Refining the Production Date of Historical Palestinian Garments Through Dye Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Textiles and Samples
2.2. Dye Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cardon, D. Natural Dyes: Sources, Tradition, Technology and Science; Archetype Publications Ltd.: London, UK, 2007. [Google Scholar]
- Tamburini, D.; Sabatini, F.; Berbers, S.; van Bommel, M.R.; Degano, I. An Introduction and Recent Advances in the Analytical Study of Early Synthetic Dyes and Organic Pigments in Cultural Heritage. Heritage 2024, 7, 1969–2010. [Google Scholar] [CrossRef]
- Hagan, E.; Poulin, J. Statistics of the early synthetic dye industry. Herit. Sci. 2021, 9, 33. [Google Scholar] [CrossRef]
- Travis, A.S. The Rainbow Makers: The Origins of the Synthetic Dyestuffs Industry in Western Europe; Lehigh University Press: Bethlehem, PA, USA, 1993. [Google Scholar]
- Beer, J.J. The Emergence of the German Dye Industry; University of Illinois Press: Champaign, IL, USA, 1959. [Google Scholar]
- Simon, C. The transition from natural dyestuffs to synthetic dyestuffs: The case of Basel, 1850–1940. In Natural Dyestuffs and Industrial Culture in Europe, 1750–1880; Fox, R., Nieto-Galan, A., Eds.; Science History Publications: Canton, MA, USA, 1999; pp. 313–338. [Google Scholar]
- Tamburini, D.; Klink-Hoppe, Z.; McCarthy, B. New insights into the dyes of Central Asian ikat textiles. J. Cult. Herit. 2024, 66, 343–355. [Google Scholar] [CrossRef]
- Do, K.L.; Mushtaq, A.; Liu, J.; Zhao, F.; Su, M. Unveiling the Use of Natural and Early Synthetic Dyes in Indonesian Historical Silk Textiles. Fibers Polym. 2024, 6, 2233–2244. [Google Scholar] [CrossRef]
- Tamburini, D.; Kim-Marandet, M.; Kim, S.-A. Dye Identification in Mounting Textiles of Traditional Korean Paintings from the Late Joseon Dynasty. Heritage 2023, 6, 44–66. [Google Scholar] [CrossRef]
- Tamburini, D.; Dyer, J.; Cartwright, C.; Green, A. Changes in the production materials of Burmese textiles in the 19th century–dyes, mordants and fibres of Karen garments from the British Museum’s collection. Herit. Sci. 2023, 11, 150. [Google Scholar] [CrossRef]
- Tamburini, D.; Dyer, J.; Cartwright, C. First evidence and characterisation of rare chrome-based colourants used on 19th-century textiles from Myanmar. Dye. Pigment. 2023, 218, 111472. [Google Scholar] [CrossRef]
- Petroviciu, I.; Teodorescu, I.C.; Vasilca, S.; Albu, F. Transition from Natural to Early Synthetic Dyes in the Romanian Traditional Shirts Decoration. Heritage 2023, 6, 505–523. [Google Scholar] [CrossRef]
- Petroviciu, I.; Teodorescu, I.; Vasilca, S.; Albu, F.; Medvedovici, A. Liquid chromatography as analytical tool for the study of natural and early synthetic dyes in traditional Saxon textiles. Herit. Sci. 2023, 11, 164. [Google Scholar] [CrossRef]
- Longoni, M.; Gavazzi, M.; Monti, D.; Bruni, S. Early synthetic textile dyes of the late 19th century from the “Primo Levi” Chemistry Museum (Rome): A multi-technique analytical investigation. J. Cult. Herit. 2023, 59, 131–139. [Google Scholar] [CrossRef]
