3D-Printed Antibacterial Scaffolds for the Regeneration of Alveolar Bone in Severe Periodontitis
Abstract
:1. Introduction
2. Results
2.1. Scaffold Morphological Characteristics
2.2. Drug Loaded Scaffolds—Entrapment and In Vitro Release Evaluation
2.3. Physicochemical Characterisation of Scaffolds
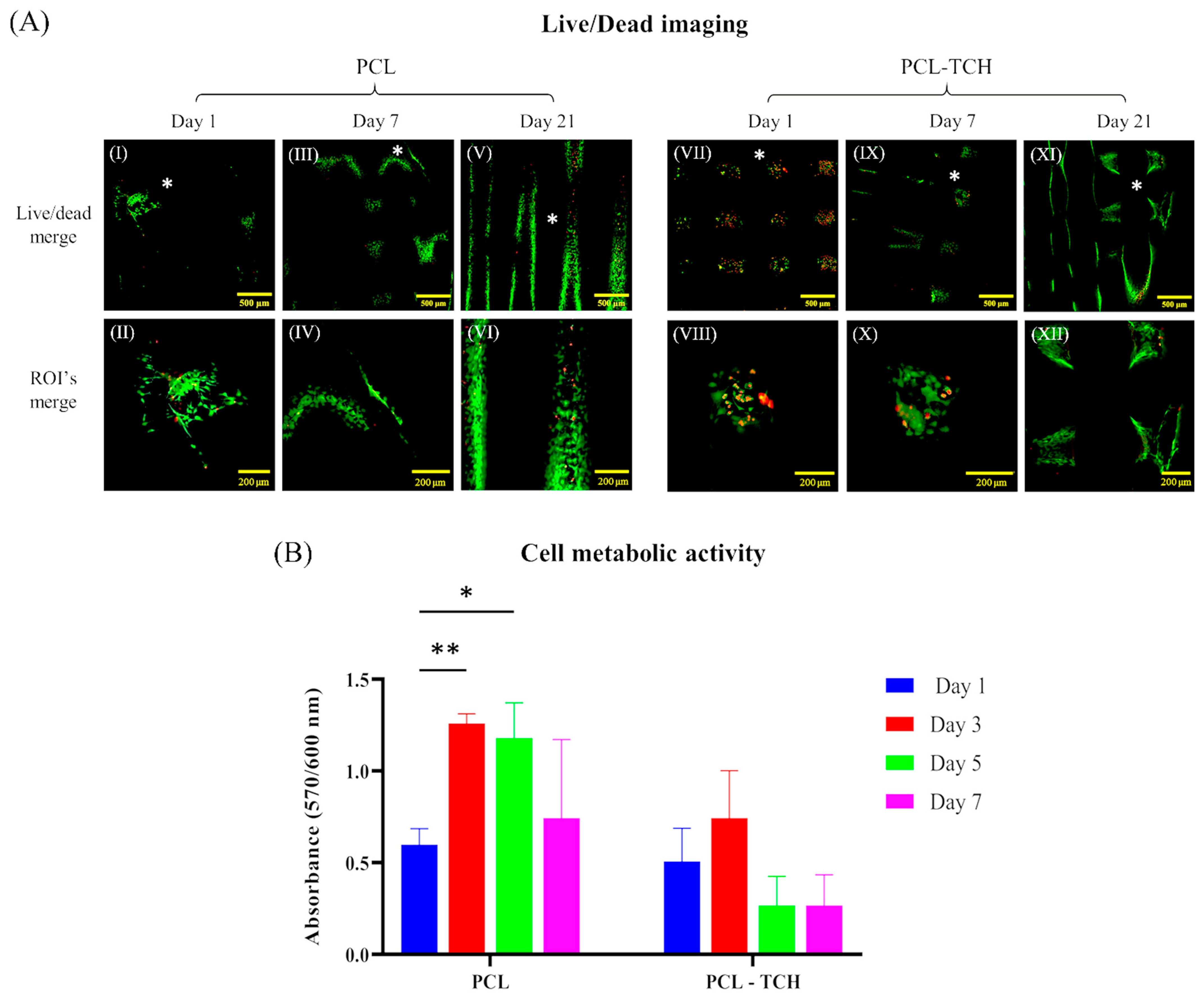
2.4. Cell Growth and Proliferation within Scaffolds
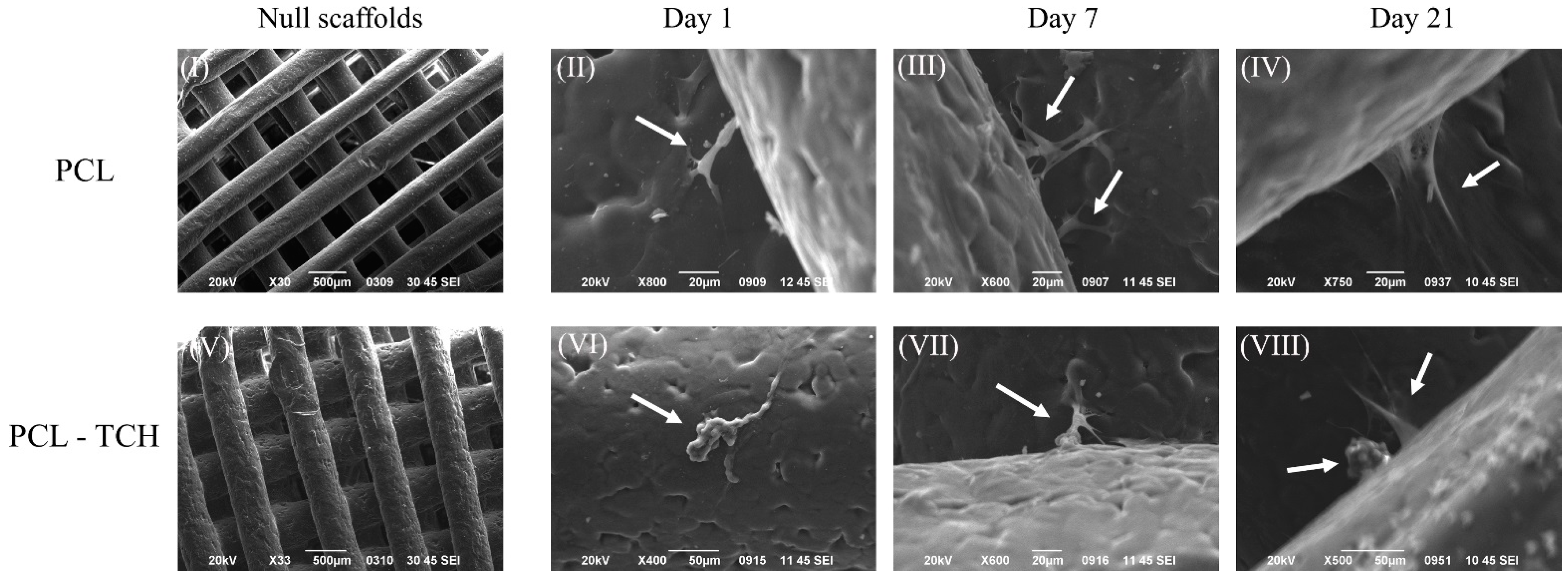
2.5. SEM Analysis of Cell-Seeded Scaffolds
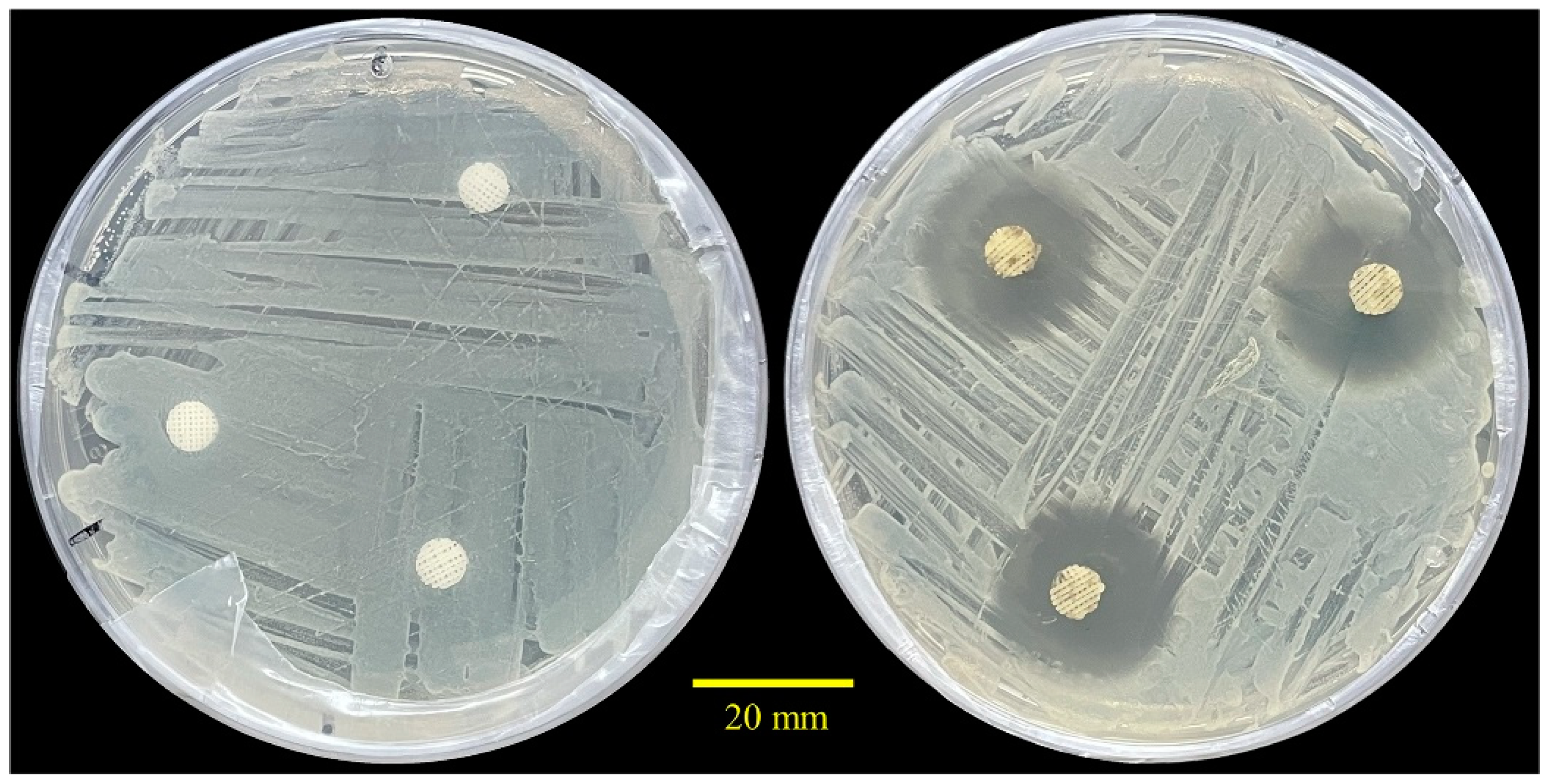
2.6. Antibacterial Performance
2.7. Osteogenic Differentiation and Bone Formation in Scaffolds
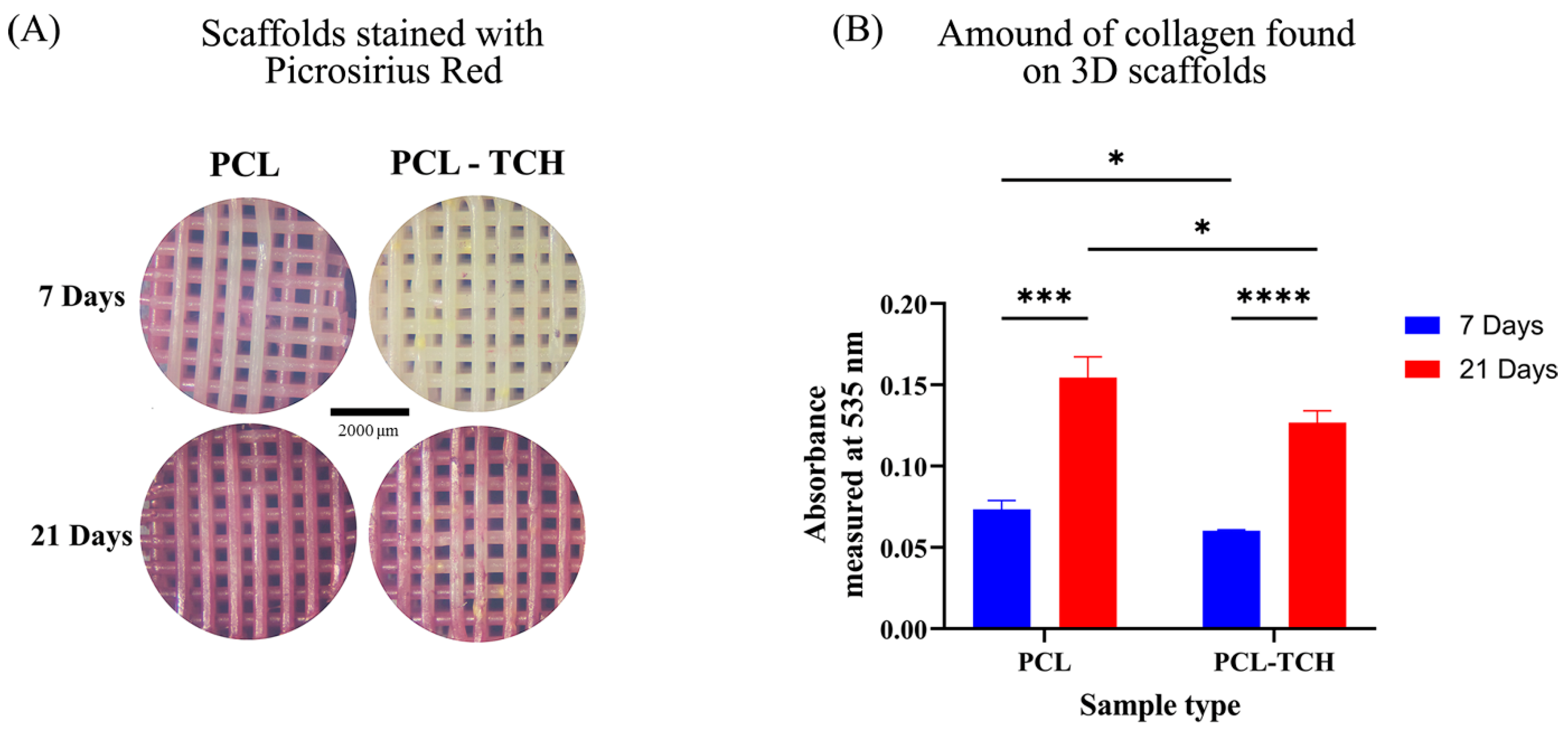
2.7.1. Collagen Content
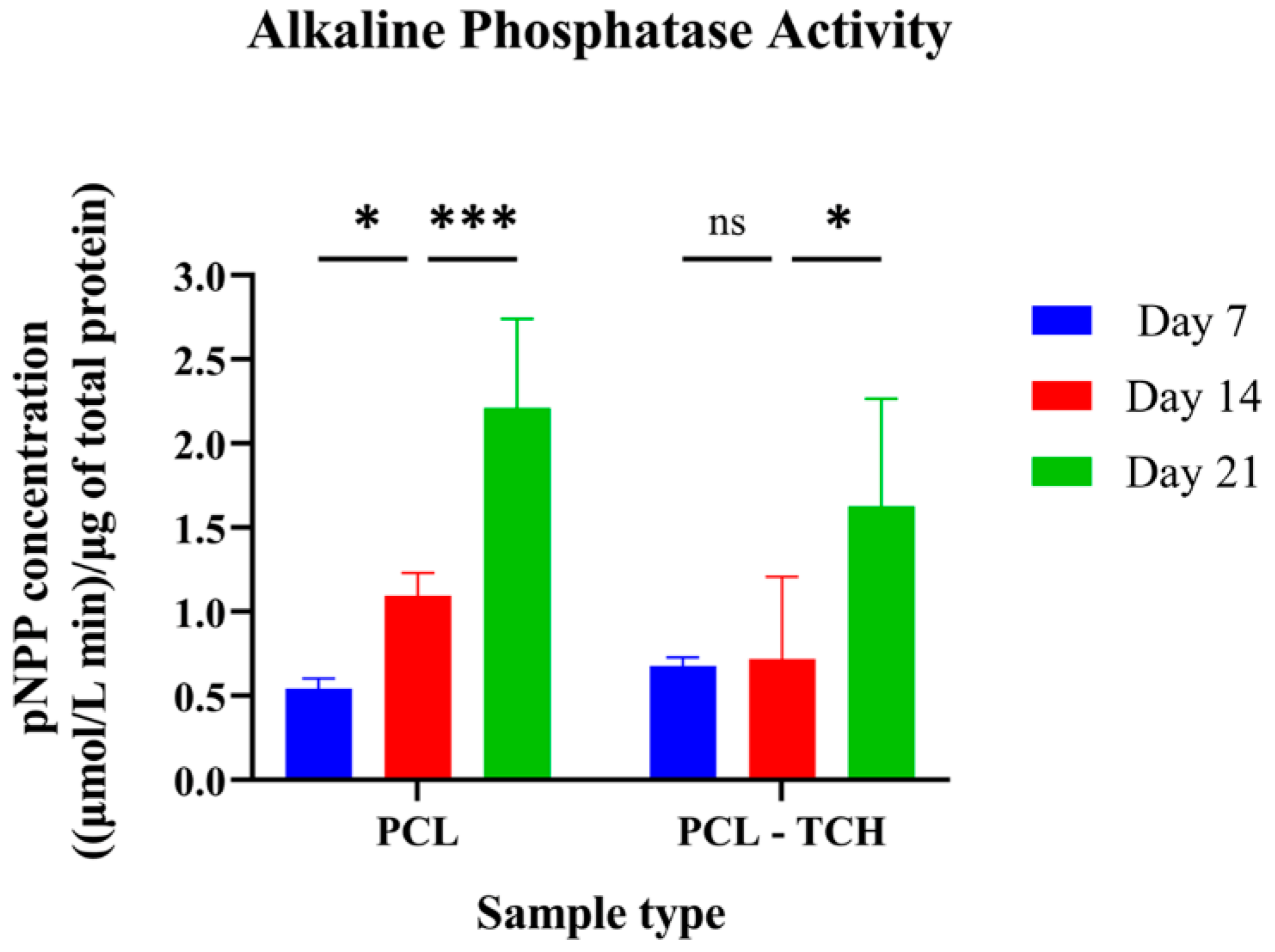
2.7.2. Determination of the ALP Activity
2.7.3. Bone Tissue Formation and Labelling
3. Discussion
3.1. Bioactive Scaffolds and Their Physiochemical Characteristics
3.2. Bioactive Scaffolds and Their Potential to Bone Regeneration
3.3. Challenges and Future Perspectives
4. Materials and Methods
4.1. Material Preparation, for Neat and Drug-Loaded Scaffolds
4.2. Drug Entrapment Efficiency
4.3. In Vitro Release Kinetics
4.4. Scaffold Characterisation
4.4.1. Morphological Characterisation
4.4.2. Thermal Analysis
4.5. Cell Seeding, Proliferation and Osteogenic Induction
4.6. Cell Viability Assessment
4.6.1. Cell Viability Assessment with the AlamarBlue™
4.6.2. Cell Viability Assessment by Live/Dead Staining
4.7. Cell-Seeded Scaffolds Morphology
4.8. Preosteoblasts Differentiation and Osteogenesis
4.8.1. Alkaline Phosphatase Activity
4.8.2. Collagen Content
4.8.3. Mineralised Bone Tissue Labeling
4.9. Antibacterial Assessment
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Song, K.S.; Marao, H.F.; Mao, J.J. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral Biol. 2016, 18, 1–8. [Google Scholar]
