Uses of Gas Sorption and Mercury Porosimetry Methods in Studies of Heritage Materials
Abstract
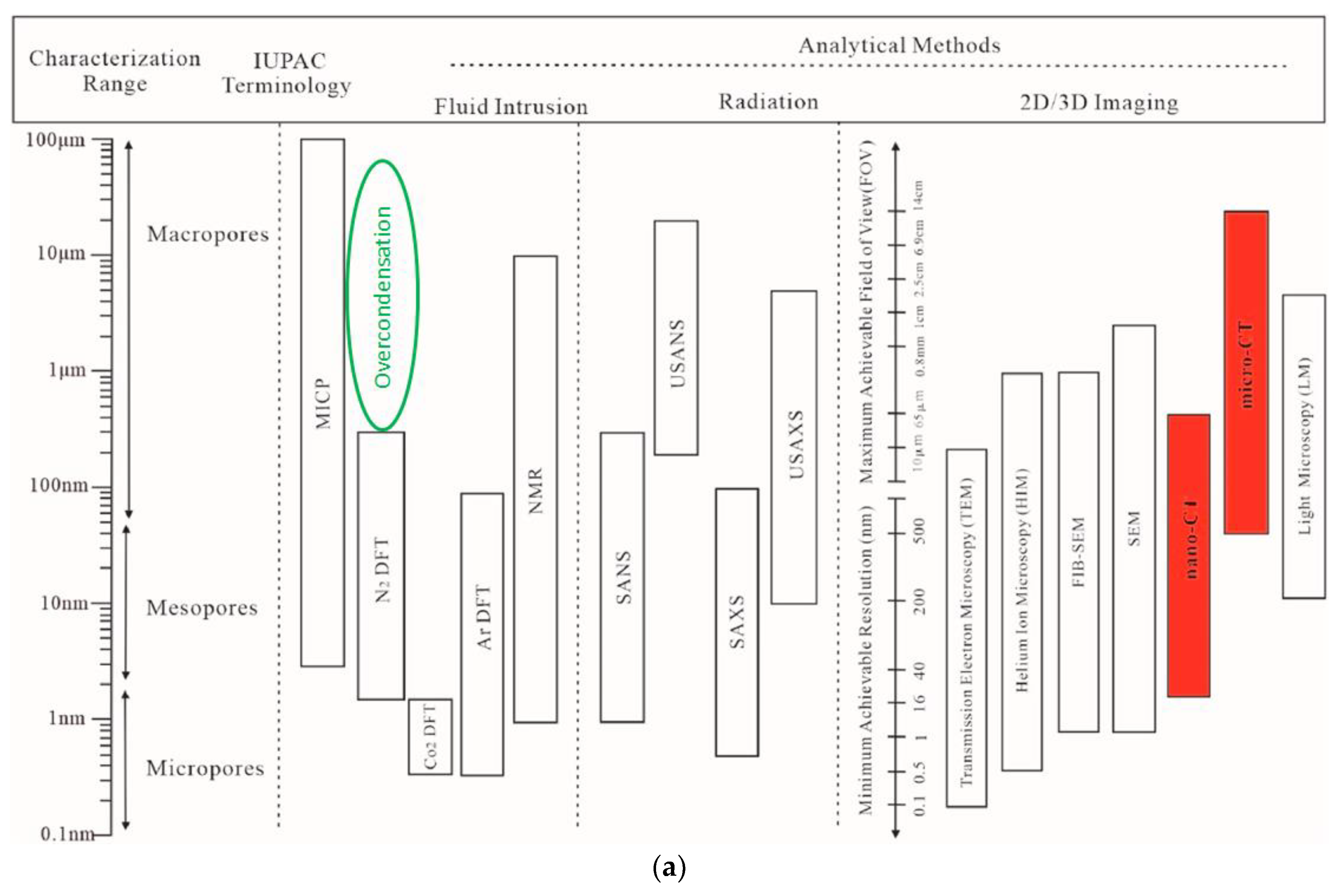
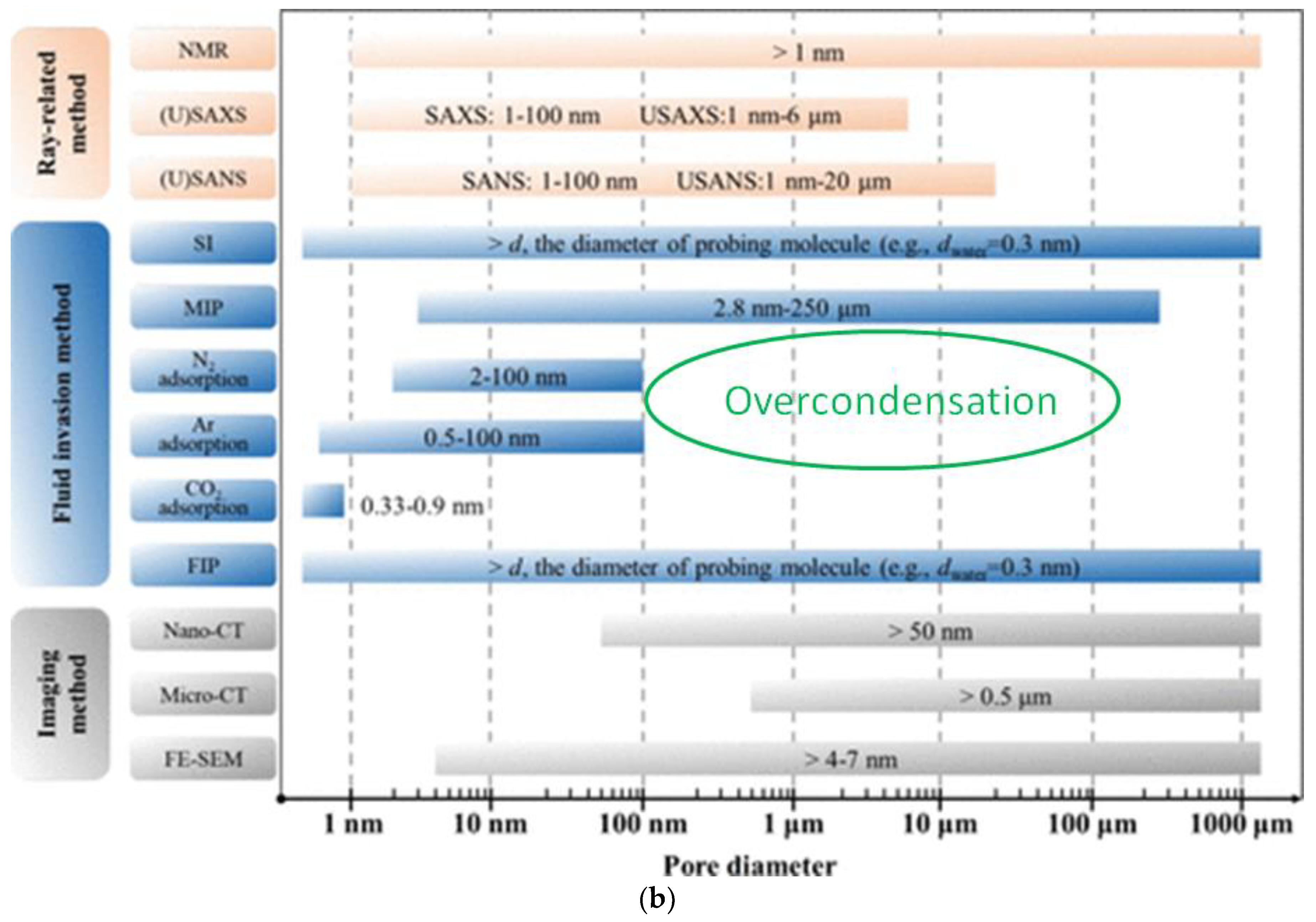
1. Introduction
2. Methodology
2.1. Equipment
2.2. Sampling
2.3. Sample Preparation
2.4. Experiment Protocols and Parameters
2.4.1. Basic Experiment
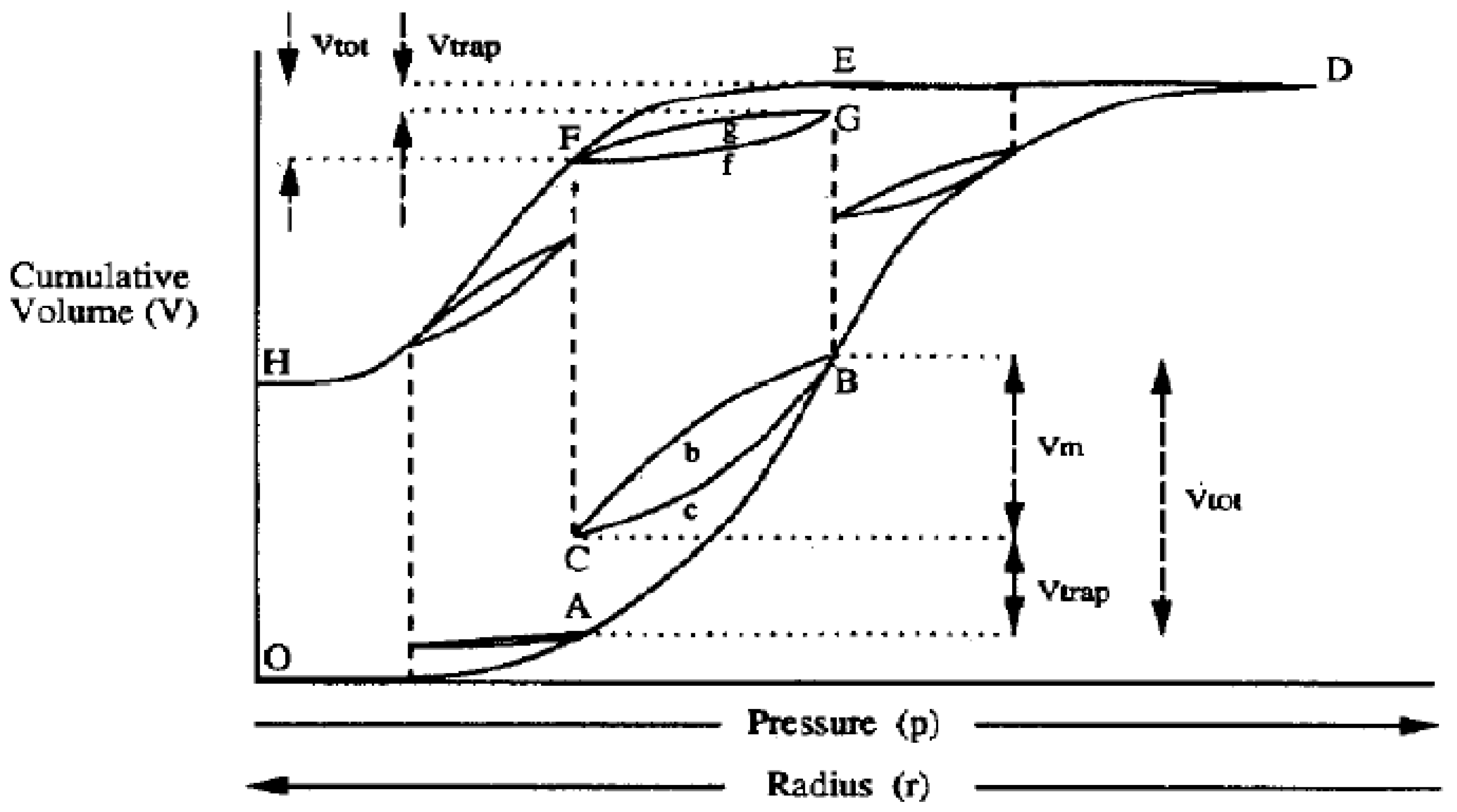
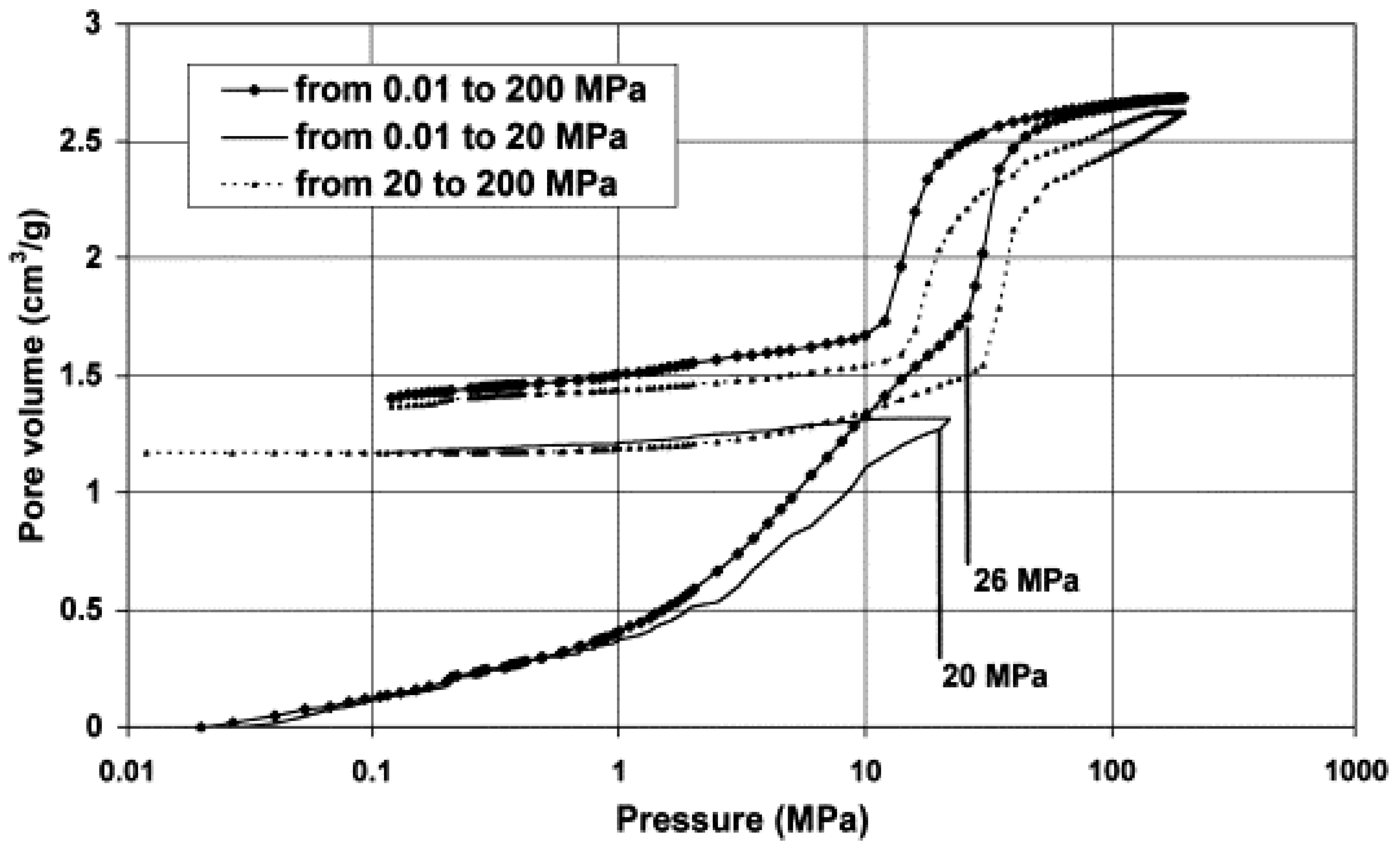
2.4.2. Scanning Curves (SCs) and Scanning Loops (SL)
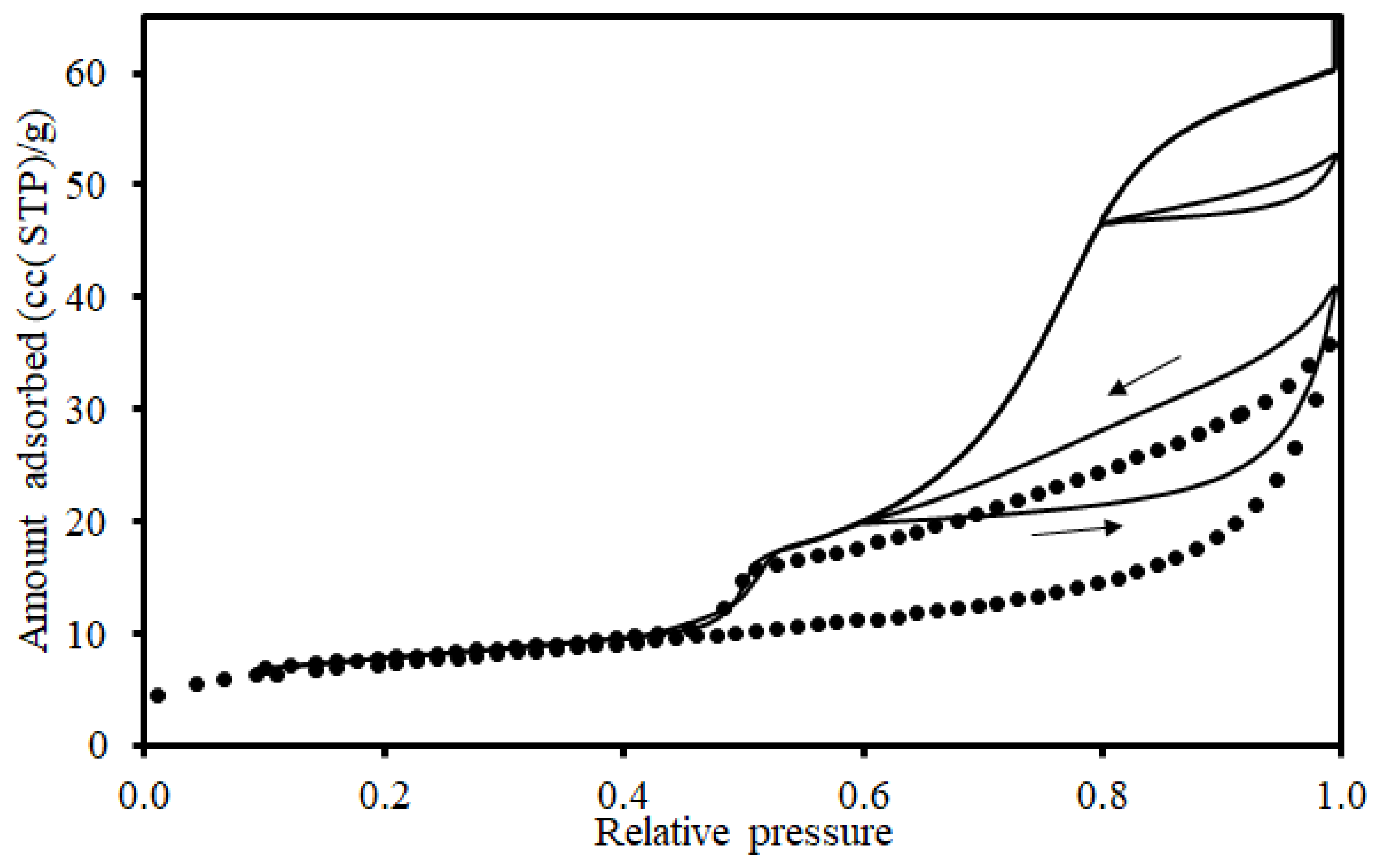
2.4.3. Over-Condensation (OC)
2.5. Hybrid Experiments
2.6. Data Analysis
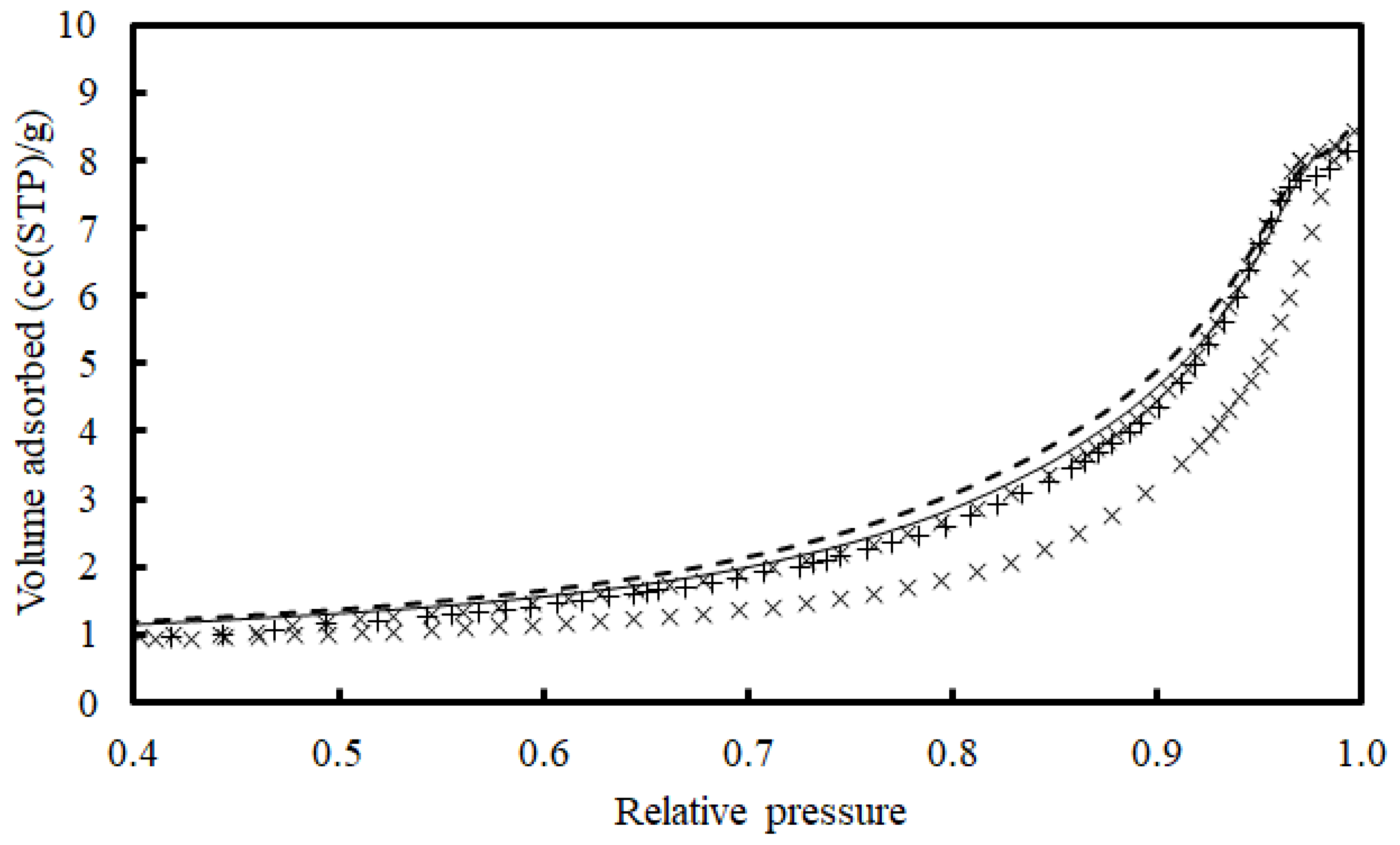
2.6.1. Specific Surface Area
2.6.2. Pore Size Distribution (PSD)
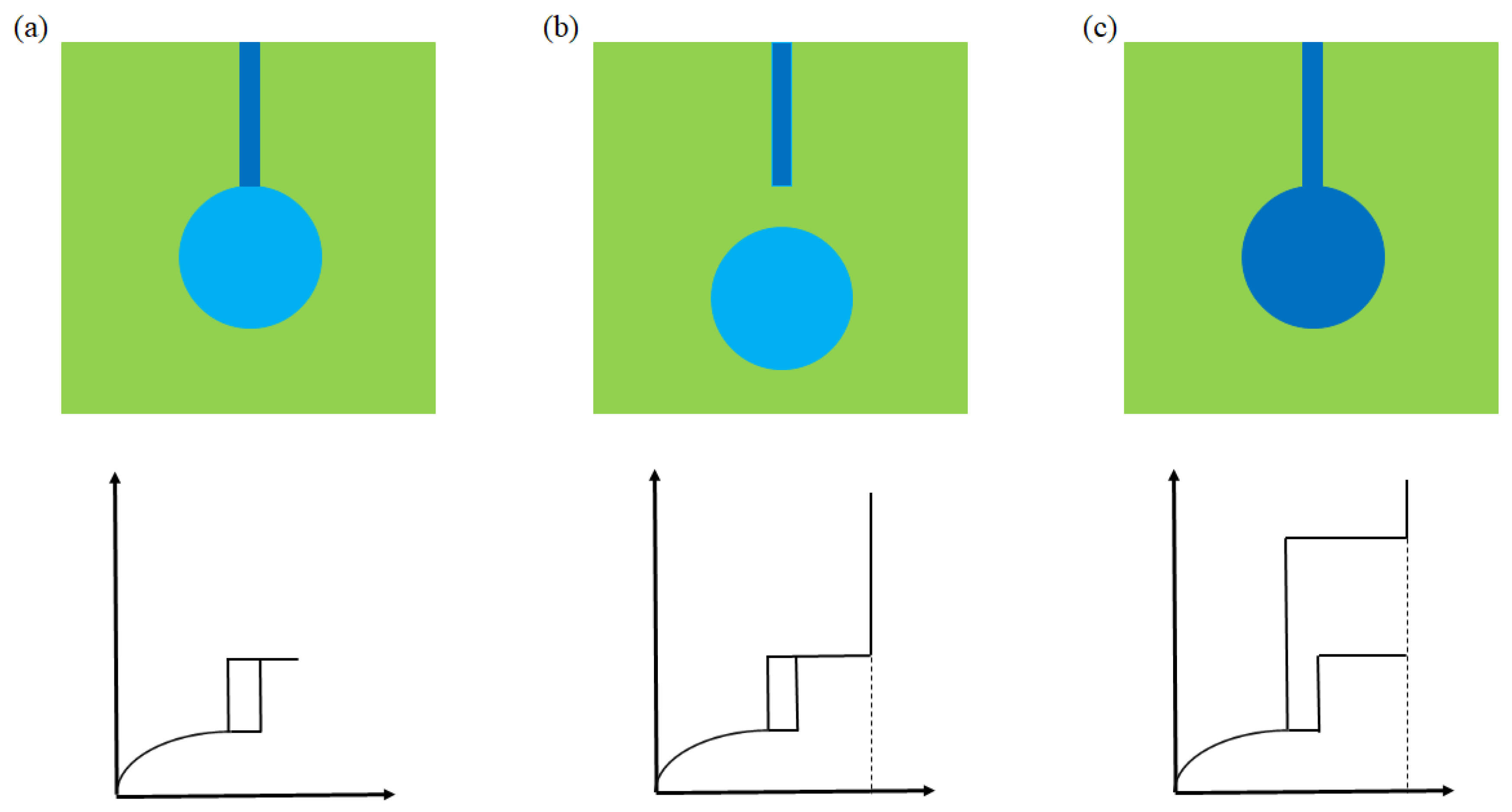
2.6.3. Pore Connectivity
3. Materials
3.1. Ancient Glass
3.2. Ancient Ceramics
3.3. Building Materials
3.4. Resilience and Conservation of Heritage Materials