- Vermeulen, M.; Tamburini, D.; McGeachy, A.C.; Meyers, R.D.; Walton, M.S. Multiscale characterization of shellfish purple and other organic colorants in 20th-century traditional enredos from Oaxaca, Mexico. Dye. Pigment. 2022, 206, 110663. [Google Scholar] [CrossRef]
- Chen, V.J.; Smith, G.D.; Whitaker, M.R.; von Rabenau, B. Identification of Red Dyes in Selected Textiles from Chin and Karen Ethnic Groups of Myanmar by LC-DAD-ESI-MS. In Dyes in History and Archaeology 33/34; Kirby, J., Ed.; Archetype Publications: London, UK, 2021; pp. 92–101. [Google Scholar]
- Cesaratto, A.; Luo, Y.-B.; Smith, H.D.; Leona, M. A timeline for the introduction of synthetic dyestuffs in Japan during the late Edo and Meiji periods. Herit. Sci. 2018, 6, 22. [Google Scholar] [CrossRef]
- Tamburini, D.; Breitung, E.; Mori, C.; Kotajima, T.; Clarke, M.L.; McCarthy, B. Exploring the transition from natural to synthetic dyes in the production of 19th-century Central Asian ikat textiles. Herit. Sci. 2020, 8, 114. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Zhao, F.; Peng, Z.; Wang, S. Identification of early synthetic dyes in historical Chinese textiles of the late nineteenth century by high-performance liquid chromatography coupled with diode array detection and mass spectrometry. Color. Technol. 2016, 132, 177–185. [Google Scholar] [CrossRef]
- Chen, V.J.; Smith, G.D.; Holden, A.; Paydar, N.; Kiefer, K. Chemical analysis of dyes on an Uzbek ceremonial coat: Objective evidence for artifact dating and the chemistry of early synthetic dyes. Dye. Pigment. 2016, 131, 320–332. [Google Scholar] [CrossRef]
- Degano, I.; Ribechini, E.; Modugno, F.; Colombini, M.P. Analytical Methods for the Characterization of Organic Dyes in Artworks and in Historical Textiles. Appl. Spectrosc. Rev. 2009, 44, 363–410. [Google Scholar] [CrossRef]
- Grillini, F.; de Ferri, L.; Pantos, G.A.; George, S.; Veseth, M. Reflectance imaging spectroscopy for the study of archaeological pre-Columbian textiles. Microchem. J. 2024, 200, 110168. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Forleo, T.; Pojana, G.; Lagioia, G.; Mangone, A.; Giannossa, L.C. Characterization of silk-cotton and wool-cotton blends pattern books by fibre optic reflectance spectroscopy. The booming market of first synthetic textile dyes in early 20th century. Microchem. J. 2022, 175, 107178. [Google Scholar] [CrossRef]
- Ding, L.; Gong, T.; Wang, B.; Yang, Q.; Liu, W.; Pemo, R.; Metok, T. Non-invasive study of natural dyes in textiles of the Qing Dynasty using fiber optic reflectance spectroscopy. J. Cult. Herit. 2021, 47, 69–78. [Google Scholar] [CrossRef]
- Tamburini, D.; Dyer, J. Fibre optic reflectance spectroscopy and multispectral imaging for the non-invasive investigation of Asian colourants in Chinese textiles from Dunhuang (7th-10th century AD). Dye. Pigment. 2019, 162, 494–511. [Google Scholar] [CrossRef]
