [CrossRef]
- Pollard, A.M.; Thomas, R.G.; Williams, P.A. The synthesis and composition of georgeite and its reactions to form other secondary copper(II) carbonates. Mineral. Mag. 1991, 55, 163–166. [Google Scholar] [CrossRef]
- Leyssens, K.; Adriaens, M.; Pantos, E.; Degrigny, C. Study of corrosion potential measurements as a means to monitor the storage and stabilisation processes of archaeological copper artefacts. In Proceedings of Metal 2004; National Museum of Australia: Canberra, Australia, 2004. [Google Scholar]
- Trentelman, K.; Stodulski, L.; Back, M.; Stock, S.; Strahan, D.; Drews, A.R.; O’Neill, A.; Weber, W.H.; Chen, A.E.; Garrett, S.J. The Characterization of a new pale blue corrosion product found on copper alloy artifacts. Stud. Conserv. 2002, 47, 217–227. [Google Scholar] [CrossRef]
- Boccia Paterakis, A. The hidden secrets of copper alloy artifacts in the Athenian Agora. Am. Inst. Conserv. Hist. Artist. Work. 1996, 6, 70–76. [Google Scholar]
- Thickett, D.; Oldyha, M. Note on the identification of an unusual pale blue corrosion product from Egyptian copper alloy articfacts. Stud. Conserv. 2000, 45, 63–67. [Google Scholar] [CrossRef]
- Paterakis, A.B. The Formation of Acetate Corrosion on Bronze Antiquities: Characterisation and Conservation. Ph.D. Thesis, University College London, London, UK, 2010. [Google Scholar]
- Gibson, L.-T.; Watt, C.-M. Acetic and formic acids emitted from wood samples and their effect on selected materials in museum environments. Corros. Sci. 2010, 52, 172–178. [Google Scholar] [CrossRef]
- Eggert, G.; Fischer, A. The formation of formates: A review of metal formates on heritage objects. Herit. Sci. 2021, 9, 26. [Google Scholar] [CrossRef]
- Sengupta, A.K.; Nandi, A.K. Complex carbonates of copper(II). J. Inorg. Nucl. Chem. 1974, 36, 2479–2484. [Google Scholar] [CrossRef]
- Gröger, M. Uber die Alkalikupfercarbonate. Berichte Des Dtsch. Chem. Ges. 1901, 34, 429–432. [Google Scholar] [CrossRef]
- RRUFF. Available online: https://rruff.info/)/R160004 (accessed on 10 April 2024).
- Ito, K.; Bernstein, H.J. The vibrational spectra of the formate, acetate and oxalate ions. Can. J. Chem. 1956, 34, 170–178. [Google Scholar] [CrossRef]
- Paterakis Boccia, A. The Influence of Conservation Treatments and Environmental Storage Factors on Corrosion of Copper Alloys in the Ancient Athenian Agora. J. Am. Inst. Conserv. 2003, 42, 313–339. [Google Scholar] [CrossRef]
- Fontaine, C.; Lemoine, S.; Pelé-Meziani, C.; Guilminot, E. The use of gels in localized dechlorination treatments of metallic cultural heritage objects. Herit. Sci. 2022, 10, 117. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelé-Meziani, C.; Raimon, A.; Mevellec, J.-Y.; Guilminot, E. Experimental Study of Chalconatronite: From Its Identification to the Treatment of Copper Alloy Objects. Heritage 2024, 7, 2866-2879. https://doi.org/10.3390/heritage7060135
Pelé-Meziani C, Raimon A, Mevellec J-Y, Guilminot E. Experimental Study of Chalconatronite: From Its Identification to the Treatment of Copper Alloy Objects. Heritage. 2024; 7(6):2866-2879. https://doi.org/10.3390/heritage7060135
Chicago/Turabian StylePelé-Meziani, Charlène, Aymeric Raimon, Jean-Yves Mevellec, and Elodie Guilminot. 2024. "Experimental Study of Chalconatronite: From Its Identification to the Treatment of Copper Alloy Objects" Heritage 7, no. 6: 2866-2879. https://doi.org/10.3390/heritage7060135
APA StylePelé-Meziani, C., Raimon, A., Mevellec, J.-Y., & Guilminot, E. (2024). Experimental Study of Chalconatronite: From Its Identification to the Treatment of Copper Alloy Objects. Heritage, 7(6), 2866-2879. https://doi.org/10.3390/heritage7060135






