Preparation of Adsorbent from Mechanochemical Reaction-Based Waste Seashell with Sodium Oxalate and Its Application in Pb Ion Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Scallop Shell
2.1.2. Chemicals
2.2. Preparation of Adsorbent
2.3. Characterization of OS Adsorbent
2.3.1. Scanning Electron Microscopy Image
2.3.2. Measurement of Particle Size Distribution
2.3.3. Measurement of X-ray Diffraction Pattern
2.3.4. FT-IR Measurement
2.4. Procedure of Adsorption Experiment
3. Results and Discussion
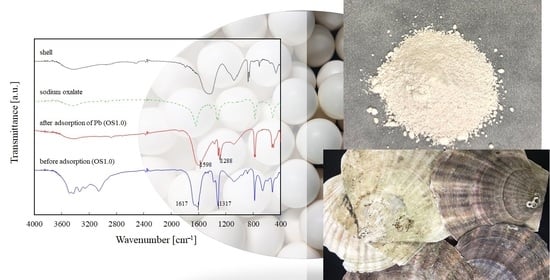
3.1. Characterization of OS Adsorbent
3.2. Adsorption Kinetics of Pb Ions onto OS Adsorbents
3.3. Adsorption Equilibrium Relationship between Pb Ions and Surface of OS Adsorbents
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Ce | equilibrium concentration of Pb ions [mol/m3] |
| Ci | initial concentration of of Pb ions [mol/m3] |
| Ct | concentration of Pb ions at time, t [mol/m3] |
| K | equilibrium adsorption constant defined in Equation (4) [m3/mol] |
| ka | adsorption rate constant defined in Equation (3) [m3/(mol min)] |
| kd | desorption rate constant defined in Equation (3) [min−1] |
| m | mass of adsorbent [kg] |
| t | time [min] |
| Xe | equilibrium amount of Pb ions adsorbed [mol/kg] |
| Xs | saturated amount of Pb ions adsorbed defined in Equation (4) [mol/kg] |
| Xt | amount of Pb ions adsorbed at time, t [mol/kg] |
| V | volume of liquid [m3] |
References
- Ou, Z.; Li, J.; Wang, Z. Application of mechanochemistry to metal recovery from second-hand resources: A technical overview. Environ. Sci. Process. Impacts 2015, 17, 1522–1530. [Google Scholar] [CrossRef]
- Tsuzuki, T. Mechanochemical synthesis of metal oxide nanoparticles. Commun. Chem. 2021, 4, 143. [Google Scholar] [CrossRef]
- Dubadi, R.; Huang, S.D.; Jaroniec, M. Mechanochemical synthesis of nanoparticles for potential antimicrobial applications. Materials 2023, 16, 1460. [Google Scholar] [CrossRef]
- Alrbaihat, M. A review of solid state mechanochemistry for drug synthesis and modification. AIP Conf. Proc. 2023, 2834, 030019. [Google Scholar]
- Wu, K.; Ju, T.; Deng, Y.; Xi, J. Mechanochemical assisted extraction: A novel, efficient, eco-friendly technology. Trends Food Sci. Technol. 2017, 66, 166–175. [Google Scholar] [CrossRef]
- Fan, L.; Fan, W.; Mei, Y.; Liu, L.; Li, L.; Wang, Z.; Yang, L. Mechanochemical assisted extraction as a green approach in preparation of bioactive components extraction from natural products—A review. Trends Food Sci. Technol. 2022, 129, 98–110. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical Synthesis of Catalytic Materials. Chem. Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Yu, C.; Fang, H.; Li, Z. Mechanochemical preparation of single atom catalysts for versatile catalytic applications: A perspective review. Mater. Today 2023, 63, 288–312. [Google Scholar] [CrossRef]
- Seyedi, M.; Haratian, S.; Khaki, J.V. Mechanochemical Synthesis of Fe2O3 Nanoparticles. Procedia Mater. Sci. 2015, 11, 309–313. [Google Scholar] [CrossRef]
- Molla, A.; Youk, J.H. Mechanochemical synthesis of graphitic carbon nitride/graphene oxide nanocomposites for dye sorption. Dye. Pigment. 2023, 220, 111725. [Google Scholar] [CrossRef]
