Abstract
Mitochondrial energy metabolism declines during aging. PGC-1α is a transcription coactivator that plays a key role in the regulation of energetic metabolism and mitochondrial biogenesis in the cells. The aim of this study was to compare the PPARGC1A gene expression level in normal human dermal fibroblasts (NHDF) derived from young and old donors. A PGC-1α-derived peptide was then synthetized and its ability to affect the PPARGC1A gene expression and mitochondrial function was tested. We assessed changes in PPARGC1A gene expression using quantitative RT-PCR. The effect of the PGC-1α-derived peptide on energy production was determined using an ATP bioluminescent assay kit. We also studied changes in mitochondrial membrane potential using JC-1 fluorescent dye and the level of reactive oxygen species (ROS) using DCFH-DA dye in NHDF cells after UVA/B irradiation alone and in combination with a peptide treatment. The PPARGC1A gene expression decreased in an aged human dermal fibroblast. The PGC-1α-derived peptide was synthetized and increased the PPARGC1A gene expression and ATP levels in cells. Furthermore, the mitochondrial membrane potential in UVA/B irradiated cells treated with the tested PGC-1α-derived peptide was increased compared to irradiated controls. Moreover, the ROS levels in UVA/B irradiated cells treated with the PGC-1α-derived peptide decreased. On the basis of our results, PGC-1α emerges as an interesting target to combat decreasing energetic metabolism in aging skin cells. Indeed, the PGC-1α-derived peptide increasing the PPARGC1A gene expression improved the mitochondrial function and increased energy production in the cells.
1. Introduction
Skin aging is a process coupled with a reduction in the functional capacity of cells resulting in physical disorders of the skin. At subcellular levels, the involvement of mitochondria in aging processes is at the centre of interest. Actually, the mitochondrial theory of aging is still considered to be the one of the most widespread and generally accepted theories [1]. Mitochondria are major producers of cellular energy in the form of ATP (adenosine triphosphate). On the other hand, they also generate high levels of reactive oxygen species (ROS), which are eliminated by their own antioxidant system. A decline in mitochondria number or function, connected with a decrease in ATP production and an increase in ROS production contributes to skin aging processes [2,3].
The important regulators of mitochondria biogenesis and energy metabolism are the members of PGC-1 (peroxisome proliferator-activated receptor-γ coactivator 1) family of transcription coactivators. This family includes the PGC-1α, PGC-1β and PGC-related coactivators. PGC-1α has been the most characterized so far and its linkage to aging due to its association with factors influencing organismal longevity has been demonstrated [4].
PGC-1α is encoded by the PPARGC1A gene. This protein interacts with a wide range of different transcription factors and cellular receptors influencing various biological processes, such as adaptive thermogenesis, glucose and fatty acid metabolism, the circadian clock, adipogenesis, peroxisome biogenesis and, in particular, mitochondrial biogenesis and mitochondrial oxidative metabolism [5,6]. PGC-1α coactivates nuclear respiratory factors 1 and 2 (NRF1 and 2) and therefore activates the expression of mitochondrial transcription factor A (mtTFA) and other subunits of the electron transport chain. mtTFA also stimulates mitochondrial DNA replication and gene expression. Hence, the elevated expression of PGC-1α causes an increase in mitochondrial mass and enhances the function therof [7,8]. Moreover, PGC-1α influences the level of ROS in cells. It stimulates the expression of ROS-detoxifying enzymes and uncoupling proteins (UCP), which decrease electrochemical potential across the inner mitochondrial membrane [9].
This protein is present in many cell types, but it is more expressed in mitochondria-rich tissues with high energy demand, such as heart, brain, skeletal muscle, brown adipose tissue, liver and pancreatic islets [5,10]. During aging, PGC-1α signalling pathways are reduced in some tissues, and this reduction leads to the impairment of the mitochondrial function. Decreased levels of PGC-1α cause different health disorders emerging in aging, such as obesity, cardiac disease and diabetes [4]. The repression of PGC-1α transcription also contributes to the development of neurodegenerative diseases [11,12]. Furthermore, the age-dependent decrease in PGC-1α was studied in different tissues. For example, reduced levels of PGC-1α in the hearts of aged mice compared with young mice were found [13]. Moreover, an age-related decrease of PGC-1α gene expression and protein levels has also been found in skeletal muscles [14,15]. On the other hand, the overexpression of the PPARGC1A gene in 3T3 mouse embryonic fibroblasts stimulated mitochondrial biogenesis, followed by increased resistance to oxidative stress [16]. In addition, the overexpression of Drosophila PGC-1 homologue increased mitochondrial activity and extended the lifespan of a fly [17]. PGC-1α is activated during caloric restriction, exercise, hypoxia and after treatment with certain drugs, such as bezafibrate, rosiglitazone, AICAR, metformin and resveratrol [18,19,20,21]. The gene expression of PGC-1α in human dermal fibroblasts related to aging has not been studied yet.
The aim of this work was to identify, whether the expression of PGC-1α changes in normal human dermal fibroblasts derived from young and old donors. After that, we proposed and synthetized a PGC-1α-derived peptide and evaluated its effect on the expression of this protein and the mitochondrial biogenesis and function.