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rigby, S.P. Use of Computerised X-ray Tomography in the Study of the Fabrication Methods and Conservation of Ceramics, Glass and Stone Building Materials. Heritage 2024, 7, 5687–5722. [Google Scholar] [CrossRef]
- Bronitsky, G. The use of materials science techniques in the study of pottery construction and use. Adv. Archaeol. Method Theory 1986, 9, 209–276. [Google Scholar] [CrossRef]
- Sobott, R.; Bente, K.; Kittel, M. Comparative porosity measurements on ceramic materials. Old Potter’s Alm. 2014, 19, 18–25. [Google Scholar]
- Rigby, S.P.; Himona, E. Methods of Pore Structural Characterisation of Sedimentary Rocks and Their Constituent Minerals. Minerals 2024, 14, 756. [Google Scholar] [CrossRef]
- Heimann, R. Firing technologies and their possible assessment by modern analytical methods. In Archaeological Ceramics; Olin, J., Franklin, A., Eds.; Smithsonian Institute: Washington, DC, USA, 1982; pp. 89–96. [Google Scholar]
- Manohar, S.; Santhanam, M. Correlation between physical-mineralogical properties and weathering resistance using characterisation case studies in historic Indian bricks. Int. J. Archit. Herit. 2022, 16, 667–680. [Google Scholar] [CrossRef]
- Giesche, H. Mercury porosimetry: A general (practical) overview. Part. Part. Syst. Charact. 2006, 23, 9–19. [Google Scholar] [CrossRef]
- Rigby, S.P. Structural Characterisation of Natural and Industrial Porous Materials: A Manual; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Aukett, P.N.; Jessop, C.A. Assessment of connectivity in mixed meso/macroporous solids using nitrogen sorption. In Fundamentals of Adsorption; Kluwer Academic Publishers: Boston, MA, USA, 1996; pp. 59–66. [Google Scholar]
- Murray, K.L.; Seaton, N.A.; Day, M.A. An Adsorption-Based Method for the Characterization of Pore Networks Containing Both Mesopores and Macropores. Langmuir 1999, 15, 6728–6737. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, M.; Sun, X.; Liu, B.; Ostadhassan, M.; Huang, W.; Chen, X.; Pan, Z. Pore network characterization of shale reservoirs through state-of-the-art X-ray computed tomography: A review. Gas Sci. Eng. 2023, 113, 204967. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H. Advances in Microscopic Pore Structure Characterization of Fine-Grained Mudrocks. Energy Fuels 2023, 37, 1495. [Google Scholar]
- Van Brakel, J.; Modrý, S.; Svatá, M. Mercury porosimetry: State of the art. Powder Technol. 1981, 29, 1–12. [Google Scholar] [CrossRef]
- Drieu, L.; Horgnies, M.; Binder, D.; Pétrequin, P.; Pétrequin, A.-M.; Peche-Quilichini, K.; Lachenal, T.; Regert, M. Influence of porosity on lipid preservation in the wall of archaeological pottery. Archaeometry 2019, 61, 1081–1096. [Google Scholar] [CrossRef]
- Watt-Smith, M.; Edler, K.J.; Rigby, S.P. An Experimental Study of Gas Adsorption on Fractal Surfaces. Langmuir 2005, 21, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, E.G.; Fletcher, R.S.; Large, D.J.; Rigby, S.P. Pore structure–transport relationships in the Bowland Shale. In The Bowland Shale Formation, UK: Processes and Resources; Emmings, J.F., Parnell, J., Stephenson, M.H., Lodhia, B.E., Eds.; The Geological Society: London, UK, 2024; pp. 183–200. [Google Scholar]
- Volzone, C.; Zagorodny, N. Mercury intrusion porosimetry (MIP) study of archaeological pottery from Hualfin Valley, Catamarca, Argentina. Appl. Clay Sci. 2014, 91–92, 12–15. [Google Scholar] [CrossRef]
- Ning, C.; Jiang, Z.; Gao, Z.; Li, Z.; Zhu, R.; Su, S.; Li, T.; Wang, Z.; Huang, R.; Chen, L. Quantitative evaluation of pore connectivity with nuclear magnetic resonance and high pressure mercury injection: A case study of the lower section of Es3 in Zhanhua sag. J. China Univ. Min. Technol. 2017, 46, 578–584. [Google Scholar] [CrossRef]
- BS ISO 9277:2010; Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. International Standards Organisation (ISO): Geneva, Switzerland, 2010.