- Listgarten, M.A. Pathogenesis of periodontitis. J. Clin. Periodontol. 1986, 13, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L.M.; Walker, C.B. Treating chronic periodontitis: Current status, challenges, and future directions. Clin. Cosmet. Investig. Dent. 2010, 2, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Decker, A.; Nibali, L.; Pilipchuk, S.; Berglundh, T.; Giannobile, W. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2015, 95, 255–266. [Google Scholar] [CrossRef]
- Sufaru, I.-G.; Macovei, G.; Stoleriu, S.; Martu, M.-A.; Luchian, I.; Kappenberg-Nitescu, D.-C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Won, J.-Y.; Park, J.-H.; Bae, J.-H.; Ahn, G.; Kim, C.-H.; Lim, D.-H.; Cho, D.-W.; Yun, W.-S.; Bae, E.-B.; et al. Effects of 3D-Printed Polycaprolactone/β-Tricalcium Phosphate Membranes on Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-Dimensional Printed Multiphase Scaffolds for Regeneration of Periodontium Complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94 (Suppl. 9), 153S–157S. [Google Scholar] [CrossRef]
- Park, C.H. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. Int. J. Mol. Sci. 2019, 20, 4364. [Google Scholar] [CrossRef]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater. Sci. Eng. C 2016, 58, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeei, S.; Moghadam, F.; Asare-Addo, K.; Nokhodchi, A. Design of a nanofibrous guided tissue regeneration carrier as a potential drug delivery system for tetracycline hydrochloride in the management of periodontitis. J. Drug Deliv. Sci. Technol. 2022, 75, 103722. [Google Scholar] [CrossRef]
- LlindheT, J.; Heijl, L.; Goodson, J.M.; Socransky, S.S. Local tetracycline delivery using hollow fiber devices in periodontal therapy. J. Clin. Periodontol. 1979, 6, 141–149. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; de Annunzio, S.R.; Carmello, J.C.; Pavarina, A.C.; Fontana, C.R.; Correa, D.S. Combining Coaxial Electrospinning and 3D Printing: Design of Biodegradable Bilayered Membranes with Dual Drug Delivery Capability for Periodontitis Treatment. ACS Appl. Bio Mater. 2022, 5, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Khodir, W.W.A.; Guarino, V.; Alvarez-Perez, M.; Cafiero, C.; Ambrosio, L. Trapping tetracycline-loaded nanoparticles into polycaprolactone fiber networks for periodontal regeneration therapy. J. Bioact. Compat. Polym. 2013, 28, 258–273. [Google Scholar] [CrossRef]