2. Materials and Methods
2.1. Synthesis, Purification and Identification of the PGC-1α-Derived Peptide
The PGC-1α-derived peptide (TPPTTP) was synthetized manually following standard Fmoc-protocols using 2-chlorotrityl chloride resin (Iris Biotech, Marktredwitz, Germany). Fmoc-amino acids and chemicals used for synthesis and cleavage were obtained from Iris Biotech (Marktredwitz, Germany). Other solvents used for resin washing were purchased from Lachner (Neratovice, Czech Republic). The amino acids were coupled in ratio Fmoc-protected amino acid/HBTU/DIPEA (2/2/4). The PGC-1α-derived peptide was cleaved from the resin by a mixture containing TFA/DCM/anisole/thioanisole (85/10/3/2). The resin was washed with TFA and the peptide was precipitated with DEE. The crude peptide was re-dissolved in water/propan-2-ol and purified with LC-MS 2020 (Shimadzu Europa, Duisburg, Germany), on Jupiter® 10u Proteo 90A, 21.2 × 250 mm (Phenomenex, Torrance, CA, USA). The pure peptide (98%) was lyophilised and the structure was confirmed with LC-MS 2020 (Shimadzu Europa, Duisburg, Germany), on Jupiter® 4u Proteo 90A, 10 × 250 mm (Phenomenex, Torrance, CA, USA), the spectrum showed dominant ion at 613.5 m/z [M+]. There are no more ions in MS spectrum in the range of 250–800 m/z.
2.2. Cell Culture, Peptides Treatment and UVA/B Irradiation
Normal human dermal fibroblasts (NHDF) were derived from facial skin removed during cosmetic plastic surgery and isolated by digestion method [22]. Cell cultures were maintained in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, Saint Louis, MO, USA) supplemented with a 10% fetal bovine serum, L-glutamine, D-glucose, penicillin and streptomycin at 37 °C in a humidified atmosphere of 5% CO2. Appropriate amounts of stock solution of PGC-1α-derived peptide dissolved in PBS were added to culture medium to achieve the final concentration of 0.1–250 µg mL−1. The cells were allowed to settle for 24 h before every experiment.
2.3. Cell Viability
For assessing the cytotoxic effect of tested compound on cell viability, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromid (Sigma-Aldrich, Saint Louis, MO, USA) reagent was used. 3T3 cells were seeded in 96-well microplates (5 × 103 cells/well) and allowed to settle overnight at 37 °C and in 5% CO2. Next, cultures were exposed to different concentrations of peptide for differing time intervals. At the end of each interval, MTT assay was carried out as described previously [23].
2.4. Determination of PPARGC1A Gene Expression
Total RNA was isolated from NHDF cells using RNAzol reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) according to the manufacturer’s instructions. NHDF cells were seeded in 6-well microplates (300 × 103 cells/well) and allowed to settle overnight at 37 °C and in 5% CO2. Next, cultures were exposed to different concentrations of peptide. RNA (1 µg) was reverse transcribed to cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) in Miniopticon RT-PCR system (Bio-Rad, Hercules, CA, USA). A quantitative real-time PCR(qPCR) was performed using Gene Expression TaqMan Assays (PPARGC1A: Hs01016719_m1; house-keeping gene: RPL13A: Hs03043885_g1) at universal cycling conditions (15 min at 95 °C, 40 cycles of 15 s at 95 °C and 1 min at 60 °C) in a StepOne real time PCR cycler (Applied Biosystems, Foster City, CA, USA). The threshold cycle (Ct) was determined for the genes of interest, and the relative mRNA level in each sample was calculated using the 2-ΔΔCt method.
2.5. ATP Assay
ATP content in NHDF cells was determined by CellTiter-Glo® Luminescent assay kit (Promega Corporation, Madison, WI, USA) according to the manufacturer’s instructions.
2.6. Measurement of Mitochondrial Membrane Potential
Mitochondrial membrane potential was assessed in NHDF cells (96-well plate, 5 × 103 cells/well, allowed to settle for 24 h) irradiated with UVA/B and treated with the PGC-1α-derived peptide. For UVA/B irradiation treatment, the cells were irradiated in culture plates using a 1000 W xenon solar UV-simulator (Oriel Instruments, Newport, NY, USA). JC-1 dye (Sigma-Aldrich, Saint Louis, MO, USA) was added to the NHDF cells to achieve the final concentration of 5 µM. After 20 min of incubation, cells were washed with PBS and red fluorescence was measured by EnSightTM Multimode Plate Reader (PerkinElmer, Waltham, MA, USA) [24].
2.7. ROS Assay
For measurement of the ROS production in NHDF cells (96-well plate, 5 × 103 cells/well, allowed to settle for 24 h) irradiated with UVA/B and treated with the tested compound, we used the DCFH-DA (2′-7′-dichlorodihydrofluorescein diacetate; Sigma-Aldrich, Saint Louis, MO, USA) method. For UVA/B irradiation treatment, the cells were irradiated in culture plates using a 1000 W xenon solar UV-simulator (Oriel Instruments, Newport, NY, USA). DCFH-DA is a membrane permeable non-fluorescent precursor that is cleaved by cellular esterases and converted to fluorescent DCF (2′,7′-dichlorofluorescein) in the presence of ROS [25]. At the end of the treatment, NHDF cells were incubated with DCFH-DA (15 µM for 30 min and the fluorescence was measured by an EnSightTM Multimode Plate Reader (PerkinElmer, Waltham, MA, USA) at λex 485 nm and λem 530 nm.
2.8. Statistical Analysis
The data are expressed as means ± SEM of at least three independent experiments and statistical analysis was performed using a Student’s t-test.