- Selberg, S.; Vanker, E.; Peets, P.; Wright, K.; Tshepelevitsh, S.; Pagano, T.; Vahur, S.; Herodes, K.; Leito, I. Non-invasive analysis of natural textile dyes using fluorescence excitation-emission matrices. Talanta 2023, 252, 123805. [Google Scholar] [CrossRef] [PubMed]
- Ciccola, A.; McClure, K.R.; Serafini, I.; Vincenti, F.; Montesano, C.; Gentili, A.; Curini, R.; Favero, G.; Postorino, P. The 20th century and its new colours: Investigating the molecular structures of historical synthetic dyes using Raman spectroscopy. J. Raman Spectrosc. 2024, 55, 324–335. [Google Scholar] [CrossRef]
- Haddad, A.; Nakie-Miller, T.; Jenks, J.B.; Kowach, G. Andy Warhol and His Amazing Technicolor Shoes: Characterizing the Synthetic Dyes Found in Dr. Ph. Martin’s Synchromatic Transparent Watercolors and Used in À la Recherche du Shoe Perdu. Colorants 2023, 2, 1–21. [Google Scholar] [CrossRef]
- Sessa, C.; Steuer, C.; Quintero Balbas, D.; Sciutto, G.; Prati, S.; Stege, H. Analytical studies on commercial artists’ colour charts from Das Deutsche Farbenbuch (1925)—Identification of synthetic and natural organic colourants by Raman microscopy, surface-enhanced Raman spectroscopy and metal underlayer ATR-FTIR spectroscopy. Herit. Sci. 2022, 10, 109. [Google Scholar] [CrossRef]
- Quintero Balbas, D.; Lanterna, G.; Cirrincione, C.; Ricci, M.; Becucci, M.; Fontana, R.; Striova, J. Non-invasive identification of turmeric and saffron dyes in proteinaceous textile fibres using Raman spectroscopy and multivariate analysis. J. Raman Spectrosc. 2022, 53, 593. [Google Scholar] [CrossRef]
- Pirok, B.W.J.; den Uijl, M.J.; Moro, G.; Berbers, S.V.J.; Croes, C.J.M.; van Bommel, M.R.; Schoenmakers, P.J. Characterization of Dye Extracts from Historical Cultural-Heritage Objects using State-of-the-Art Comprehensive Two-Dimensional Liquid Chromatography and Mass Spectrometry with Active Modulation and Optimized Shifting Gradients. Anal. Chem. 2019, 91, 3062–3069. [Google Scholar] [CrossRef]
- Souto, C. Analysis of Early Synthetic Dyes with HPLC-DAD-MS-An Important Database for Analysis of Colorants Used in Cultural Heritage. Master’s Thesis, Univerisy of Lisbon, Lisbon, Portugal, 2010. [Google Scholar]
- Tamburini, D. On the reliability of historic books as sources of reference samples of early synthetic dyes–The case of “The Coal Tar Colours of the Farbwerke vorm. Meister, Lucius & Brüning, Höchst on the Main, Germany–A General Part” (1896). Dye. Pigment. 2024, 221, 111796. [Google Scholar] [CrossRef]
- Tamburini, D. Investigating Asian colourants in Chinese textiles from Dunhuang (7th–10th century AD) by high performance liquid chromatography tandem mass spectrometry–Towards the creation of a mass spectra database. Dye. Pigment. 2019, 163, 454–474. [Google Scholar] [CrossRef]
- Degano, I. Liquid chromatography: Current applications in Heritage Science and recent developments. Phys. Sci. Rev. 2019, 4, 20180009. [Google Scholar] [CrossRef]
- Vasileiadou, A.; Karapanagiotis, I.; Zotou, A. Determination of Tyrian purple by high performance liquid chromatography with diode array detection. J. Chromatogr. A 2016, 1448, 67–72. [Google Scholar] [CrossRef]