- Tamjidi, S.; Ameri, A. A review of the application of sea material shells as low cost and effective bio-adsorbent for removal of heavy metals from wastewater. Environ. Sci. Pollut. Res. 2020, 27, 31105–31119. [Google Scholar] [CrossRef]
- AbiD, B.A.; BrbootI, M.M.; Al-ShuwaikI, N.M. Removal of heavy metals using chemicals precipitation. Eng. Technol. J. 2011, 29, 595–612. [Google Scholar] [CrossRef]
- Al-Enezi, G.; Hamoda, M.F.; Fawzi, N. Ion exchange extraction of heavy metals from wastewater sludges. J. Environ. Sci. Health Part A 2011, 39, 455–464. [Google Scholar] [CrossRef]
- Birniwa, A.H.; Habibu, S.; Abdullahi, S.S.; Mohammad, R.E.A.; Hussaini, A.; Magaji, H.; Al-Dhawi, B.N.S.; Noor, A.; Jagaba, A.H. Membrane technologies for heavy metals removal from water and wastewater: A mini review. Case Stud. Chem. Environ. Eng. 2024, 9, 100538. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Pan, S.-Y.; Zhu, S.; Yu, Y.; Zheng, H. Performance evaluation and optimization of flocculation process for removing heavy metal. Chem. Eng. J. 2019, 385, 123911. [Google Scholar] [CrossRef]
- Yang, L.; Hu, W.; Chang, Z.; Liu, T.; Fang, D.; Shao, P.; Shi, H.; Luo, X. Electrochemical recovery and high value-added reutilization of heavy metal ions from wastewater: Recent advances and future trends. Environ. Int. 2021, 152, 106512. [Google Scholar] [CrossRef]
- Bozbaş, S.K.; Boz, Y. Low-cost biosorbent: Anadara inaequivalvis shells for removal of Pb(II) and Cu(II) from aqueous solution. Process. Saf. Environ. Prot. 2016, 103, 144–152. [Google Scholar] [CrossRef]
- Khankhaje, E.; Kim, T.; Jang, H.; Rafieizonooz, M. Laboratory evaluation of heavy metal removal from stormwater runoff by pervious concrete pavement containing seashell and oil palm kernel shell. Constr. Build. Mater. 2023, 400, 132648. [Google Scholar] [CrossRef]
- Bulut, A.; Yusan, S.; Aytas, S.; Sert, S. The use of sea shell (Donax trunculus) powder to remove Sr(II) ions from aqueous solutions. Water Sci. Technol. 2018, 78, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, F.; Ouyang, X.-K.; Yang, L.-Y.; Wang, Y. Adsorption of Pb(II) from Aqueous Solution by Mussel Shell-Based Adsorbent: Preparation, Characterization, and Adsorption Performance. Materials 2021, 14, 741. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Tamjidi, S.; Abed, M. Removal of Cu(II), Co(II) and Pb(II) from synthetic and real wastewater using calcified Solamen Vaillanti snail shell. Desalination Water Treat. 2020, 174, 324–335. [Google Scholar] [CrossRef]
- Allaoui, M.; Berradi, M.; Bensalah, J.; Es-Sahbany, H.; Dagdag, O.; Ibn Ahmed, S. Study of the adsorption of nickel ions on the sea shells of Mehdia: Kinetic and thermodynamic study and mathematical modelling of experimental data. Mater. Today Proc. 2021, 45, 7494–7500. [Google Scholar] [CrossRef]
- Li, T.; Xin, R.; Wang, D.; Yuan, L.; Wu, D.; Wu, X. Research Progress on the Applications of Seashell Adsorption Behaviors in Cement-Based Materials. Buildings 2023, 13, 1289. [Google Scholar] [CrossRef]
- Ketwong, C.; Trisupakitti, S.; Nausri, C.; Senajuk, W. Removal of heavy metal from synthetic wastewater using calcined golden apple snail shells. Naresuan Univ. J. Sci. Technol. 2018, 26, 61–70. [Google Scholar] [CrossRef]