3. Results
3.1. PPARGC1A Gene Expression in NHDF from Young and Old Donors
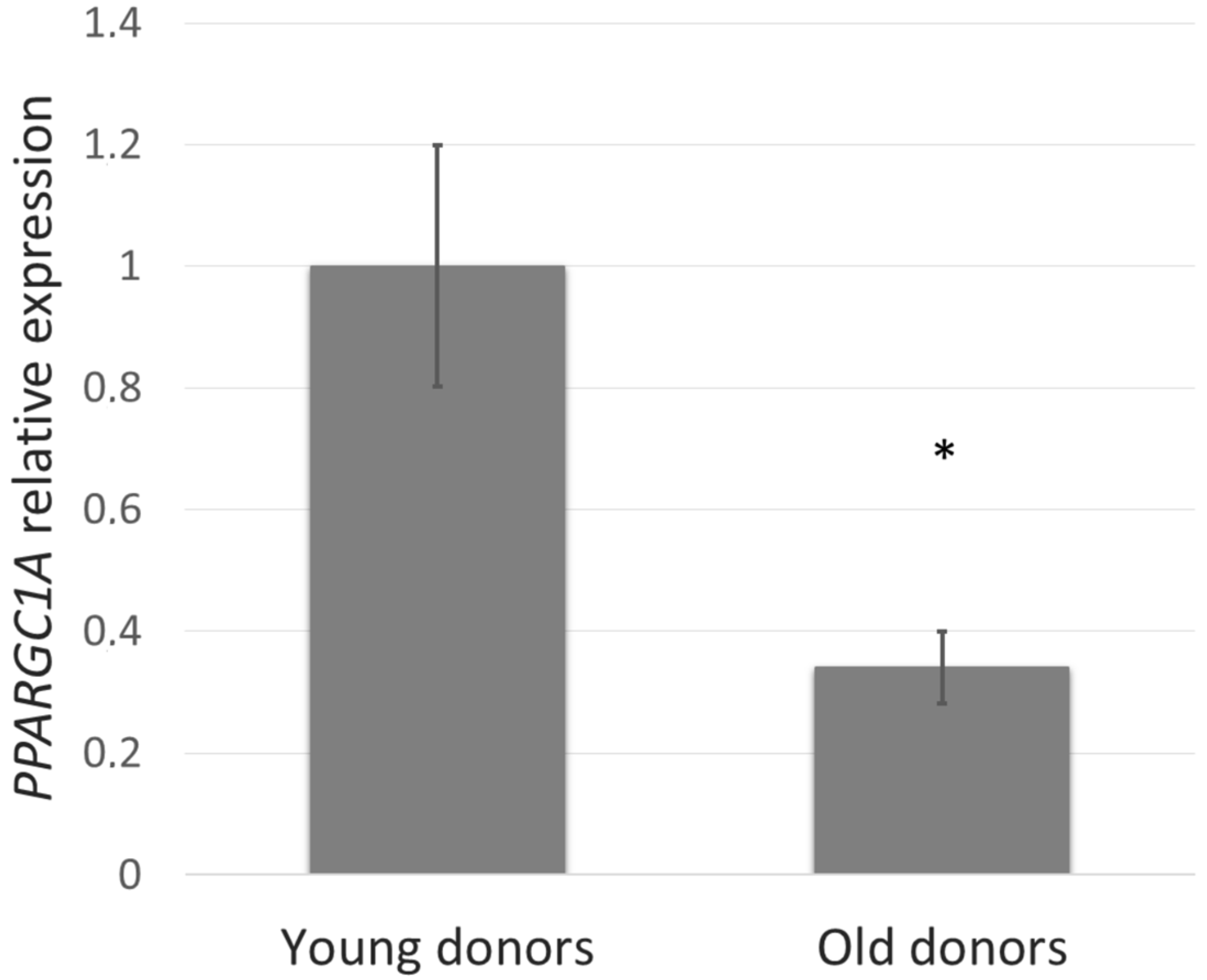
First, we studied whether the PGC-1α level depends on age and thus could be involved in the skin aging process. To assess this, PPARGC1A gene expression in NHDF cells from young (5–9 years) compared with old (44–60 years) donors was determined. In the fibroblasts derived from old donors, PPARGC1A expression was reduced by more than 60% (Figure 1).
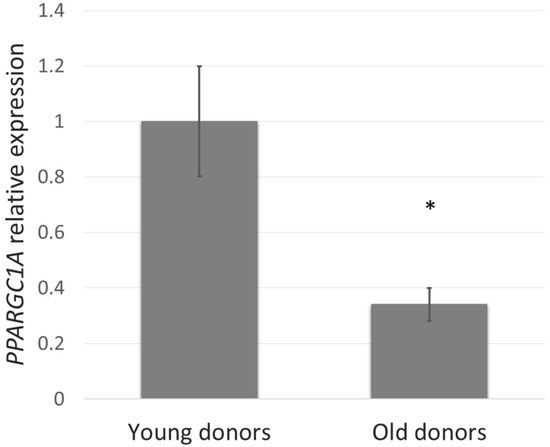
Figure 1.
PPARGC1A gene expression in normal human dermal fibroblasts (NHDF) cells from young (5–9 years) and old (44–60 years) donors was determined by quantitative RT-PCR. The values represent means ± SEM of NHDF cells from 5 donors for each group (* p < 0.05 compared with young donors).
3.2. Proposal and Synthesis of PGC-1α-Derived Peptide
Since we proved an age-dependent decrease in PPARGC1A gene expression, our next aim was to propose and synthetize peptides that could affect this gene expression and therefore increase the energy metabolism in NHDF cells. We proposed several peptides (fragments of PGC-1α protein) and studied their effect on the PPARGC1A gene expression (data not shown). One peptide was shown to increase PPARGC1A activity and was chosen for further studies (data not shown). This approach had been used earlier for the induction of the extracellular component gene expression. The induction of type I collagen production in cells treated with a subfragment of the type I collagen propetide was shown [26,27] and negative or positive feedback regulation of extracellular matrix production was proposed. To the best of our knowledge, the induction of intracellular components by usage of their subfragment has not yet been studied.