- Lech, K.; Wilicka, E.; Witowska-Jarosz, J.; Jarosz, M. Early synthetic dyes–a challenge for tandem mass spectrometry. J. Mass Spectrom. 2013, 48, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, D.; Shimada, C.M.; McCarthy, B. The molecular characterization of early synthetic dyes in E. Knecht et al’s textile sample book “A Manual of Dyeing” (1893) by high performance liquid chromatography-Diode array detector-Mass spectrometry (HPLC-DAD-MS). Dye. Pigment. 2021, 190, 109286. [Google Scholar] [CrossRef]
- Berbers, S.V.J.; Gaibor, A.N.P.; Ligterink, F.; Neevel, J.G.; Reissland, B.; van der Werf, I.D. Shades of violet: Study of the compositional variability of historical Methyl violet dyes. J. Cult. Herit. 2024, 66, 464–475. [Google Scholar] [CrossRef]
- Sabatini, F.; Degano, I.; van Bommel, M. Investigating the in-solution photodegradation pathway of Diamond Green G by chromatography and mass spectrometry. Color. Technol. 2021, 137, 456–467. [Google Scholar] [CrossRef]
- Lech, K.; Nawała, J.; Popiel, S. Mass Spectrometry for Investigation of Natural Dyes in Historical Textiles: Unveiling the Mystery behind Safflower-Dyed Fibers. J. Am. Soc. Mass Spectrom. 2021, 32, 2552–2566. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Newsome, G.A.; Janssens, K. High-Resolution Mass Spectrometry and Nontraditional Mass Defect Analysis of Brominated Historical Pigments. Anal. Chem. 2021, 93, 14851–14858. [Google Scholar] [CrossRef]
- Degano, I.; Sabatini, F.; Braccini, C.; Colombini, M.P. Triarylmethine dyes: Characterization of isomers using integrated mass spectrometry. Dye. Pigment. 2019, 160, 587–596. [Google Scholar] [CrossRef]
- Weir, S. Palestinian Costume; British Museum Publications: London, UK, 1989. [Google Scholar]
- Ghnaim, W. Tatreez in Time-The Memory, Meaning, and Makers of Palestinian Embroidery; The Metropolitan Museum of Art: New York, NY, USA, 2024. [Google Scholar]
- Skinner, M. Palestinian Embroidery Motifs: A Treasury of Stitches 1850–1950; Melisende Publishing and Rimal Publications: Woodbridge, CT, USA, 2007. [Google Scholar]
- Tamburini, D.; Cartwright, C.R.; Pullan, M.; Vickers, H. An investigation of the dye palette in Chinese silk embroidery from Dunhuang (Tang dynasty). Archaeol. Anthropol. Sci. 2019, 11, 1221–1239. [Google Scholar] [CrossRef]
- Frignani, E.; D’Eusanio, V.; Grandi, M.; Pigani, L.; Roncaglia, F. A Continuous Extraction Protocol for the Characterisation of a Sustainably Produced Natural Indigo Pigment. Life 2024, 14, 59. [Google Scholar] [CrossRef]
- Mouri, C.; Laursen, R. Identification of anthraquinone markers for distinguishing Rubia species in madder-dyed textiles by HPLC. Microchim. Acta 2012, 179, 105–113. [Google Scholar] [CrossRef]
- Torgan Güzel, E. Ottoman palace weavings between different periods: Material characterization, comparison and suggestions for conservation. Herit. Sci. 2023, 11, 179. [Google Scholar] [CrossRef]
