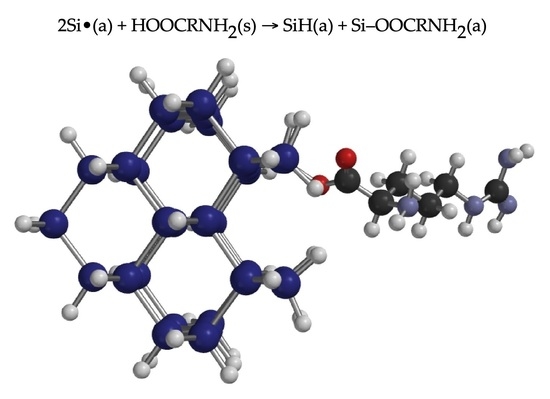
Characterization of Mechanochemical Modification of Porous Silicon with Arginine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mechanochemical Synthesis
2.2. Dynamic Light Scattering
2.3. Photoluminescence
2.4. Infrared Spectroscopy
3. Results
3.1. Rinsing and Separation
3.2. Particle Size and Zeta Potential
3.3. Photoluminescence
3.4. FTIR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moretta, R.; De Stefano, L.; Terracciano, M.; Rea, I. Porous Silicon Optical Devices: Recent Advances in Biosensing Applications. Sensors 2021, 21, 1336. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; O’Dwyer, C.; Macklin, W.J.; Holmes, J.D. Evaluating the performance of nanostructured materials as lithium-ion battery electrodes. Nano Res. 2014, 7, 1–62. [Google Scholar] [CrossRef] [Green Version]
- Dai, F.; Yi, R.; Yang, H.; Zhao, Y.; Luo, L.; Gordin, M.L.; Sohn, H.; Chen, S.; Wang, C.; Zhang, S.; et al. Minimized Volume Expansion in Hierarchical Porous Silicon upon Lithiation. ACS Appl. Mater. Interfaces 2019, 11, 13257–13263. [Google Scholar] [CrossRef] [PubMed]
- Alhmoud, H.; Brodoceanu, D.; Elnathan, R.; Kraus, T.; Voelcker, N.H. A MACEing silicon: Towards single-step etching of defined porous nanostructures for biomedicine. Prog. Mater. Sci. 2021, 116, 100636. [Google Scholar] [CrossRef]
- Kolasinski, K.W.; Gimbar, N.J.; Yu, H.; Aindow, M.; Mäkilä, E.; Salonen, J. Regenerative Electroless Etching of Silicon. Angew. Chem. Int. Ed. Engl. 2017, 56, 624–627. [Google Scholar] [CrossRef]
- Kolasinski, K.W. Porous silicon formation by stain etching. In Handbook of Porous Silicon, 2nd ed.; Canham, L.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–21. [Google Scholar] [CrossRef]
- Huo, C.; Wang, J.; Fu, H.; Li, X.; Yang, Y.; Wang, H.; Mateen, A.; Farid, G.; Peng, K.-Q. Metal-Assisted Chemical Etching of Silicon in Oxidizing HF Solutions: Origin, Mechanism, Development, and Black Silicon Solar Cell Application. Adv. Func. Mater. 2020, 30, 2005744. [Google Scholar] [CrossRef]
- Kolasinski, K.W. Metal-Assisted Catalytic Etching (MACE) for Nanofabrication of Semiconductor Powders. Micromachines 2021, 12, 776. [Google Scholar] [CrossRef]
- Canham, L. Introductory lecture: Origins and applications of efficient visible photoluminescence from silicon-based nanostructures. Faraday Discuss. 2020, 222, 10–81. [Google Scholar] [CrossRef]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for In Vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef]
- Furey, B.J.; Silbaugh, D.A.; Yu, Y.; Guillaussier, A.C.; Estrada, A.D.; Stevens, C.; Maynard, J.A.; Korgel, B.A.; Downer, M.C. Measurement of Two-Photon Absorption of Silicon Nanocrystals in Colloidal Suspension for Bio-Imaging Applications. Phys. Status Solidi (b) 2018, 255, 1700501. [Google Scholar] [CrossRef]
- Kim, D.; Kang, J.; Wang, T.; Ryu, H.G.; Zuidema, J.M.; Joo, J.; Kim, M.; Huh, Y.; Jung, J.; Ahn, K.H.; et al. Two-Photon In Vivo Imaging with Porous Silicon Nanoparticles. Adv. Mater. 2017, 29, 1703309. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, Z.; Fontana, F.; Ding, Y.; Liu, D.; Hirvonen, J.T.; Santos, H.A. Tailoring Porous Silicon for Biomedical Applications: From Drug Delivery to Cancer Immunotherapy. Adv. Mater. 2018, 30, 1703740. [Google Scholar] [CrossRef]