3.3. Cell Viability
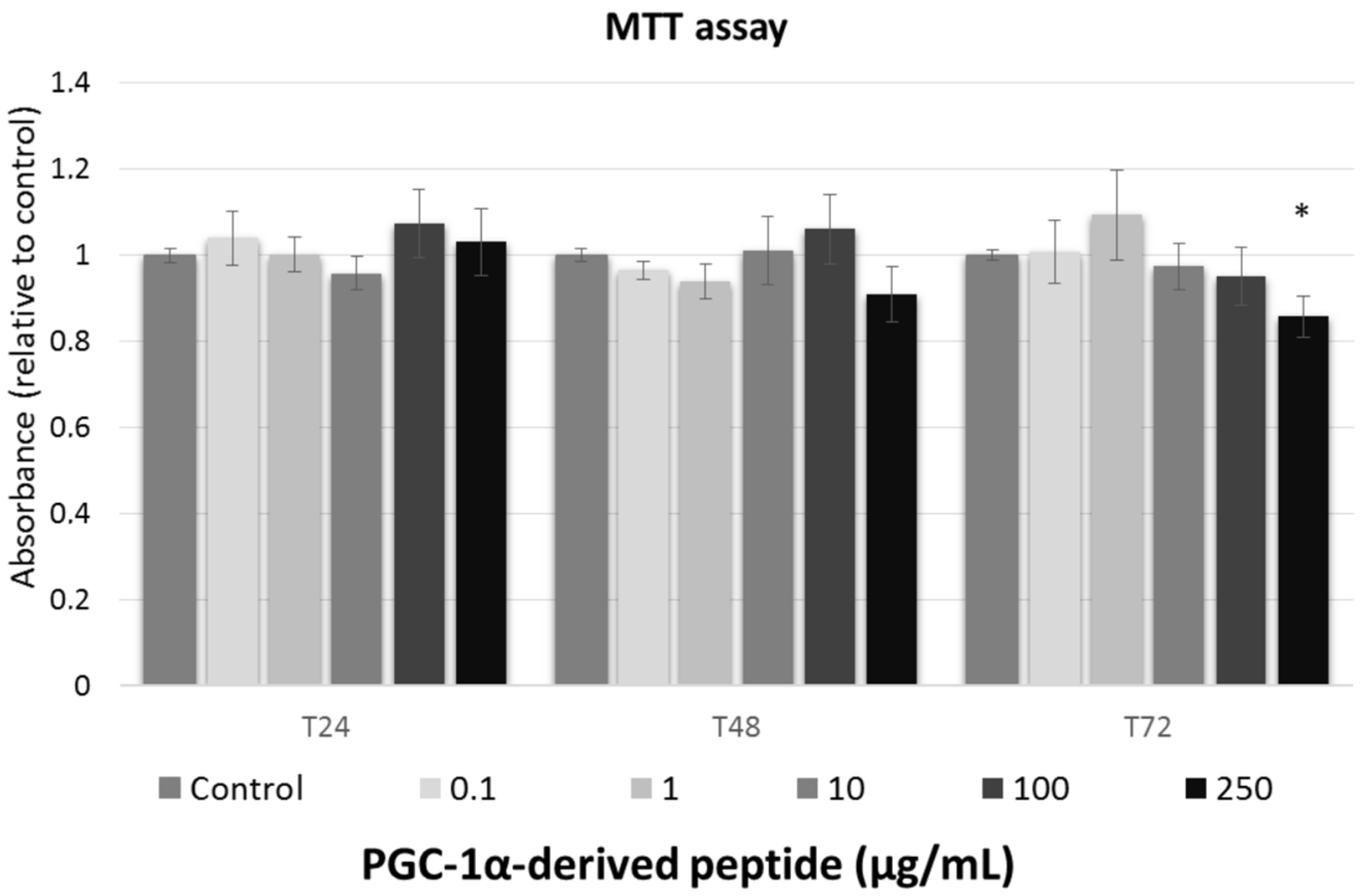
We studied the effect of tested peptide at various concentrations (0, 0.1, 1, 10, 100, and 250 µg/mL) on the cell viability of the 3T3 mouse fibroblast at different times of exposure (24, 48 and 72 h). To assessment the cell viability after treatment with tested compounds, MTT test was used.
Against the ISO 10993-5 [28], the substances are considered as non-toxic, when the cell viability is above 80%, weak toxic (cell viability 60–80%), moderate toxic (40–60%) and strong toxic (below 40%), respectively. As shown in Figure 2, tested peptide caused no cytoxic effect to mouse fibroblasts and thus, it could be considered safe for anti-aging usage.
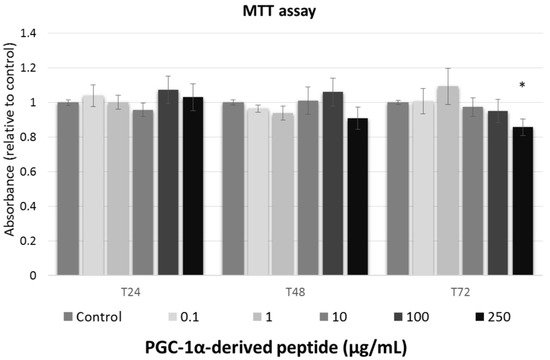
Figure 2.
The cell viability of Swiss 3T3 mouse fibroblasts. Cultured cells were treated with PGC-1α-derived peptide at various concentrations for 24, 48 and 72 h, then examined using MTT test. Values represent means ± SEM of at least 3 independent experiments (* p < 0.05 compared with untreated control group).