- ASTM D4404–10; Standard Test Method for Determination of Pore Volume and Pore Volume Distribution of Soil and Rock by Mercury Intrusion Porosimetry. STM International: West Conshohocken, PA, USA, 2010.
- Facchini, A.; Malara, C.; Bazzani, G.; Cavallotti, P.L. Ancient Parchment Examination by Surface Investigation Methods. J. Colloid Interface Sci. 2000, 231, 213–220. [Google Scholar] [CrossRef]
- Zouridakis, N.M.; Economou, I.G.; Tzevelekos, K.P.; Kikkinides, E.S. Investigation of the physicochemical characteristics of ancient mortars by static and dynamic studies. Cem. Concr. Res. 2000, 30, 1151–1155. [Google Scholar] [CrossRef]
- Rispoli, C.; Esposito, R.; Guerriero, L.; Cappelletti, P. Ancient Roman Mortars from Villa del Capo di Sorrento: A Multi-Analytical Approach to Define Microstructural and Compositional Features. Minerals 2021, 11, 469. [Google Scholar] [CrossRef]
- Mousa, S.; Baron, K.; Fletcher, R.S.; Rigby, S.P. Triangulation of pore structural characterisation of disordered mesoporous silica using novel hybrid methods involving dual-probe porosimetries. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130026. [Google Scholar] [CrossRef]
- Theodoridou, M.; Ioannou, I.; Philokyprou, M. New evidence of early use of artificial pozzolanic material in mortars. J. Archaeol. Sci. 2013, 40, 3263–3269. [Google Scholar] [CrossRef]
- Rigby, S.P.; Jahan, H.; Stevens, L.; Uguna, C.; Snape, C.; Macnaughton, B.; Large, D.J.; Fletcher, R.S. Pore structural evolution of shale following thermochemical treatment. Mar. Pet. Geol. 2020, 112, 104058. [Google Scholar] [CrossRef]
- Portsmouth, R.L.; Gladden, L.F. Determination of pore connectivity by mercury porosimetry. Chem. Eng. Sci. 1991, 46, 3023–3036. [Google Scholar] [CrossRef]
- Kanth, A.P.; Singh, M.R.; Ganaraj, K. Spectroscopic and X-ray based microstructural investigation of the early-Harappan potsherds and estimation of firing temperature from Kunal archaeological site, India. Int. J. Conserv. Sci. 2022, 13, 131–146. [Google Scholar]
- Shi, J.; Chun, Q.; Feng, S.; Liu, C.; Liu, Z.; Wang, D.; Zhang, Y. Pore structure and fractal dimension analysis of ancient city wall bricks in China. J. Build. Eng. 2023, 76, 107324. [Google Scholar]
- Salvatore, A.; Vai, S.; Caporali, S.; Caramelli, D.; Lari, M.; Carretti, E. Evaluation of Diammonium hydrogen phosphate and Ca(OH)2 nanoparticles for consolidation of ancient bones. J. Cult. Herit. 2020, 41, 1–12. [Google Scholar]
- Gong, Y.; Qiao, C.; Yu, X.; Wang, J.; Gong, D. Study on the ancient putty from the site of the Ming Dynasty (1368–1644 CE) Baochuanchang Shipyard, Nanjing, China. J. Archaeol. Sci. Rep. 2019, 23, 189–195. [Google Scholar]
- De Boer, J.H. The Shapes of Capillaries. In The Structure and Properties of Porous Solids; Everett, D.H., Stone, F.S., Eds.; Butterworths Scientific Publications: London, UK, 1958; p. 68. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press Inc.: London, UK, 1982. [Google Scholar]
- Rigby, S.P. Novel hybrid methods provide greatly increased information on this important class of solids. Joh. Matthey Technol. Rev. 2018, 62, 296–312. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Walker, W.C.; Zettlemoyer, A.C. A dual-surface BET adsorption theory. J. Phys. Phys. Chem. 1948, 52, 47–58. [Google Scholar]
- Rigby, S.P.; Stevens, L.; Meersmann, T.; Pavloskaya, G.E.; Rees, G.J.; Henderson, J.; Bryant, S.J.; Edler, K.J.; Fletcher, R.S. Structural and chemical heterogeneity in ancient glass probed using gas overcondensation, X-ray tomography, and solid-state NMR. Mater. Charact. 2020, 167, 110467. [Google Scholar]
- Mahnke, M.; Mögel, H.J. Fractal Analysis of Physical Adsorption on Material Surfaces. Colloids Surf. A 2003, 216, 215–228. [Google Scholar]
- Pfeifer, P.; Wu, Y.J.; Cole, M.W.; Krim, J. Multilayer adsorption on a fractally rough surface. Phys. Rev. Lett. 1989, 62, 1997–2000. [Google Scholar]
- Urosevic, M.; Pardo, E.S.; Rutz-Agudo, E.; Cardell, C. Physical properties of carbonate rocks used as a modern and historic construction material in Eastern Andalusia, Spain. Mater. Constr. 2011, 61, 93–114. [Google Scholar]
- Goli, V.S.N.S.; Yadav, R.; Singh, M.R. Forensic investigations on 1900 years old brick and mortar samples from Buddhist stupa located at Nalasopara, India. Constr. Build. Mater. 2023, 367, 130281. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Neimark, A.V.; Ravikovitch, P.I. Capillary Condensation in MMS and Pore Structure Characterization. Microporous Mesoporous Mater. 2001, 44, 697–707. [Google Scholar]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273. [Google Scholar]