- Shahid, M.; Wertz, J.; Degano, I.; Aceto, M.; Khan, M.I.; Quye, A. Analytical methods for determination of anthraquinone dyes in historical textiles: A review. Anal. Chim. Acta 2019, 1083, 58–87. [Google Scholar] [CrossRef] [PubMed]
- Hagan, E.; Castro-Soto, I.; Breault, M.; Poulin, J. The lightfastness of early synthetic organic dyes. Herit. Sci. 2022, 10, 50. [Google Scholar] [CrossRef]
- Knecht, E.; Rawson, C.; Loewenthal, R. A Manual of Dyeing: For the Use of Practical Dyers, Manufacturers, Students, and All Interested in the Art of Dyeing (Volume I); Charles Griffin and Company: London, UK, 1893; Volume 1. [Google Scholar]
- Welham, R.D. The Early History of the Synthetic Dye Industry. J. Soc. Dye. Colour. 1963, 79, 146–152. [Google Scholar] [CrossRef]
- Knecht, E.; Rawson, C.; Loewenthal, R. A Manual of Dyeing: For the Use of Practical Dyers, Manufacturers, Students, and All Interested in the Art of Dyeing (Volume II); Charles Griffin and Company: London, UK, 1893; Volume 2. [Google Scholar]
- Chen, V.; Minto, R.; Manicke, N.; Smith, G. Structural elucidation of two Congo red derivatives on dyed historical objects indicative of formaldehyde exposure and the potential for chemical fading. Dye. Pigment. 2022, 201, 110173. [Google Scholar] [CrossRef]
- Knecht, E.; Rawson, C.; Loewenthal, R. A Manual of Dyeing: For the Use of Practical Dyers, Manufacturers, Students, and All Interested in the Art of Dyeing (Specimen of Dyed Fabrics); Charles Griffin and Company: London, UK, 1893. [Google Scholar]
- Mouri, C.; Mozaffarian, V.; Zhang, X.; Laursen, R. Characterization of flavonols in plants used for textile dyeing and the significance of flavonol conjugates. Dye. Pigment. 2014, 100, 135–141. [Google Scholar] [CrossRef]
- Liu, J.; Ji, L.; Chen, L.; Pei, K.; Zhao, P.; Zhou, Y.; Zhao, F. Identification of yellow dyes in two wall coverings from the Palace Museum: Evidence for reconstitution of artifacts. Dye. Pigment. 2018, 153, 137–143. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Cuoco, G.; Mathe, C.; Vieillescazes, C. Liquid chromatographic analysis of flavonol compounds in green fruits of three Rhamnus species used in Stil de grain. Microchem. J. 2014, 115, 130–137. [Google Scholar] [CrossRef]
- Sabatini, F.; La Nasa, J.; Degano, I.; Campanella, B.; Legnaioli, S.; Saccani, I.; Modugno, F. Fluorescent Paints in Contemporary Murals: A Case Study. Heritage 2023, 6, 5689–5699. [Google Scholar] [CrossRef]
- Ferreira, B.R.V.; Correa, D.N.; Eberlin, M.N.; Vendramini, P.H. Fragmentation Reactions of Rhodamine B and 6G as Revealed by High Accuracy Orbitrap Tandem Mass Spectrometry. J. Braz. Chem. Soc. 2017, 28, 136–142. [Google Scholar] [CrossRef]

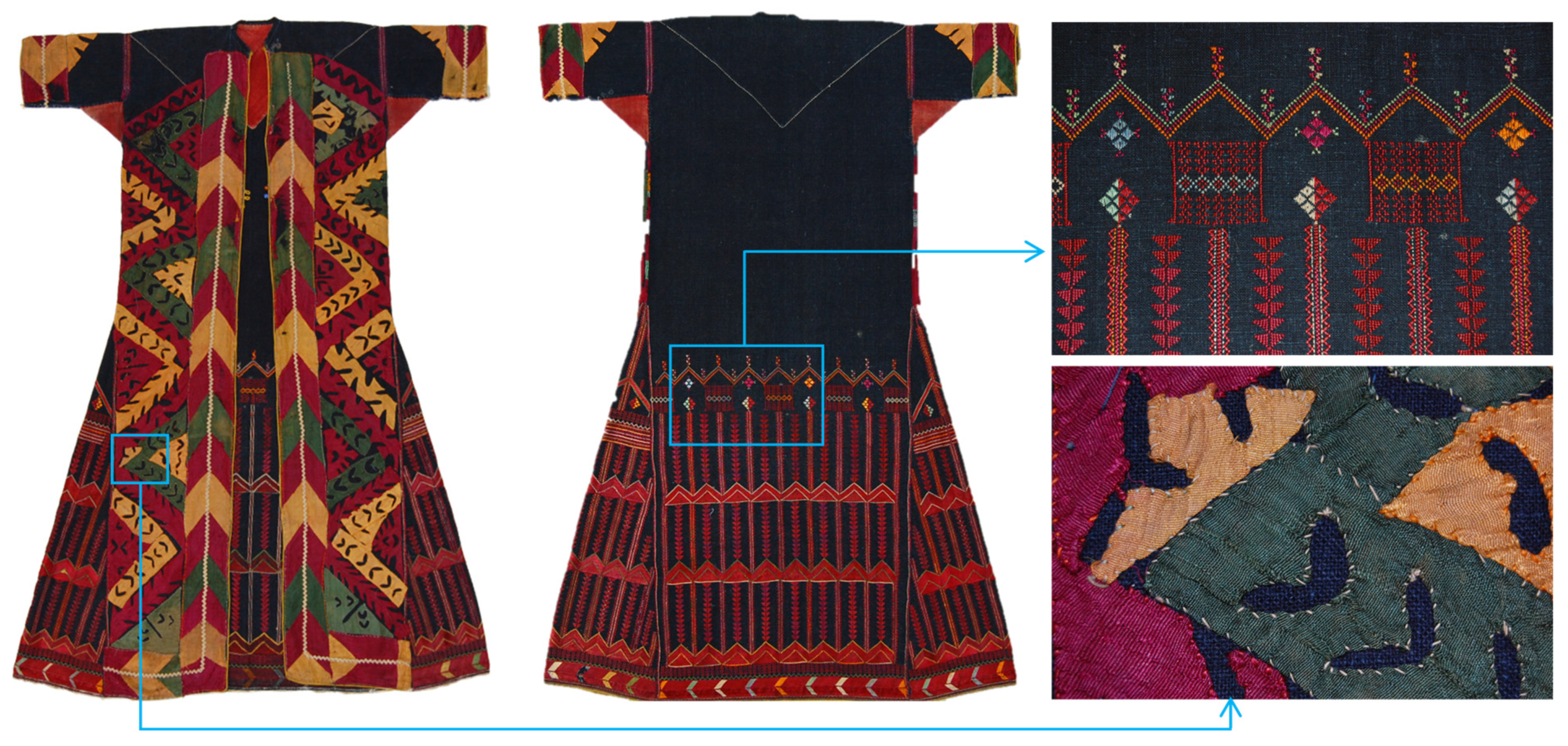
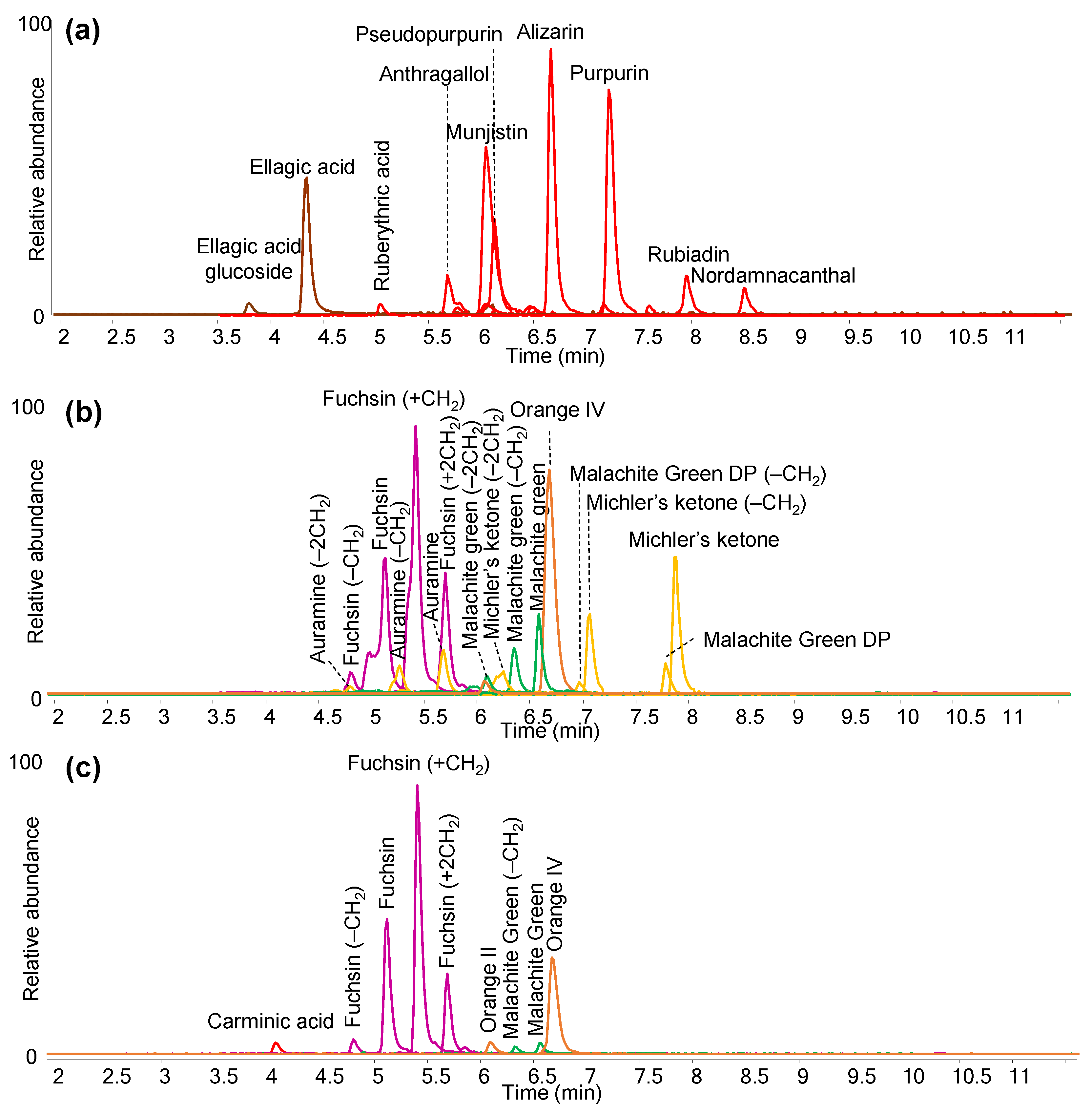



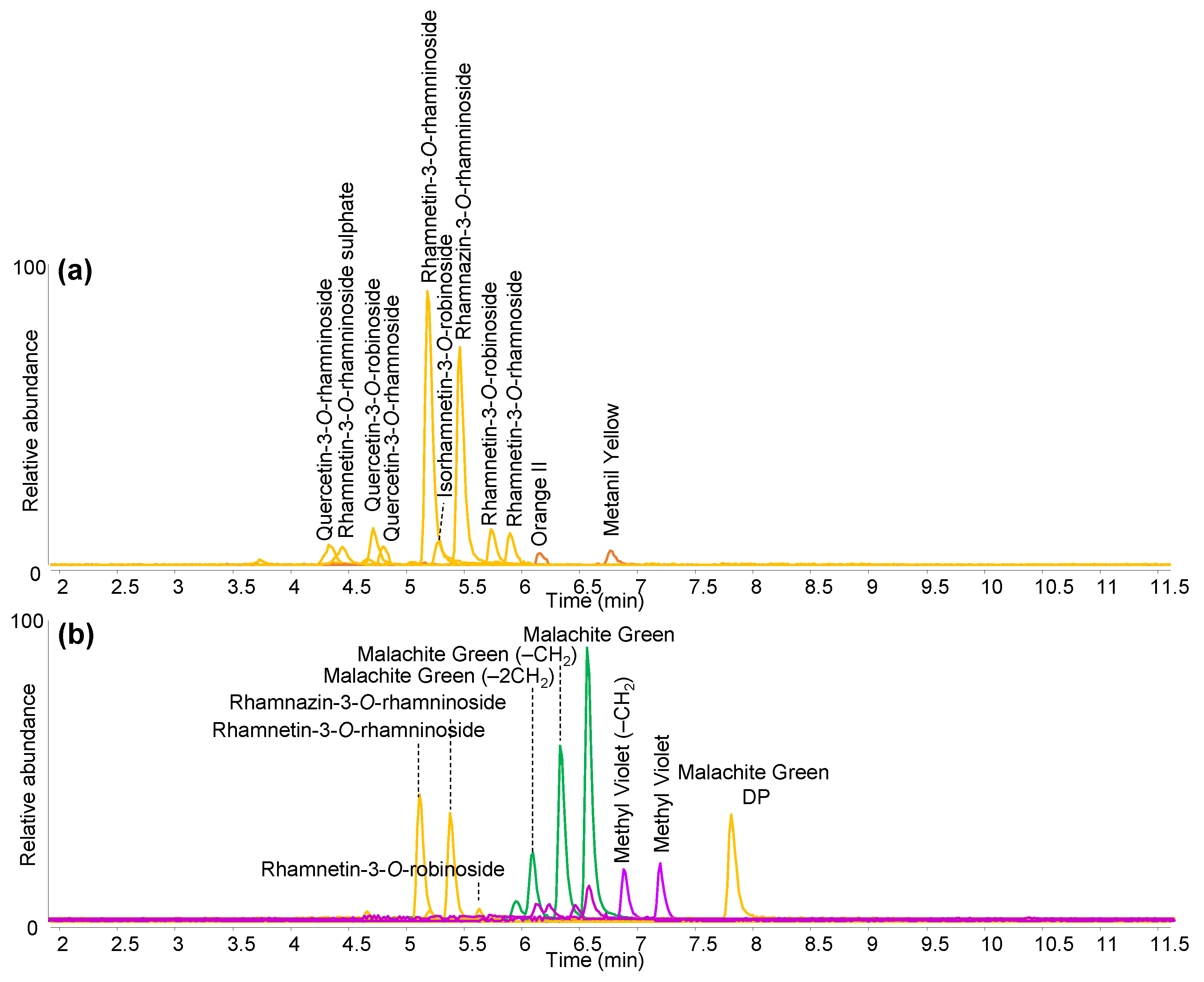
| Colour | Dye Identified | Date of First Synthesis | |
|---|---|---|---|
 | Blue | Indigo/woad (natural) | - |
 | Red from bottom panel | Tannins | - |
| Madder (Rubia tinctorum) | - | ||
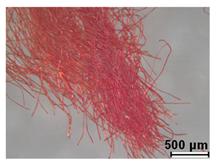 | Red from central chest panel | Tannins | |
| Madder (Rubia tinctorum) | |||