- Kumeria, T.; McInnes, S.J.P.; Maher, S.; Santos, A. Porous silicon for drug delivery applications and theranostics: Recent advances, critical review and perspectives. Expert Opin. Drug Deliv. 2017, 14, 1407–1422. [Google Scholar] [CrossRef]
- Mäkilä, E.; Kivelä, H.; Shrestha, N.; Correia, A.; Kaasalainen, M.; Kukk, E.; Hirvonen, J.; Santos, H.A.; Salonen, J. Influence of Surface Chemistry on Ibuprofen Adsorption and Confinement in Mesoporous Silicon Microparticles. Langmuir 2016, 32, 13020–13029. [Google Scholar] [CrossRef]
- Turner, C.T.; Hasanzadeh Kafshgari, M.; Melville, E.; Delalat, B.; Harding, F.; Mäkilä, E.; Salonen, J.J.; Cowin, A.J.; Voelcker, N.H. Delivery of Flightless I siRNA from Porous Silicon Nanoparticles Improves Wound Healing in Mice. ACS Biomater. Sci. Eng. 2016, 2, 2339–2346. [Google Scholar] [CrossRef]
- Martins, J.P.; D’Auria, R.; Liu, D.; Fontana, F.; Ferreira, M.P.A.; Correia, A.; Kemell, M.; Moslova, K.; Mäkilä, E.; Salonen, J.; et al. Engineered Multifunctional Albumin-Decorated Porous Silicon Nanoparticles for FcRn Translocation of Insulin. Small 2018, 14, 1800462. [Google Scholar] [CrossRef]
- Jin, Y.S.; Kim, D.; Roh, H.; Kim, S.; Hussain, S.; Kang, J.Y.; Pack, C.G.; Kim, J.K.; Myung, S.J.; Ruoslahti, E.; et al. Tracking the Fate of Porous Silicon Nanoparticles Delivering a Peptide Payload by Intrinsic Photoluminescence Lifetime. Adv. Mater. 2018, 30, 1802878. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Ivask, A.; Sporleder, E.; Kaur, I.; Assan, Y.; Rao, S.; Warther, D.; Prestidge, C.A.; Durand, J.-O.; Voelcker, N.H. Dual-Action Cancer Therapy with Targeted Porous Silicon Nanovectors. Small 2017, 13, 1701201. [Google Scholar] [CrossRef]
- Henstock, J.R.; Canham, L.T.; Anderson, S.I. Silicon: The evolution of its use in biomaterials. Acta Biomater. 2015, 11, 17–26. [Google Scholar] [CrossRef]
- Low, S.; Voelcker, N.; Canham, L.; Williams, K. The biocompatibility of porous silicon in tissues of the eye. Biomaterials 2009, 30, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of highly porous materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2016, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Russo, L.; Colangelo, F.; Cioffi, R.; Rea, I.; Stefano, L.D. A Mechanochemical Approach to Porous Silicon Nanoparticles Fabrication. Materials 2011, 4, 1023–1033. [Google Scholar] [CrossRef]
- Kolasinski, K.W.; Swanson, J.D.; Roe, B.; Lee, T. Response of Photoluminescence of H-Terminated and Hydrosilylated Porous Si Powders to Rinsing and Temperature. Surfaces 2020, 3, 366–380. [Google Scholar] [CrossRef]
- Shao, Y.; Gan, Z.; Epifanovsky, E.; Gilbert, A.T.B.; Wormit, M.; Kussmann, J.; Lange, A.W.; Behn, A.; Deng, J.; Feng, X.; et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 2015, 113, 184–215. [Google Scholar] [CrossRef] [Green Version]
- Yakin, F.E.; Barisik, M.; Sen, T. Pore Size and Porosity Dependent Zeta Potentials of Mesoporous Silica Nanoparticles. J. Phys. Chem. C 2020, 124, 19579–19587. [Google Scholar] [CrossRef]
- Kolasinski, K.W.; Hartline, J.D.; Kelly, B.T.; Yadlovskiy, J. Dynamics of Porous Silicon Formation by Etching in HF + V2O5 Solutions. Mol. Phys. 2010, 108, 1033–1043. [Google Scholar] [CrossRef]
- Kolasinski, K.W.; Unger, B.A.; Ernst, A.T.; Aindow, M. Crystallographically Determined Etching and Its Relevance to the Metal-Assisted Catalytic Etching (MACE) of Silicon Powders. Front. Chem. 2019, 6, 651. [Google Scholar] [CrossRef]