3.4. Effect of the PGC-1α-Derived Peptide on PPARGC1A Gene Expression
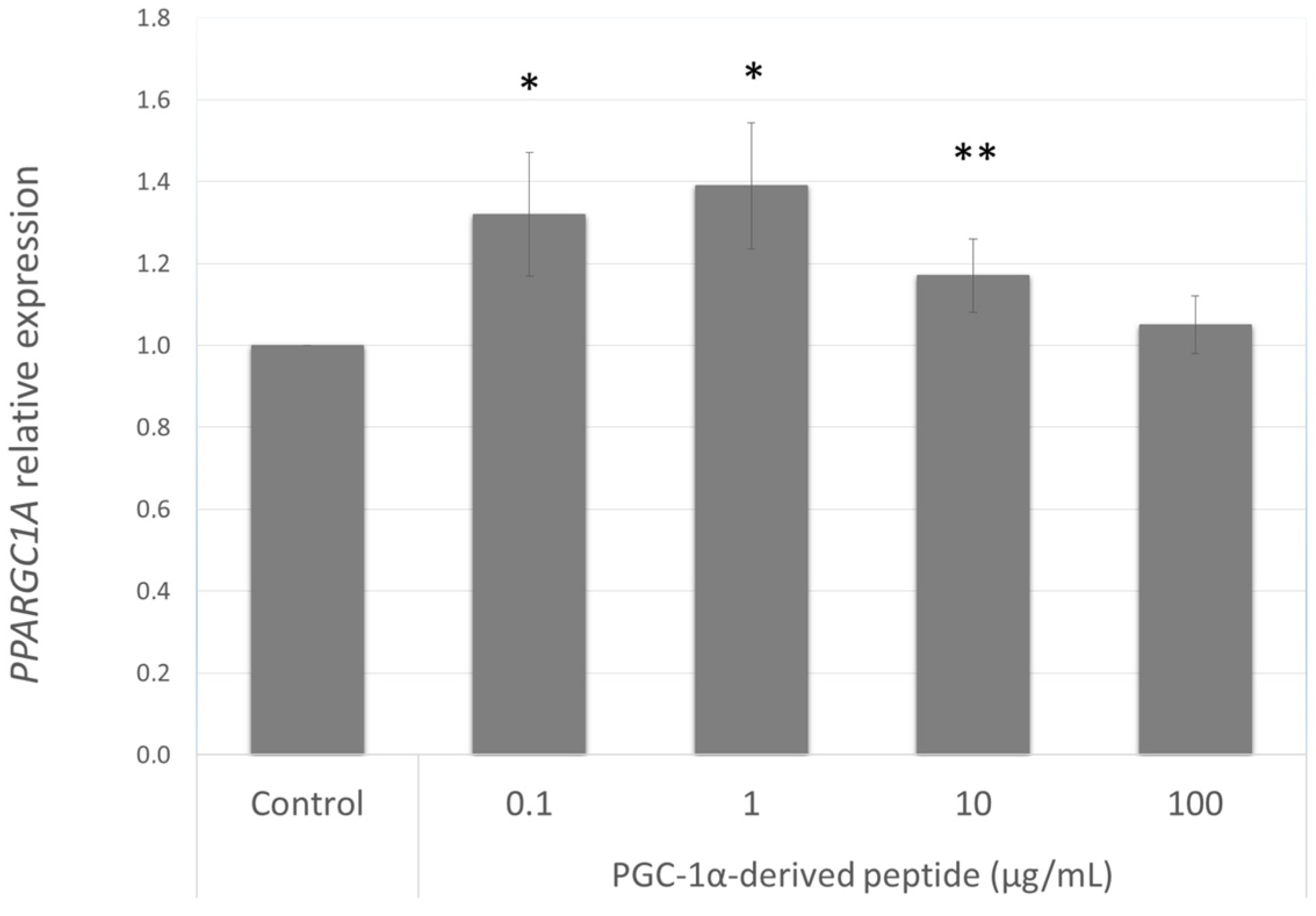
Our aim was to find a peptide that could increase the PPARGC1A gene expression in NHDF cells from old donors. Hence we further studied the effect of the PGC-1α-derived peptide at different concentrations (0.1, 1, 10 and 100 µg mL−1) after 24 h treatment on PPARGC1A gene expression. As seen in Figure 3, PGC-1α-derived peptide caused a significant increase in PPARGC1A gene expression at concentrations of 0.1, 1 and 100 µg mL−1.
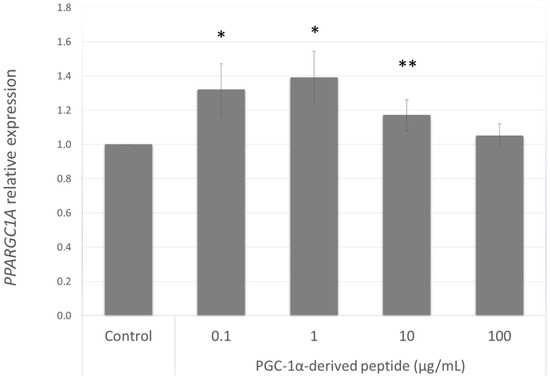
Figure 3.
PPARGC1A gene expression in NHDF cells. NHDF cells were treated with the PGC-1α-derived peptide at various concentrations for 24 h, then PPARGC1A gene expression was determined by quantitative RT-PCR. The values represent means ± SEM of at least 3 independent experiments (* p < 0.05, ** p < 0.01 compared with untreated control group).