- Shenoy, D.; Little, S.; Langer, R.; Amiji, M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: Part In vivo distribution and tumor localization studies. Pharm. Res. 2005, 22, 2107–2114. [Google Scholar] [CrossRef]
- Kuru, L.; Griffiths, G.S.; Petrie, A.; Olsen, I. Alkaline phosphatase activity is up regulated in regenerating human periodontal cells. J. Periodontal Res. 1999, 34, 123–127. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef]
- Jain, N.; Jain, G.K.; Javed, S.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Recent approaches for the treatment of periodontitis. Drug Discov. Today 2008, 13, 932–943. [Google Scholar] [CrossRef]
- Schallhorn, R.A.; McClain, P.K.; Benhamou, V.; Doobrow, J.H.; Grandin, H.M.; Kasaj, A. Application of enamel matrix derivative in conjunction with non-surgical therapy for treatment of moderate to severe periodontitis: A 12-month, randomized prospective, multicenter study. J. Periodontol. 2021, 92, 619–628. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Ramaglia, L.; Isola, G.; Blasi, A.; Salvi, G.E.; Sculean, A. Changes in clinical parameters following adjunctive local sodium hypochlorite gel in minimally invasive nonsurgical therapy (MINST) of periodontal pockets: A 6-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5331–5340. [Google Scholar] [CrossRef] [PubMed]
- Sultan, N.; Jafri, Z.; Sawai, M.; Bhardwaj, A. Minimally invasive periodontal therapy. J. Oral Biol. Craniofacial Res. 2020, 10, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Dhondt, R.; Dekeyser, C.; Quirynen, M. Treatment of aggressive periodontitis. Periodontology 2000 2014, 65, 107–133. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Muschler, G.F.; Nakamoto, C.; Griffith, L.G. Engineering principles of clinical cell-based tissue engineering. Minerva Anestesiol. 2004, 86, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Kim, I.G.; Tran, H.N.; Cho, H.; Janarthanan, G.; Noh, I.; Chung, E.-J. Three-Dimensional Printed Design of Antibiotic-Releasing Esophageal Patches for Antimicrobial Activity Prevention. Tissue Eng. Part A 2021, 27, 1490–1502. [Google Scholar] [CrossRef]