- Jarvis, K.L.; Barnes, T.J.; Prestidge, C.A. Surface chemistry of porous silicon and implications for drug encapsulation and delivery applications. Adv. Colloid Interface Sci. 2012, 175, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Rupich, S.M.; Shafiq, N.; Gartstein, Y.N.; Malko, A.V.; Chabal, Y.J. Silicon Surface Modification and Characterization for Emergent Photovoltaic Applications Based on Energy Transfer. Chem. Rev. 2015, 115, 12764–12796. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; Zuilhof, H. Rapid Surface Functionalization of Hydrogen-Terminated Silicon by Alkyl Silanols. J. Am. Chem. Soc. 2017, 139, 5870–5876. [Google Scholar] [CrossRef] [Green Version]
- Kolasinski, K.W. Silicon surface photochemistry. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; Volume 2, pp. 611–620. [Google Scholar]
- Aswal, D.K.; Koiry, S.P.; Jousselme, B.; Gupta, S.K.; Palacin, S.; Yakhmi, J.V. Hybrid molecule-on-silicon nanoelectronics: Electrochemical processes for grafting and printing of monolayers. Phys. E Low-Dimens. Syst. Nanostructures 2009, 41, 325–344. [Google Scholar] [CrossRef]
- Dudley, M.E.; Kolasinski, K.W. Stain etching with Fe(III), V(V) and Ce(IV) to form microporous silicon. Electrochem. Solid State Lett. 2009, 12, D22–D26. [Google Scholar] [CrossRef]
- van den Boom, A.F.J.; Pujari, S.P.; Bannani, F.; Driss, H.; Zuilhof, H. Fast room-temperature functionalization of silicon nanoparticles using alkyl silanols. Faraday Discuss. 2020, 222, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Pujari, S.P.; Driss, H.; Bannani, F.; van Lagen, B.; Zuilhof, H. One-Pot Gram-Scale Synthesis of Hydrogen-Terminated Silicon Nanoparticles. Chem. Mater. 2018, 30, 6503–6512. [Google Scholar] [CrossRef] [PubMed]
- Linford, M.R.; Chidsey, C.E.D. Alkyl monolayers covalently bonded to silicon surfaces. J. Am. Chem. Soc. 1993, 115, 12631–12632. [Google Scholar] [CrossRef]
- Kolasinski, K.W. Photochemical and nonthermal chemical modification of porous silicon. In Porous Silicon for Biomedical Applications; Santos, H., Ed.; Woodhead Publishing (Elsevier): Duxford, UK, 2021; pp. 51–112. [Google Scholar]
- Gräf, D.; Grundner, M.; Schulz, R.; Muhlhoff, L. Oxidation of HF-Treated Si Wafer Surfaces in Air. J. Appl. Phys. 1990, 68, 5155–5161. [Google Scholar] [CrossRef]
- Gräf, D.; Grundner, M.; Schulz, R. Reaction of Water with Hydrofluoric-Acid Treated Silicon(111) and (100) Surfaces. J. Vac. Sci. Technol. A 1989, 7, 808–813. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiPietro, J.A.; Kolasinski, K.W. Characterization of Mechanochemical Modification of Porous Silicon with Arginine. Surfaces 2022, 5, 143-154. https://doi.org/10.3390/surfaces5010007
DiPietro JA, Kolasinski KW. Characterization of Mechanochemical Modification of Porous Silicon with Arginine. Surfaces. 2022; 5(1):143-154. https://doi.org/10.3390/surfaces5010007
Chicago/Turabian StyleDiPietro, Jacklyn A., and Kurt W. Kolasinski. 2022. "Characterization of Mechanochemical Modification of Porous Silicon with Arginine" Surfaces 5, no. 1: 143-154. https://doi.org/10.3390/surfaces5010007
APA StyleDiPietro, J. A., & Kolasinski, K. W. (2022). Characterization of Mechanochemical Modification of Porous Silicon with Arginine. Surfaces, 5(1), 143-154. https://doi.org/10.3390/surfaces5010007