- Zhang, J.; Xiao, H.; Chen, Y.; Qi, J.; Xie, J.; Huang, X.; Jiang, Y. Porosity and pore size distribution of recent and ancient buried phoebe zhennan wood determined by mercury intrusion porosimetry. Wood Res. 2021, 66, 277–283. [Google Scholar]
- Liabastre, A.A.; Orr, C. Evaluation of pore structure by mercury penetration. J. Colloid Interface Sci. 1978, 64, 1–18. [Google Scholar]
- Kloubek, J. Hysteresis in porosimetry. Powder Technol. 1981, 29, 63–73. [Google Scholar]
- Rigby, S.P. New methodologies in mercury porosimetry. Stud. Surf. Sci. Cat. 2000, 144, 185–192. [Google Scholar]
- Rigby, S.P.; Edler, K.J. The influence of mercury contact angle, surface tension and retraction mechanism on the interpretation of mercury porosimetry data. J. Colloid Interface Sci. 2002, 250, 175–190. [Google Scholar] [CrossRef]
- Lawrence, R.M.; Mays, T.J.; Rigby, S.P.; Walker, P.; D’Ayala, D. Effects of carbonation on the pore structure of non-hydraulic lime mortars. Cem. Concr. Res. 2007, 37, 1059–1069. [Google Scholar]
- Perkins, E.L.; Lowe, J.P.; Edler, K.J.; Tanko, N.; Rigby, S.P. Determination of the percolation properties and pore connectivity for mesoporous solids using NMR cryodiffusometry. Chem. Eng. Sci. 2008, 63, 1929–1940. [Google Scholar]
- Wardlaw, N.C.; McKellar, M. Mercury porosimetry and the interpretation of pore geometry in sedimentary rocks and artificial models. Powder Technol. 1981, 29, 127–143. [Google Scholar]
- Tsakiroglou, C.D.; Payatakes, A.C. Mercury intrusion and retraction in model porous media. Adv. Colloid Interface Sci. 1998, 75, 215–253. [Google Scholar]
- Bafarawa, B.; Nepryahin, A.; Lu, J.; Holt, E.M.; Wang, J.; Rigby, S.P. Combining mercury thermoporometry with integrated gas sorption and mercury porosimetry to improve accuracy of pore-size distributions for disordered solids. J. Colloid Interface Sci. 2014, 426, 72–79. [Google Scholar] [CrossRef]
- Alié, C.; Pirard, R.; Pirard, J.P. Mercury porosimetry applied to porous silica materials: Successive buckling and intrusion mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 367–374. [Google Scholar]
- Pirard, R.; Alié, C.; Pirard, J.P. Characterization of porous texture of hyperporous materials by mercury porosimetry using densification equation. Powder Technol. 2002, 128, 242–247. [Google Scholar]
- Seaton, N.A. Determination of the connectivity of porous solids from nitrogen sorption measurements. Chem. Eng. Sci. 1991, 46, 1895–1909. [Google Scholar] [CrossRef]
- Ghanbarian, B.; Hunt, A.G.; Ewing, R.P.; Sahimi, M. Tortuosity in Porous Media: A Critical Review. Soil Sci. Soc. Am. J. 2013, 77, 1461–1477. [Google Scholar] [CrossRef]
- Androutsopoulos, G.P.; Salmas, C.E. A New Model for Capillary Condensation−Evaporation Hysteresis Based on a Random Corrugated Pore Structure Concept: Prediction of Intrinsic Pore Size Distributions. 1. Model Formulation. Ind. Eng. Chem. Res. 2000, 39, 3747–3763. [Google Scholar] [CrossRef]
- Lester, E.; Hilal, N.; Henderson, J. Porosity in ancient glass from Syria (c. 800 AD) using gas sorption and atomic force microscopy. Surf. Interface Anal. 2004, 36, 1323–1329. [Google Scholar] [CrossRef]
- Sanders, H.P. Pore-size distribution determinations in Neolithic, iron age, Roman and other pottery. Archaeometry 1973, 15, 159–161. [Google Scholar] [CrossRef]
- Morariu, V.; Bogdan, M.; Ardelean, I. Ancient pottery: Its pore structure. Archaeometry 1977, 19, 187–192. [Google Scholar] [CrossRef]
- Drieu, L.; Peche-Quilichini, K.; Lachenal, T.; Regert, M. Domestic activities and pottery use in the iron age Corsican settlement of Cuciurpula revealed by organic residue analysis. J. Archaeol. Sci. Rep. 2018, 19, 213–223. [Google Scholar] [CrossRef]
- Rice, P.M. Pottery Analysis: A Sourcebook; The University of Chicago Press: Chicago, IL, USA, 1987. [Google Scholar]
- Cayme, J.-M.; Palm, R.; Somelar, P.; Vahur, S.; Leito, I.; Oras, E. Influence of mineral composition and firing temperature on the micro- and mesoporosity of replicate archaeological ceramics. Clays Clay Miner. 2024, 72, 1–14. [Google Scholar] [CrossRef]
- Evershed, R.P. Experimental approaches to the interpretation of absorbed organic residues in archaeological ceramics. World Archaeol. 2008, 40, 26–47. [Google Scholar] [CrossRef]
- Namdar, D.; Stacey, R.J.; Simpson, S.J. First results on thermally induced porosity in chlorite cooking vessels from Merv (Turkmenistan) and implications for the formation and preservation of archaeological lipid residues. J. Archaeol. Sci. 2009, 36, 2507–2516. [Google Scholar] [CrossRef]