- Li, W.; Dai, F.; Zhang, S.; Xu, F.; Xu, Z.; Liao, S.; Zeng, L.; Song, L.; Ai, F. Pore Size of 3D-Printed Polycaprolactone/Polyethylene Glycol/Hydroxyapatite Scaffolds Affects Bone Regeneration by Modulating Macrophage Polarization and the Foreign Body Response. ACS Appl. Mater. Interfaces 2022, 14, 20693–20707. [Google Scholar] [CrossRef]
- Tamjid, E.; Bohlouli, M.; Mohammadi, S.; Alipour, H.; Nikkhah, M. Sustainable drug release from highly porous and architecturally engineered composite scaffolds prepared by 3D printing. J. Biomed. Mater. Res. Part A 2020, 108, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; He, Z.; Liu, Y.; Liu, X.; He, R.; Deng, G.; Peng, Z.; Liu, J.; Luo, Z.; He, X.; et al. Cryogenic 3D Printing of w/o Pickering Emulsions Containing Bifunctional Drugs for Producing Hierarchically Porous Bone Tissue Engineering Scaffolds with Antibacterial Capability. Int. J. Mol. Sci. 2022, 23, 9722. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Birnbaum, J.C.; Johnson, T.J.; Kelly-Gorham, M.R.K.; Lindenmaier, R.; Myers, T.L. Tetra-cycline Hydrochloride. In NIST Standard Reference Database 69, NIST Chemistry WebBook; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Cervini, P.; Machado, L.C.M.; Ferreira, A.P.G.; Ambrozini, B. Thermal decomposition of tetracycline and chlor-tetracycline. J. Anal. Appl. Pyrolysis 2016, 118, 317–324. [Google Scholar] [CrossRef]
- Theodoridis, K.; Aggelidou, E.; Manthou, M.-E.; Kritis, A. Hypoxia Promotes Cartilage Regeneration in Cell-Seeded 3D-Printed Bioscaffolds Cultured with a Bespoke 3D Culture Device. Int. J. Mol. Sci. 2023, 24, 6040. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, K.; Aggelidou, E.; Vavilis, T.; Manthou, M.E.; Tsimponis, A.; Demiri, E.C.; Boukla, A.; Salpistis, C.; Bakopoulou, A.; Mihailidis, A.; et al. Hyaline cartilage next generation implants from adipose-tissue-derived mesenchymal stem cells: Comparative study on 3D-printed polycaprolactone scaffold patterns. J. Tissue Eng. Regen. Med. 2019, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, K.; Aggelidou, E.; Manthou, M.; Demiri, E.; Bakopoulou, A.; Kritis, A. Assessment of cartilage regeneration on 3D collagen-polycaprolactone scaffolds: Evaluation of growth media in static and in perfusion bioreactor dynamic culture. Colloids Surfaces B Biointerfaces 2019, 183, 110403. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Kantorski, K.Z.; Dubey, N.; Daghrery, A.; Fenno, J.C.; Mishina, Y.; Chan, H.-L.; Mendonça, G.; Bottino, M.C. Personalized and Defect-Specific Antibi-otic-Laden Scaffolds for Periodontal Infection Ablation. ACS Appl. Mater. Interfaces 2021, 13, 49642–49657. [Google Scholar] [CrossRef]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef]
- Boer, R.E.; Schneekloth, J.S. Targeting Mammalian Translational Inhibition with Tetracyclines. Cell Chem. Biol. 2018, 25, 1437–1438. [Google Scholar] [CrossRef]
- Park, J.-B. Low dose of doxycyline promotes early differentiation of preosteoblasts by partially regulating the expression of es-trogen receptors. J. Surg. Res. 2012, 178, 737–742. [Google Scholar] [CrossRef]
- Park, J.-B. Effects of Doxycycline, Minocycline, and Tetracycline on Cell Proliferation, Differentiation, and Protein Expression in Osteoprecursor Cells. J. Craniofacial Surg. 2011, 22, 1839–1842. [Google Scholar] [CrossRef]
- Cheng, W.; Yue, Y.; Fan, W.; Hu, Y.; Wang, X.; Pan, X.; Zhou, X.; Qin, L.; Zhang, P. Effects of tetracyclines on bones: An ambiguous question needs to be clarified. Die Pharmazie-Int. J. Pharm. Sci. 2012, 67, 457–459. [Google Scholar]