3.5. Measuring of ATP Levels
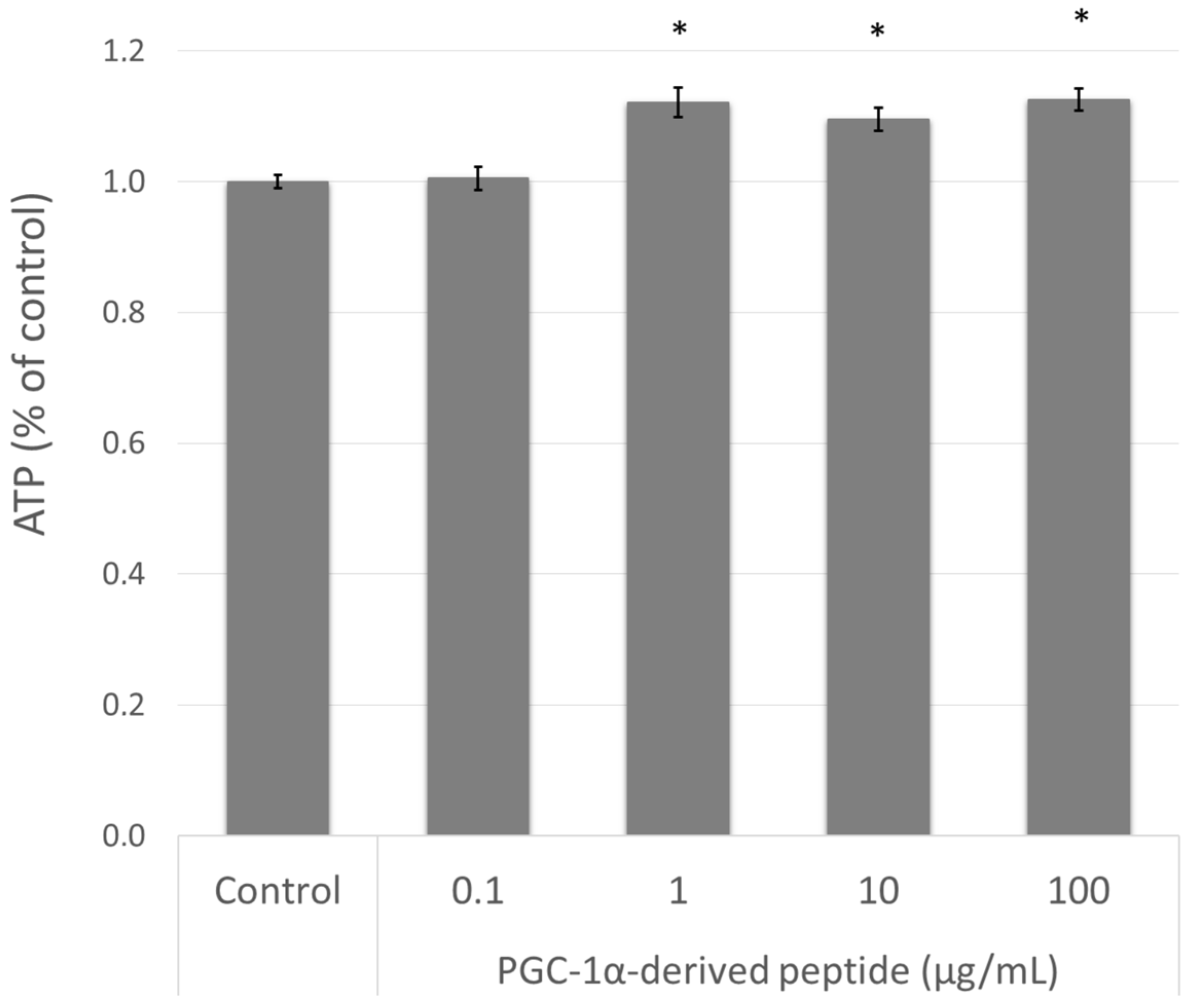
The elevation of PPARGC1A gene expression is related to mitochondrial biogenesis and therefore to intracellular ATP levels [10]. Therefore, we treated the NHDF cells with the PGC-1α-derived peptide and after 24 h we measured the ATP levels using the bioluminescent method described above. As seen in Figure 4, the PGC-1α-derived peptide at concentrations of 1, 10 and 100 µg mL−1 significantly increased the intracellular ATP content after 24-h incubation.
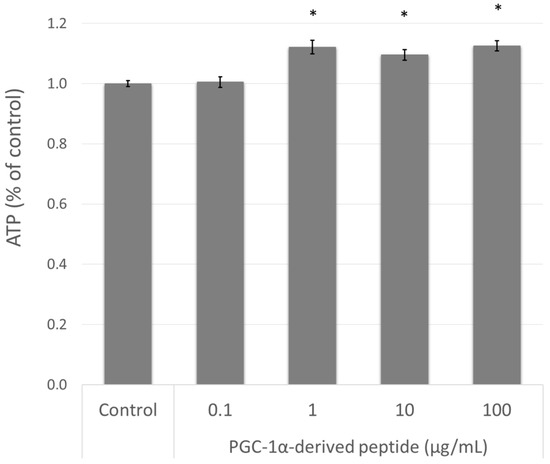
Figure 4.
The ATP (adenosine triphosphate) level in NHDF cells. NHDF cells were treated with the PGC-1α-derived peptide at various concentrations for 24 h, then the ATP was measured. The values represent means ± SEM of at least 3 independent experiments (* p < 0.05 compared with untreated control group).