 | Faded red from right sleeve | Fuchsin (C.I. 42510) | 1859 |
| Auramine O (C.I. 41000) | 1883 | ||
| Orange II (C.I. 15510) | 1877 | ||
| Orange IV (C.I. 13080) | 1877 | ||
| Malachite green (C.I. 42000) | 1877 | ||
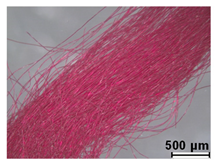 | Dark red | Cochineal (probably Dactylopius coccus) | - |
| Fuchsin (C.I. 42510) | 1859 | ||
| Orange II (C.I. 15510) | 1877 | ||
| Orange IV (C.I. 13080) | 1877 | ||
| Malachite Green (C.I. 42000) | 1877 | ||
 | Orange | Orange II (C.I. 15510) | 1877 |
| Orange IV (C.I. 13080) | 1877 | ||
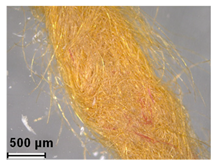 | Yellow | Metanil Yellow (C.I. 13065) | 1879 |
| Orange II (C.I. 15510) | 1877 | ||
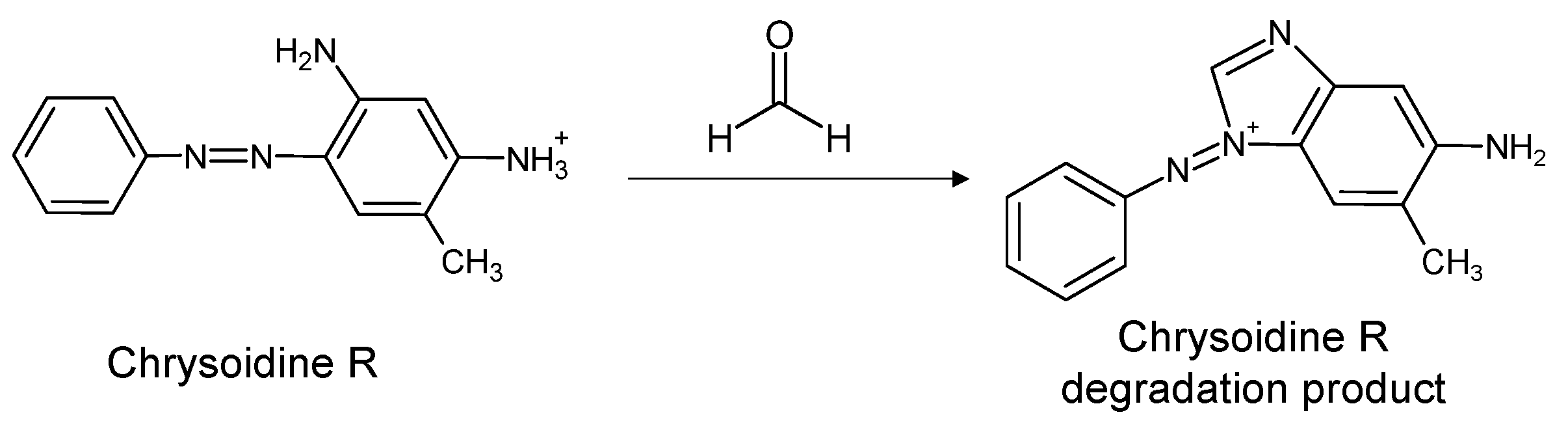
| Chrysoidine R (C.I. 11320) | 1877 | ||
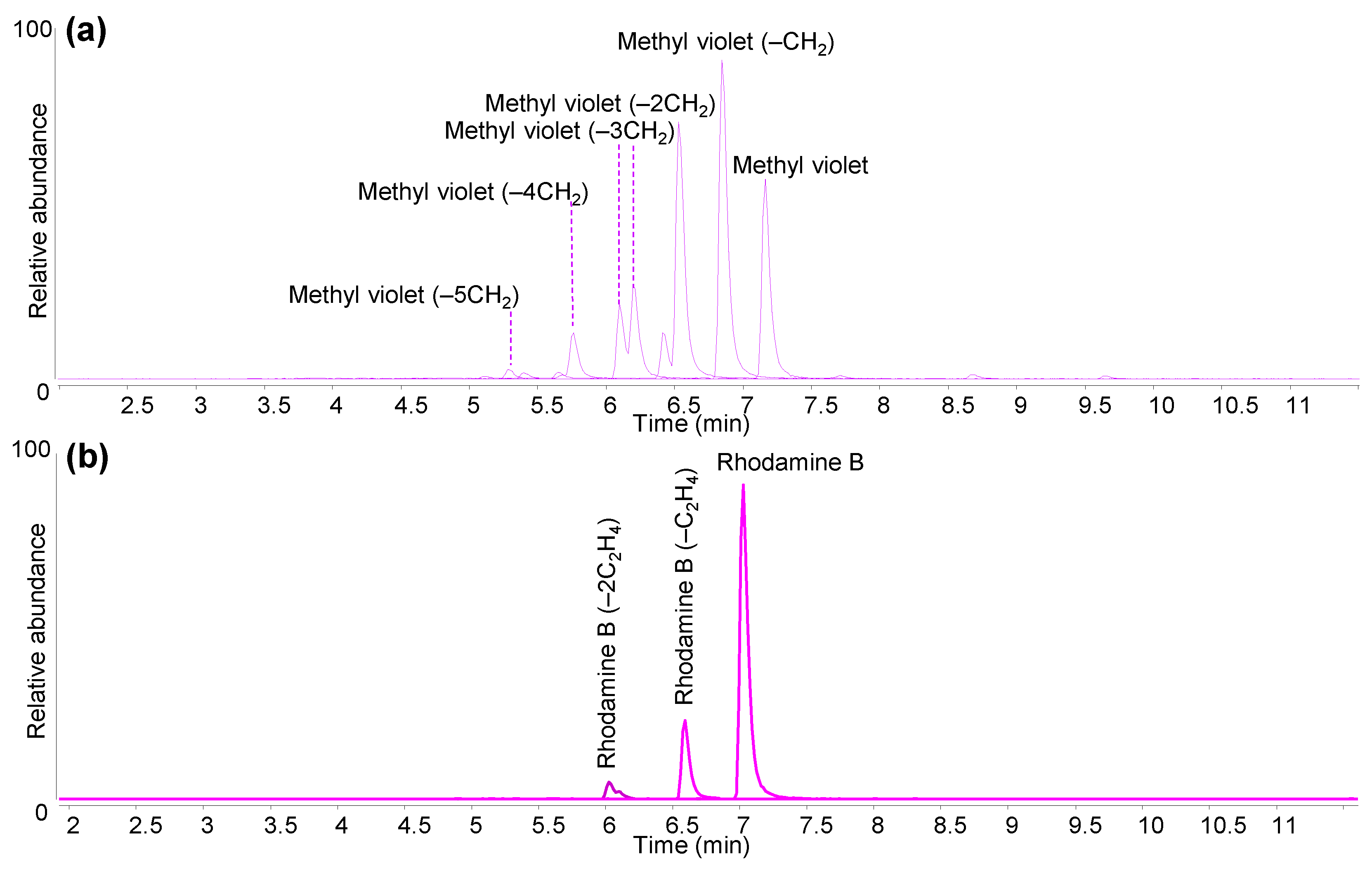
 | Pink | Rhodamine B (C.I. 45170) | 1887 |
 | Purple | Methyl violet (C.I. 42535) | 1861 |
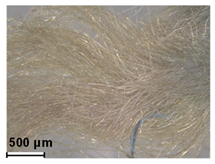 | Green | Buckthorn (Rhamnus sp.) | - |
| Malachite green (C.I. 42000) | 1877 | ||
| Methyl violet (C.I. 42535) | 1861 | ||
| Orange II (C.I. 15510) | 1877 | ||
| Metanil Yellow (C.I. 13065) | 1879 | ||
 | Lime green | Buckthorn (Rhamnus sp.) | - |
| Malachite green (C.I. 42000) | 1877 | ||
| Methyl violet (C.I. 42535) | 1861 | ||
| Orange II (C.I. 15510) | 1877 | ||
| Metanil Yellow (C.I. 13065) | 1879 |
| Colour | Dye Identified | Date of First Synthesis | |
|---|---|---|---|
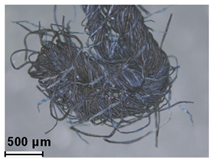 | Blue | Indigo/woad (natural) | - |
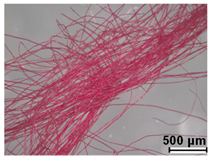 | Red | Fuchsin (C.I. 42510) | 1859 |
| Orange IV (C.I. 13080) | 1877 | ||
 | Magenta | Fuchsin (C.I. 42510) | 1859 |
| Orange II (C.I. 15510) | 1877 | ||
 | Orange | Orange II (C.I. 15510) | 1877 |
| Orange IV (C.I. 13080) | 1877 | ||
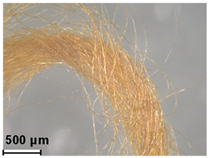 | Yellow | Buckthorn (Rhamnus sp.) | - |
| Orange II (C.I. 15510) | 1877 | ||
| Orange IV (C.I. 13080) | 1877 | ||
| Malachite green (C.I. 42000) | 1877 | ||
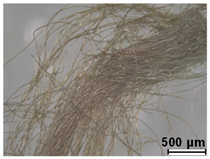 | Green | Buckthorn (Rhamnus sp.) | - |
| Malachite green (C.I. 42000) | 1877 | ||
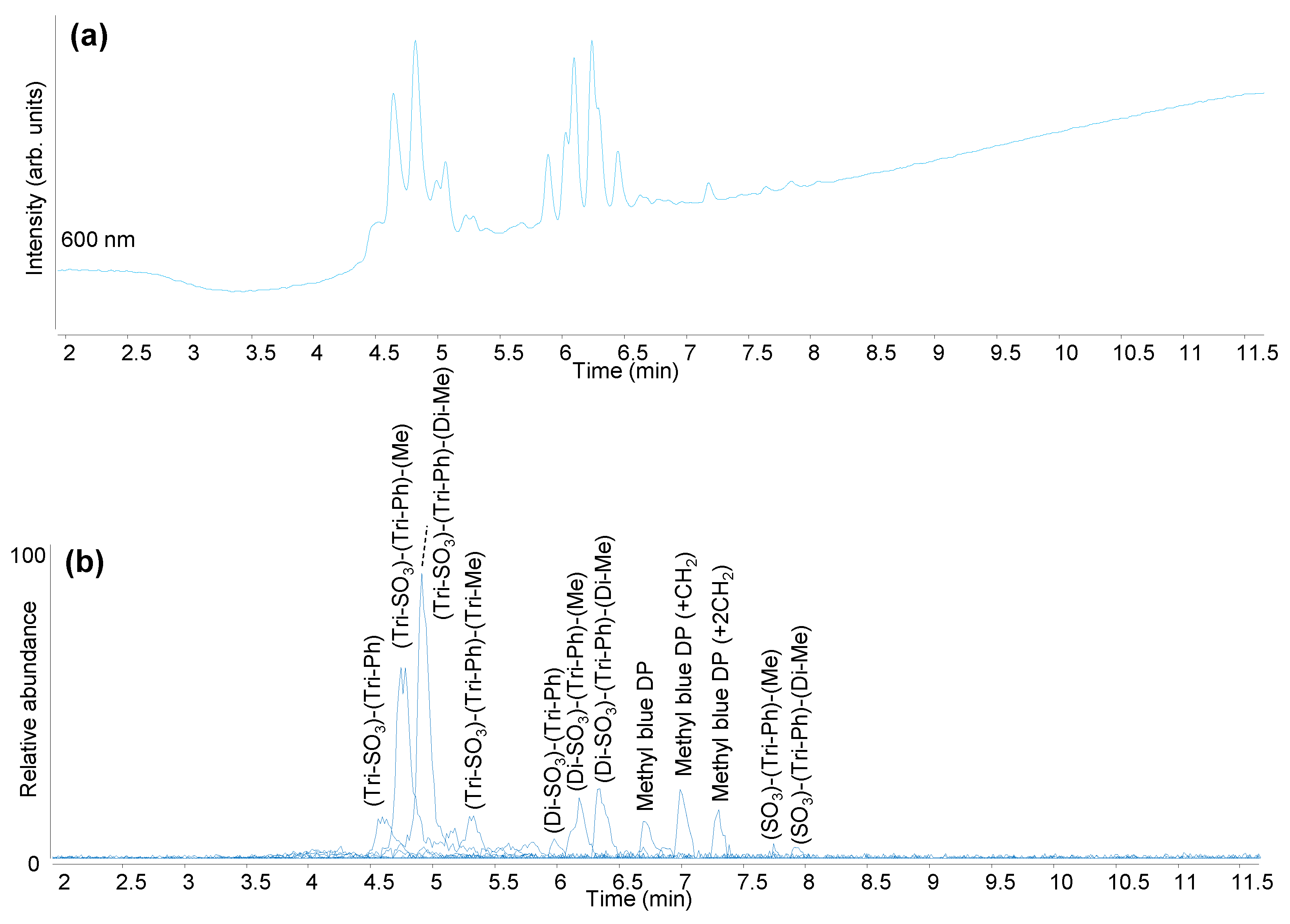
 | Light Blue | Methyl blue (C.I. 42780) | 1862 |
| Orange IV (C.I. 13080) | 1877 |
| Dye | Molecule | Formula | [M-H]− (m/z) | [M]+/[M+H]+ (m/z) |
|---|---|---|---|---|
| Buckthorn (probably Rhamnus saxatilis) | Quercetin-3-O-rhamnoside | C21H20O11 | 447.093 | 449.108 |
| Rhamnetin-3-O-rhamnoside | C22H22O11 | 461.109 | 463.124 | |
| Quercetin-3-O-robinoside * | C27H30O16 | 609.146 | 611.161 | |
| Rhamnetin-3-O-robinoside * | C28H32O16 | 623.162 | 625.176 | |
| Rhamnazin-3-O-robinoside * | C29H34O16 | 637.177 | 639.192 | |
| Rhamnocitrin-3-O-rhamninoside ** | C34H42O19 | 753.225 | 755.239 | |
| Quercetin-3-O-rhamninoside ** | C33H40O20 | 755.204 | 757.219 | |
| Rhamnetin-3-O-rhamninoside ** | C34H42O20 | 769.220 | 771.234 | |
| Rhamnazin-3-O-rhamninoside ** | C35H44O20 | 783.235 | 785.250 | |
| Rhamnetin-3-O-rhamninoside sulphate ** | C34H42O23S | 849.177 | 851.191 | |