- Martin, V.; Bettencourt, A.F.; Santos, C.; Fernandes, M.H.; Gomes, P.S. Unveiling the Osteogenic Potential of Tetracyclines: A Comparative Study in Human Mesenchymal Stem Cells. Cells 2023, 12, 2244. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Fernandes, M.H. Effect of therapeutic levels of doxycycline and minocycline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch. Oral Biol. 2007, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Donahue, H.J.; Iijima, K.; Goligorsky, M.S.; Rubin, C.T.; Rifkin, B.R. Regulation of cytoplasmic calcium concentration in tetracy-cline-treated osteoclasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1992, 7, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Ramamurthy, N.S.; Kaneko, H.; Sasaki, T.; Rifkin, B.; McNamara, T.F. Tetracycline administration prevents diabe-tes-induced osteopenia in the rat: Initial observations. Res. Commun. Chem. Pathol. Pharmacol. 1990, 68, 27–40. [Google Scholar]
- Kim, Y.; Kim, J.; Lee, H.; Shin, W.-R.; Lee, S.; Lee, J.; Park, J.-I.; Jhun, B.H.; Kim, Y.-H.; Yi, S.-J.; et al. Tetracycline Analogs Inhibit Osteoclast Differentiation by Suppressing MMP-9-Mediated Histone H3 Cleavage. Int. J. Mol. Sci. 2019, 20, 4038. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J.H.; Choi, E.H.; Han, I. Promotion of osteogenic differentiation by non-thermal biocompatible plasma treated chitosan scaffold. Sci. Rep. 2019, 9, 3712. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar]
- Tzaphlidou, M. Bone Architecture: Collagen Structure and Calcium/Phosphorus Maps. J. Biol. Phys. 2008, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Dayaghi, E.; Bakhsheshi-Rad, H.; Hamzah, E.; Akhavan-Farid, A.; Ismail, A.; Aziz, M.; Abdolahi, E. Magnesium-zinc scaffold loaded with tetracycline for tissue engineering application: In vitro cell biology and antibacterial activity assessment. Mater. Sci. Eng. C 2019, 102, 53–65. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Sahrapeyma, H.; Ehterami, A.; Goodarzi, A.; Rahmati, M.; Lakalayeh, G.A.; Ghorbani, S.; Vaez, A.; Salehi, M. Tetracycline hydrochlo-ride-containing poly (ε-caprolactone)/poly lactic acid scaffold for bone tissue engineering application: In vitro and in vivo study. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 472–479. [Google Scholar] [CrossRef]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.S.; Caseiro, A.R.; Guedes, F.; Patrício, T.; Viana, T.; et al. 3D Printed Poly(ε-caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, D.; Shim, J.-H.; Nam, W. Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci. Rep. 2020, 10, 4979. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, S.; Liu, K.; Wen, W.; Lu, L.; Ding, S.; Zhou, C.; Luo, B. 3D poly (L-lactide)/chitosan micro/nano fibrous scaffolds functionalized with quercetin-polydopamine for enhanced osteogenic and anti-inflammatory activities. Chem. Eng. J. 2020, 391, 123524. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Pan, J.; Wang, H.; Li, H.; Xu, X.; Fu, X.; Shi, R.; Luo, Z.; Li, Y.; et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. J. Mater. Chem. B 2019, 7, 619–629. [Google Scholar] [CrossRef]
- Azaman, F.A.; Fournet, M.E.B.; Ab Hamid, S.S.; Zawawi, M.S.F.; Junior, V.A.d.S.; Devine, D.M. Enhancement of Scaffold In Vivo Biodegradability for Bone Regeneration Using P28 Peptide Formulations. Pharmaceuticals 2023, 16, 876. [Google Scholar] [CrossRef]
- Hirohashi, Y.; Kamijo, S.; Khan, M.; Ikeda, M.; Oki, M.; Matin, K.; Rashed, F.; Aoki, K. Tetracycline, an Appropriate Reagent for Measuring Bone-Formation Activity in the Murine Model of the Streptococcus mutans-Induced Bone Loss. Front. Cell. Infect. Microbiol. 2021, 11, 714366. [Google Scholar] [CrossRef]
- Kohli, N.; Theodoridis, K.; Hall, T.A.G.; Sanz-Pena, I.; Gaboriau, D.C.A.; van Arkel, R.J. Bioreactor analyses of tissue ingrowth, ongrowth and remodelling around implants: An alternative to live animal testing. Front. Bioeng. Biotechnol. 2023, 11, 1054391. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.S.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Willems, A.; Tsivintzelis, I.; Papageorgiou, V.P.; Kritis, A.; Assimopoulou, A.N. Electrospun wound dressings containing bioactive natural products: Physico-chemical characterization and biological assessment. Biomater. Res. 2021, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.S.; Giannakoula, K.