3.6. Assessment of Mitochondrial Membrane Potential
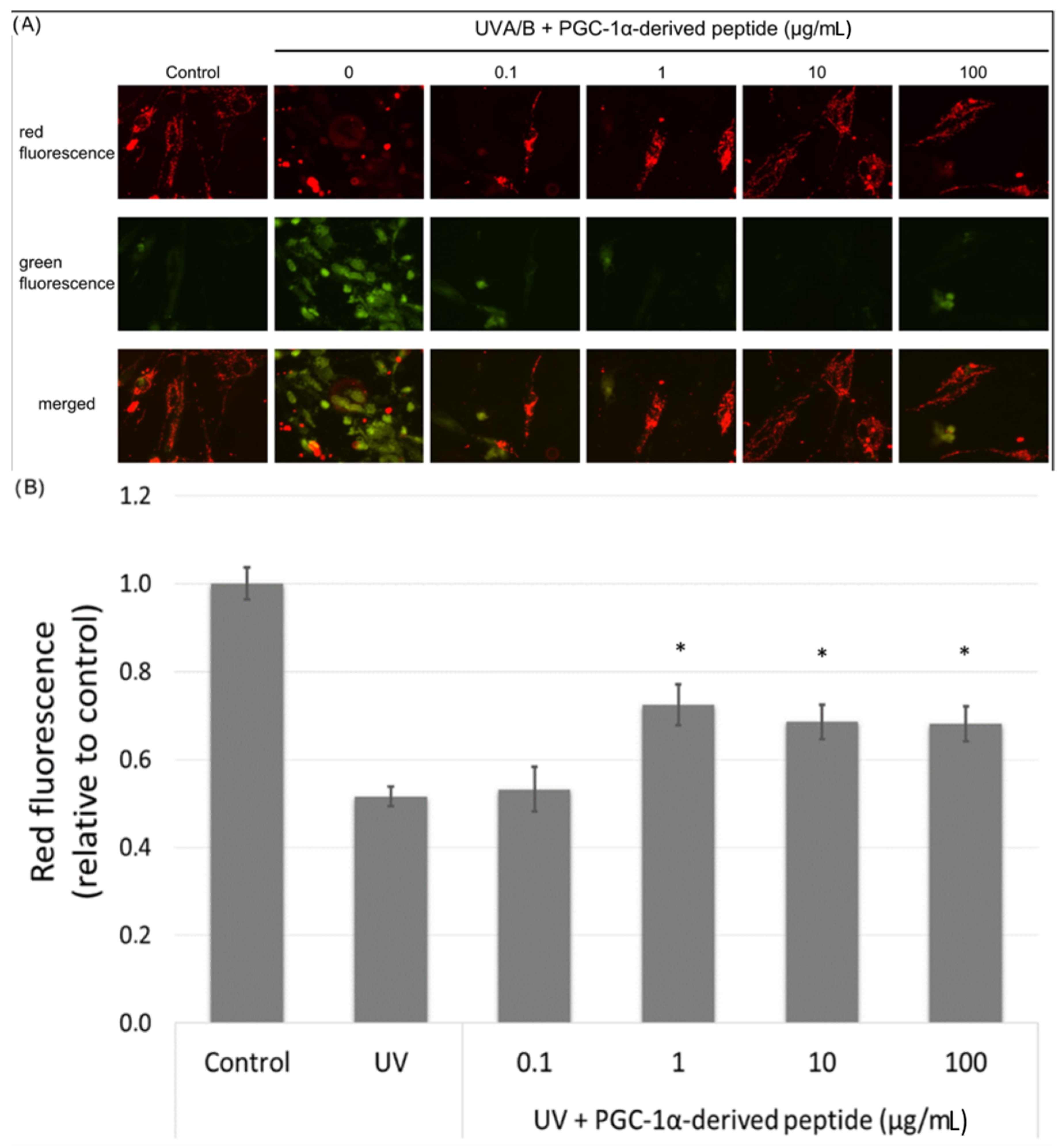
The mitochondrial membrane potential is an important parameter in the assessment of the mitochondrial function. UV radiation causes a decrease in the mitochondrial membrane potential [29]. Hence we irradiated NHDF cells with UVA/B and then treated them with the PGC-1α-derived peptide; after 24-h incubation, the changes in the mitochondrial membrane potential were measured using JC-1 dye. JC-1 dye forms orange fluorescent J-aggregates in control cells, and the depolarization of mitochondrial membrane results in the production of JC-1 green fluorescence monomers. Changes in red and green fluorescence in the treated cells were seen using a fluorescent microscope. Red fluorescence was present at high levels in control cells with unchanged mitochondrial membrane potential. On the other hand, green fluorescence increased in cells with disrupted mitochondrial membrane potential (Figure 5A). The PGC-1α-derived peptide at concentrations of 1, 10 and 100 µg mL−1 significantly elevated the mitochondrial membrane potential in UVA/B irradiated cells (Figure 5B).
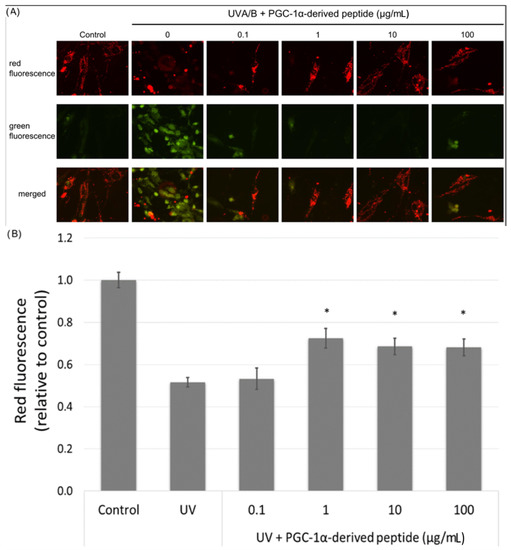
Figure 5.
Mitochondrial membrane potential in NHDF cells. Cultured cells were irradiated with UVA/B at a dose of 0.15 J/cm2 and then treated with the PGC-1α-derived peptide at various concentrations for 24 h. The mitochondrial membrane potential was determined using JC-1 dye with a fluorescence microscope (A) and a fluorescence reader (B). The values represent means ± SEM of at least 3 independent experiments (* p < 0.05 compared with UV irradiated group).