| Cochineal (probably Dactylopius coccus) | dcII (C-glucopyranoside of flavokermesic acid) | C22H20O12 | 475.088 | 477.103 |
| Carminic acid (C-glucopyranoside of kermesic acid) | C22H20O13 | 491.083 | 493.098 | |
| dcIV (C-glucofuranoside of kermesic acid) | C22H20O13 | 491.083 | 493.098 | |
| dcVII (C-glucofuranoside of kermesic acid) | C22H20O13 | 491.083 | 493.098 | |
| Madder (Rubia tinctorum) | Alizarin | C14H8O4 | 239.035 | 241.049 |
| Rubiadin | C15H10O4 | 253.051 | 255.065 | |
| Purpurin | C14H8O5 | 255.031 | 257.044 | |
| Anthragallol | C14H8O5 | 255.031 | 257.044 | |
| Nordamnacanthal | C15H8O5 | 267.030 | 269.045 | |
| Munjistin | C15H8O6 | 283.025 | 285.039 | |
| Pseudopurpurin | C15H8O7 | 299.020 | 301.034 | |
| Ruberythric acid | C25H26O13 | 533.130 | 535.145 | |
| Tannins | Ellagic acid | C14H6O8 | 300.999 | 303.014 |
| Ellagic acid glucoside | C20H16O13 | 463.052 | 465.067 | |
| Indigo | Isatin | C8H5NO2 | 146.025 | 148.039 |
| Indigotin | C16H10N2O2 | 261.067 | 263.082 | |
| Indirubin | C16H10N2O2 | 261.067 | 263.082 | |
| Fuchsin (C.I. 42510; Basic Violet 14) | Pararosaniline (Fuchsin − CH2) | C19H18N3+ | 288.150 | |
| Methylpararosaniline (Fuchsin) | C20H20N3+ | 302.166 | ||
| Dimethylpararosaniline (Fuchsin + CH2) | C21H22N3+ | 316.181 | ||
| Trimethylpararosaniline (Fuchsin + 2CH2) | C22H24N3+ | 330.196 | ||
| Methyl Violet (C.I. 42535; Basic Violet 1) | Pararosaniline | C19H18N3+ | 288.150 | |
| N-methylpararosaniline (Methyl Violet − 5CH2) | C20H20N3+ | 302.166 | ||
| N,N′-dimethylpararosaniline (Methyl Violet − 4CH2) | C21H22N3+ | 316.181 | ||
| N,N,N′-trimethylpararosaniline (Methyl Violet − 3CH2) | C22H24N3+ | 330.196 | ||
| N,N,N′,N′-tetramethylpararosaniline (Methyl Violet − 2CH2) | C23H26N3+ | 344.212 | ||
| N,N,N′,N’,N″-pentamethylpararosaniline (Methyl Violet − CH2) | C24H28N3+ | 358.228 | ||
| N,N,N′,N′,N″,N″-hexamethylpararosaniline (Methyl Violet) | C25H30N3+ | 372.243 | ||
| Malachite green (C.I. 42000; Basic Green 4) | N-methyl-4,4′-(phenylmethylene)dianiline (Malachite Green − 3CH2) | C20H19N2+ | 287.154 | |
| N,N-dimethyl-4,4′-(phenylmethylene)dianiline (Malachite Green − 2CH2) | C21H21N2+ | 301.170 | ||
| N,N,N’-trimethyl-4,4′-(phenylmethylene)dianiline (Malachite Green − CH2) | C22H23N2+ | 315.186 | ||
| N,N,N’,N’-tetramethyl-4,4′-(phenylmethylene)dianiline (Malachite Green) | C23H25N2+ | 329.201 | ||
| Degradation products of Malachite Green | 4-(aminophenyl)phenyl-methanone (Malachite Green DP − 2CH2) | C13H12NO+ | 198.091 | |
| [4-N-methyl(aminophenyl)]phenyl-methanone (Malachite Green DP − CH2) | C14H14NO+ | 212.107 | ||
| [4-N,N-dimethyl-(aminophenyl)phenyl]-methanone/[4-N-ethyl-(aminophenyl)phenyl]-methanone (Malachite Green DP) | C15H16NO+ | 226.123 | ||
| Rhodamine B (C.I. 45170; Basic Violet 10) | Bisdeethylated Rhodamine B (Rhodamine B − 2C2H4) | C24H23N2O3+ | 387.170 | |
| Deethylated Rhodamine B (Rhodamine B − C2H4) | C26H27N2O3+ | 415.202 | ||
| Rhodamine B | C28H31N2O3+ | 443.232 | ||