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Kampasakali, E.; Willems, A.; Christofilos, D.; Kritis, A.; et al. Novel electrospun poly-hydroxybutyrate scaffolds as carriers for the wound healing agents alkannins and shikonins. Regen. Biomater. 2021, 8, rbab011. [Google Scholar] [CrossRef] [PubMed]


| Scaffold Theoretical and Measured Characteristics | ||||
|---|---|---|---|---|
| Scaffold Type | Theoretical Strand Diameter (μm) | Measured Strand Diameter (μm) | Theoretical Pores Sizes (μm) | Measured Pores Sizes (μm) |
| PCL | 400 | 358.26 ± 24.09 | 325 | 355.32 ± 12.33 |
| PCL-TCH | 400 | 360.59 ± 17.09 | 325 | 364.17 ± 25.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoridis, K.; Arampatzis, A.S.; Liasi, G.; Tsalikis, L.; Barmpalexis, P.; Christofilos, D.; Assimopoulou, A.N. 3D-Printed Antibacterial Scaffolds for the Regeneration of Alveolar Bone in Severe Periodontitis. Int. J. Mol. Sci. 2023, 24, 16754. https://doi.org/10.3390/ijms242316754
Theodoridis K, Arampatzis AS, Liasi G, Tsalikis L, Barmpalexis P, Christofilos D, Assimopoulou AN. 3D-Printed Antibacterial Scaffolds for the Regeneration of Alveolar Bone in Severe Periodontitis. International Journal of Molecular Sciences. 2023; 24(23):16754. https://doi.org/10.3390/ijms242316754
Chicago/Turabian StyleTheodoridis, Konstantinos, Athanasios S. Arampatzis, Georgia Liasi, Lazaros Tsalikis, Panagiotis Barmpalexis, Dimitrios Christofilos, and Andreana N. Assimopoulou. 2023. "3D-Printed Antibacterial Scaffolds for the Regeneration of Alveolar Bone in Severe Periodontitis" International Journal of Molecular Sciences 24, no. 23: 16754. https://doi.org/10.3390/ijms242316754
APA StyleTheodoridis, K., Arampatzis, A. S., Liasi, G., Tsalikis, L., Barmpalexis, P., Christofilos, D., & Assimopoulou, A. N. (2023). 3D-Printed Antibacterial Scaffolds for the Regeneration of Alveolar Bone in Severe Periodontitis. International Journal of Molecular Sciences, 24(23), 16754. https://doi.org/10.3390/ijms242316754