- Lapp, E.C. A water absorption analysis of Nabatean, North African and other clay lamp fabrics unearthed at the Red Sea port of Roman Aila (Aqaba, Jordan). Archaeometry 2012, 54, 56–79. [Google Scholar] [CrossRef]
- Reid, K. Fire and ice: New evidence for the production and preservation of Late Archaic fiber-tempered pottery in the middle-latitude lowlands. Am. Antiq. 1984, 49, 55–76. [Google Scholar] [CrossRef]
- Xu, K.; Tremsin, A.S.; Li, J.; Ushizima, D.M.; Davy, C.A.; Bouterf, A.; Su, Y.T.; Marroccoli, M.; Mauro, A.M.; Osanna, M.; et al. Microstructure and water absorption of ancient concrete from Pompeii: An integrated synchrotron microtomography and neutron radiography characterization. Cem. Concr. Res. 2021, 139, 106282. [Google Scholar]
- Münch, B.; Holzer, L. Contradicting geometrical concepts in pore size analysis attained with electron microscopy and mercury intrusion. J. Am. Ceram. Soc. 2008, 91, 4059–4067. [Google Scholar]
- Brai, M.; Casieri, C.; De Luca, F.; Fantazzini, P.; Gombia, M.; Terenzi, C. Validity of NMR pore-size analysis of cultural heritage ancient building materials containing magnetic impurities. Solid State Nucl. Magn. Reson. 2007, 32, 129–135. [Google Scholar]
- Lopez-Acre, P.; Benavente, D.; Garcia-Guinea, J. Durability improvement of ancient bricks by cementation of porous media. J. Am. Ceram. Soc. 2005, 88, 2564–2572. [Google Scholar]
- Brunello, V.; Canevali, C.; Corti, C.; De Kock, T.; Rampazzi, L.; Recchia, S.; Sansonetti, A.; Tedeschi, C.; Cnudde, V. Understanding the Microstructure of Mortars for Cultural Heritage Using X-ray CT and MIP. Materials 2021, 14, 5939. [Google Scholar] [CrossRef]
- Kamh, G.M.E.; Koltuk, S. Micro-topographic and geotechnical investigations of sandstone wall on weathering progress, Aachen city, Germany, case study. Arab. J. Sci. Eng. 2016, 41, 2284–2294. [Google Scholar] [CrossRef]
- D’Orazio, M.; Stazi, A. Dynamic of moisture transfer in ancient plasters. J. Cult. Herit. 2006, 7, 116–122. [Google Scholar]
- Cardiano, P.; Ioppolo, S.; De Stefano, C.; Pettignano, A.; Sergi, S.; Piraino, P. Study and characterization of the ancient bricks of monastery of “San Filippo di Fragalà” in Frazzanò (Sicily). Anal. Chim. Acta 2004, 519, 103–111. [Google Scholar]
- Manohar, S.; Santhanam, M.; Chockalingam, N. Performance and microstructure of bricks with protective coatings subjected to salt weathering. Constr. Build. Mater. 2019, 226, 94–105. [Google Scholar]
- Manohar, S.; Chockalingam, N.; Santhanam, M. Experimental comparison between salt weathering testing procedures on different types of bricks. J. Mater. Civ. Eng. 2021, 33, 04021305. [Google Scholar] [CrossRef]
- Manohar, S.; Bala, K.; Santhanam, M.; Menon, A. Characteristics and deterioration mechanisms in coral stones used in a historical monument in a saline environment. Constr. Build. Mater. 2020, 241, 118102. [Google Scholar]
- Manohar, S.; Santhanam, M. Use of poulticing in desalination of masonry units–implications on salt-deteriorated structures. Curr. Sci. 2021, 121, 1307–1315. [Google Scholar]
- Hansen, W.; Kung, J.W. Pore structure and frost durability of clay bricks. Mater. Struct. 1988, 21, 443–447. [Google Scholar]
- Tang, Y.; Shao, Z.; Xu, T. Pore structure of ancient Chinese bricks under environmental vicissitudes. KSCE J. Civ. Eng. 2016, 20, 1895–1902. [Google Scholar]
- Maage, M. Frost resistance and pore size distribution in bricks. Mater. Str. 1983, 17, 345–350. [Google Scholar] [CrossRef]
- Stryszewska, T.; Kańka, S. Forms of Damage of Bricks Subjected to Cyclic Freezing and Thawing in Actual Conditions. Materials 2019, 12, 1165. [Google Scholar] [CrossRef]
- Wesolowska, M.; Kaczmarek, A.; Hola, J. The Influence of External Environmental Conditions on Properties of Ceramic Building Materials with Waste Material Additives. Materials 2021, 14, 2982. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigby, S.P. Uses of Gas Sorption and Mercury Porosimetry Methods in Studies of Heritage Materials. Heritage 2025, 8, 132. https://doi.org/10.3390/heritage8040132
Rigby SP. Uses of Gas Sorption and Mercury Porosimetry Methods in Studies of Heritage Materials. Heritage. 2025; 8(4):132. https://doi.org/10.3390/heritage8040132
Chicago/Turabian StyleRigby, Sean P. 2025. "Uses of Gas Sorption and Mercury Porosimetry Methods in Studies of Heritage Materials" Heritage 8, no. 4: 132. https://doi.org/10.3390/heritage8040132
APA StyleRigby, S. P. (2025). Uses of Gas Sorption and Mercury Porosimetry Methods in Studies of Heritage Materials. Heritage, 8(4), 132. https://doi.org/10.3390/heritage8040132