| Auramine O (C.I. 41000; Basic Yellow 2) | Bisdemethylated Auramine O (Auramine − 2CH2) | C15H18N3+ | 240.150 | |
| Demethylated Auramine O (Auramine − CH2) | C16H20N3+ | 254.165 | ||
| 4,4′-Carbonimidoylbis(N,N-dimethylaniline) (Auramine) | C17H22N3+ | 268.181 | ||
| Degradation products of Auramine O | Bisdemethylated Michler’s ketone (Michler’s ketone - 2CH2) | C15H17N2O+ | 241.134 | |
| Demethylated Michler’s ketone (Michler’s ketone − CH2) | C16H19N2O+ | 255.149 | ||
| Bis [4-(dimethylamino)phenyl]methanone (Michler’s ketone) | C17H21N2O+ | 269.165 | ||
| Methyl Blue (C.I. 42780; Acid Blue 93) | Disulphonated N,N,N-triphenyl-pararosaniline (Di-SO3)-(Tri-Ph) | C37H29N3O6S2 | 674.143 | 676.157 |
| Disulphonated N,N,N-triphenyl-N-methyl-pararosaniline (Di-SO3)-(Tri-Ph)-(Me) | C38H31N3O6S2 | 688.158 | 690.173 | |
| Disulphonated N,N,N-triphenyl-N,N-dimethyl-pararosaniline (Di-SO3)-(Tri-Ph)-(Di-Me) | C39H33N3O6S2 | 702.174 | 704.188 | |
| Trisulphonated N,N,N-triphenyl-pararosaniline (Tri-SO3)-(Tri-Ph) | C37H29N3O9S3 | 754.099/376.546 [M-2H]2− | 756.114 | |
| Trisulphonated N,N,N-triphenyl-N-methyl-pararosaniline (Tri-SO3)-(Tri-Ph)-(Me) | C38H31N3O9S3 | 768.115/383.225 [M-2H]2− | 770.130 | |
| Trisulphonated N,N,N-triphenyl-N,N-dimethyl-pararosaniline (Tri-SO3)-(Tri-Ph)-(Di-Me) | C39H33N3O9S3 | 782.131/390.562 [M-2H]2− | 784.145 | |
| Degradation products of Methyl Blue | 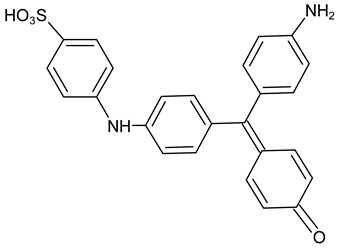 Methyl Blue DP | C25H20N2O4S | 443.107 | 445.1217 |
| Methyl Blue DP + CH2 | C26H22N2O4S | 457.123 | 459.1373 | |
| Methyl Blue DP + 2CH2 | C27H24N2O4S | 471.138 | 473.153 | |
| Orange II (C.I. 15510; Acid Orange 7) | 4-(2-Hydroxy-1-naphthylazo)benzenesulfonic acid (Orange II) | C16H12N2O4S | 327.045 | 329.059 |
| Orange IV (C.I. 13080; Acid Orange 5) | 4-[(4-Anilinophenyl)azo]benzenesulfonic acid (Orange IV) | C18H15N3O3S | 352.076 | 354.091 |
| Metanil Yellow (C.I. 13065, Acid Yellow 36) | 3-[(4-Anilinophenyl)diazenyl]benzenesulfonic acid (Metanil Yellow) | C18H15N3O3S | 352.076 | 354.091 |
| Chrysoidine R (C.I. 11320, Basic Orange 1) | 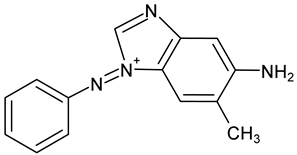 Chrysoidine R degradation product | C14H13N4+ | 237.114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburini, D.; Durand, L.; Klink-Hoppe, Z. Refining the Production Date of Historical Palestinian Garments Through Dye Identification. Heritage 2025, 8, 28. https://doi.org/10.3390/heritage8010028
Tamburini D, Durand L, Klink-Hoppe Z. Refining the Production Date of Historical Palestinian Garments Through Dye Identification. Heritage. 2025; 8(1):28. https://doi.org/10.3390/heritage8010028
Chicago/Turabian StyleTamburini, Diego, Ludovic Durand, and Zeina Klink-Hoppe. 2025. "Refining the Production Date of Historical Palestinian Garments Through Dye Identification" Heritage 8, no. 1: 28. https://doi.org/10.3390/heritage8010028
APA StyleTamburini, D., Durand, L., & Klink-Hoppe, Z. (2025). Refining the Production Date of Historical Palestinian Garments Through Dye Identification. Heritage, 8(1), 28. https://doi.org/10.3390/heritage8010028