- Pérez, B.F.; Espina, J.A.; Fernández González, M.L.Á. Adsorption of Heavy Metals Ions from Mining Metallurgical Tailings Leachate Using a Shell-Based Adsorbent: Characterization, Kinetics and Isotherm Studies. Materials 2022, 15, 5315. [Google Scholar] [CrossRef]
- Rehan, U.S.M.; Zahir, E.; Asghar, M.A. A prudent approach for the removal of copper (II) and cadmium (II) ions from aqueous solutions using indigenous Mactra aequisulcata shells. J. Serbian Chem. Soc. 2021, 86, 767–780. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Z.; Li, X.; Xu, H.; Li, X. Recycling of shell wastes into nanosized calcium carbonate powders with different phase compositions. J. Clean. Prod. 2015, 92, 223–229. [Google Scholar] [CrossRef]
- Lu, J.; Cong, X.; Li, Y.; Hao, Y.; Wang, C. Scalable recycling of oyster shells into high purity calcite powders by the mechanochemical and hydrothermal treatments. J. Clean. Prod. 2018, 172, 1978–1985. [Google Scholar] [CrossRef]
- Kurosaki, Y. Graduate School of Fisheries Sciences. Master’s Thesis, Hokkaido University, Hokkaido, Japan, 2021. (In Japanese). [Google Scholar]
- Sugata, N.; Watanabe, S. Poous Concrete with Crashed Scallop Shell; Japan Society of Civil Engineers: Tokyo, Japan, 2007; Volume 64, p. E-6. (In Japanese) [Google Scholar]
- Kobayashi, K.; Nakafusa, S.; Matsumoto, K.; Kobayashi, N.; Morii, T.; Saka, E. Fundamental Study on Strength Oriperties and Particle Breakage of the Scallop Shell, Laboratory Technical Report (Tobishima Corporation). 2013; Volume 62, pp. 75–76. Available online: https://www.tobishima.co.jp/laboratory/technique/pdf/62/gihou_62-2013-15.pdf (accessed on 30 January 2024). (In Japanese).
- Giordano, L.; Ferraro, L.; Caroppo, C.; Rubino, F.; Buonocunto, F.; Maddalena, P. A method for bivalve shells characterization by FT-IR photoacoustic spectroscopy as a tool for environmental studies. MethodsX 2022, 9, 101672. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.N.; Bin Abu Bakar, M.Z.; Ali, M.E.; Bin Hussein, M.Z.; Noordin, M.M.; Loqman, M.Y.; Miah, G.; Wahid, H.; Hashim, U. A novel method for the synthesis of calcium carbonate (aragonite) nanoparticles from cockle shells. Powder Technol. 2013, 235, 70–75. [Google Scholar] [CrossRef]
- Islam, K.N.; Bin Abu Bakar, Z.; Noordin, M.M.; Bin Hussein, M.Z.; Rahman, N.S.B.A.; Ali, E. Characterisation of calcium carbonate and its polymorphs from cockle shells (Anadara granosa). Powder Technol. 2011, 213, 188–191. [Google Scholar] [CrossRef]
- Leukel, S.; Panthöfer, M.; Mondeshki, M.; Kieslich, G.; Wu, Y.; Krautwurst, N.; Tremel, W. Tremel, Mechanochemical access to defect-stabilized amorphous calcium carbonate. Chem. Mater. 2018, 30, 6040–6052. [Google Scholar] [CrossRef]
- Ouyang, J.-M.; Duan, L.; Tieke, B. Effects of carboxylic acids on the crystal growth of calcium oxalate nanoparticles in lecithin-water liposome systems. Langmuir 2003, 19, 8980–8985. [Google Scholar] [CrossRef]
- Parekh, B.B.; Vyas, P.M.; Vasant, S.R.; Joshi, M.J. Thermal, FT–IR and dielectric studies of gel grown sodium oxalate single crystals. Bull. Mater. Sci. 2008, 31, 143–147. [Google Scholar] [CrossRef]
- Tang, S.-F.; Zhou, H.; Tan, W.-T.; Huang, J.-G.; Zeng, P.; Gu, J.-F.; Liao, B.-H. Adsorption characteristics and mechanisms of Fe-Mn oxide modified biochar for Pb(II) in wastewater. Int. J. Environ. Res. Public Health 2022, 19, 8420. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, M.M.; Yatim, N.I.; Hamzah, S. Synthesis and charaterization of hydroxyapatite from bulk seashells and its potential usage as lead ions adsorbent. Malays. J. Anal. Sci. 2017, 21, 571–584. [Google Scholar] [CrossRef]
- Harripersadth, C.; Musonge, P.; Isa, Y.M.; Morales, M.G.; Sayago, A. The application of eggshells and sugarcane bagasse as potential biomaterials in the removal of heavy metals from aqueous solutions. S. Afr. J. Chem. Eng. 2020, 34, 142–150. [Google Scholar] [CrossRef]
- Okolo, B.I.; Oke, E.O.; Agu, C.M.; Adeyi, O.; Nwoso-Obieogu, K.; Akatobi, K.N. Adsorption of lead(II) from aqueous solution using Africa elemi seed, mucuna shell and oyster shell as adsorbents and optimization using Box–Behnken design. Appl. Water Sci. 2020, 10, 201. [Google Scholar] [CrossRef]
- Feng, N.-C.; Guo, X.-Y.; Liang, S. Kinetic and thermodynamic studies on biosorption of Cu(II) by chemically modified orange peel. Trans. Nonferrous Met. Soc. China 2009, 19, 1365–1370. [Google Scholar] [CrossRef]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Milojković, J.; Mihajlović, M.; Stanojević, M.; Stanković, S. Removal of Pb2+ ions by raw corn silk (Zea mays L.) as a novel biosorbent. J. Taiwan Inst. Chem. Eng. 2016, 58, 407–416. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.-S. Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Bi, F.-Z.; Wang, Q.-J.; Wang, W.-L.; Liu, B.; Lutts, S.; Wei, W.; Zhao, Y.-P.; Wang, G.-X.; Han, R.-M. Characteristics and influencing factors of cadmium biosorption by the stem powder of the invasive plant species Solidago canadensis. Ecol. Eng. 2018, 121, 12–18. [Google Scholar] [CrossRef]






| Langmuir Adsorption Kinetics Parameters | |||||
|---|---|---|---|---|---|
| ka [L/(mol min)] | kd [min−1] | Xe [mol/g] | ka/kd [L/mol] | R2 | |
| OS0.5 | 22.48 | 6.99 × 10−4 | 5.21 × 10−3 | 3.21 × 104 | 0.997 |
| OS1.0 | 18.78 | 3.50 × 10−4 | 5.80 × 10−3 | 5.36 × 104 | 0.994 |
| OS2.0 | 23.43 | 3.51 × 10−4 | 5.93 × 10−3 | 6.67 × 104 | 0.995 |
| shell (control) | 12.54 | 8.99 × 10−4 | 6.60 × 10−3 | 1.40 × 104 | 0.579 |
| Langmuir isotherm parameters | |||||
| K [L/mol] | Xs [mol/g] | R2 | |||
| OS0.5 | 3.45 × 104 | 5.45 × 10−3 | 0.991 | ||
| OS1.0 | 5.39 × 104 | 5.75 × 10−3 | 0.961 | ||
| OS2.0 | 6.29 × 104 | 6.23 × 10−3 | 0.960 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, H. Preparation of Adsorbent from Mechanochemical Reaction-Based Waste Seashell with Sodium Oxalate and Its Application in Pb Ion Adsorption. Surfaces 2024, 7, 208-224. https://doi.org/10.3390/surfaces7020014
Maruyama H. Preparation of Adsorbent from Mechanochemical Reaction-Based Waste Seashell with Sodium Oxalate and Its Application in Pb Ion Adsorption. Surfaces. 2024; 7(2):208-224. https://doi.org/10.3390/surfaces7020014
Chicago/Turabian StyleMaruyama, Hideo. 2024. "Preparation of Adsorbent from Mechanochemical Reaction-Based Waste Seashell with Sodium Oxalate and Its Application in Pb Ion Adsorption" Surfaces 7, no. 2: 208-224. https://doi.org/10.3390/surfaces7020014
APA StyleMaruyama, H. (2024). Preparation of Adsorbent from Mechanochemical Reaction-Based Waste Seashell with Sodium Oxalate and Its Application in Pb Ion Adsorption. Surfaces, 7(2), 208-224. https://doi.org/10.3390/surfaces7020014