3.7. Assessment of ROS Levels
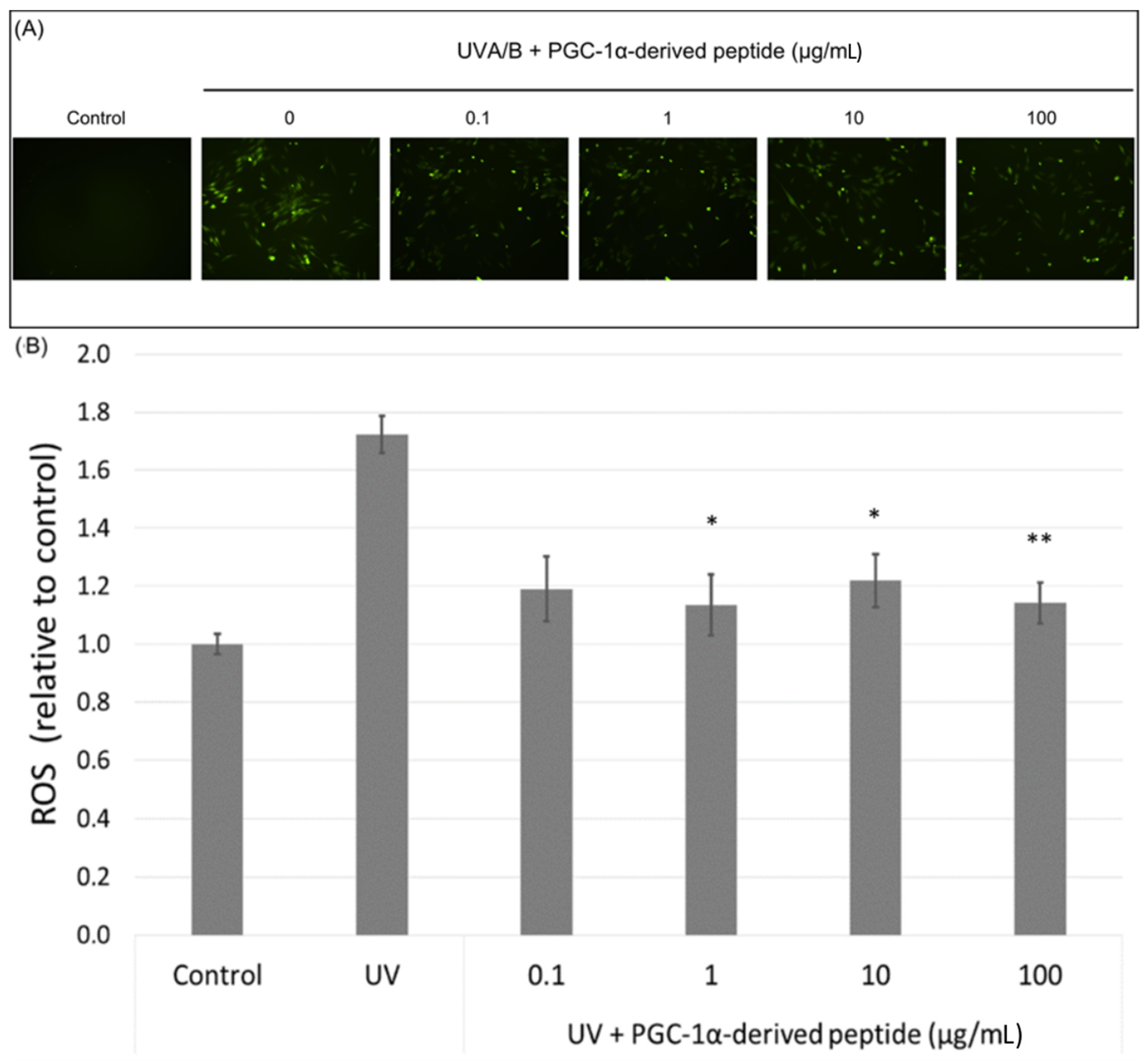
PGC-1α also plays a role in ROS detoxification in cells. Therefore, we assessed ROS levels in UVA/B irradiated NHDF cells (a dose of 0.15 J cm−2) treated with the PGC-1α-derived peptide at different concentrations. Our results show a significant decrease in the ROS level in UVA/B irradiated cells treated with the PGC-1α-derived peptide at concentrations of 1, 10 and 100 µg mL−1 after 1-h incubation (Figure 6A). A higher amount of ROS was also seen in UVA/B irradiated cells under a fluorescence microscope with a decrease in the ROS level in cells treated with the PGC-1α-derived peptide (Figure 6B).
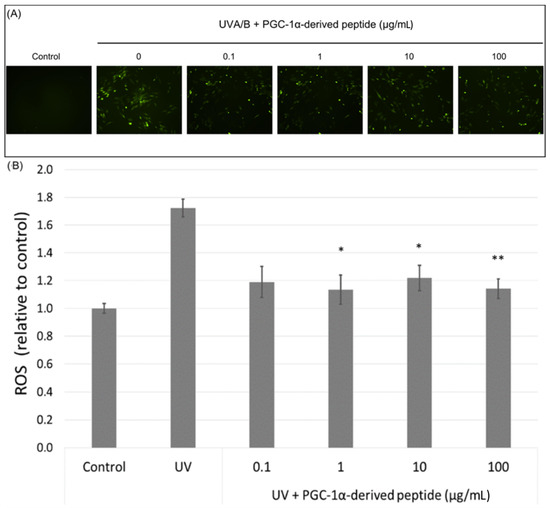
Figure 6.
The reactive oxygen species (ROS) level in NHDF cells. Cultured cells were irradiated with UVA/B at a dose of 0.15 J/cm2 and then treated with the PGC-1α-derived peptide at various concentrations for 1 h. The ROS level was determined using DCFH-DA dye with a fluorescent microscope (A) and a fluorescence reader (B). Values represent means ± SEM of at least 3 independent experiments (* p < 0.05, ** p < 0.01 compared with UV irradiated group).
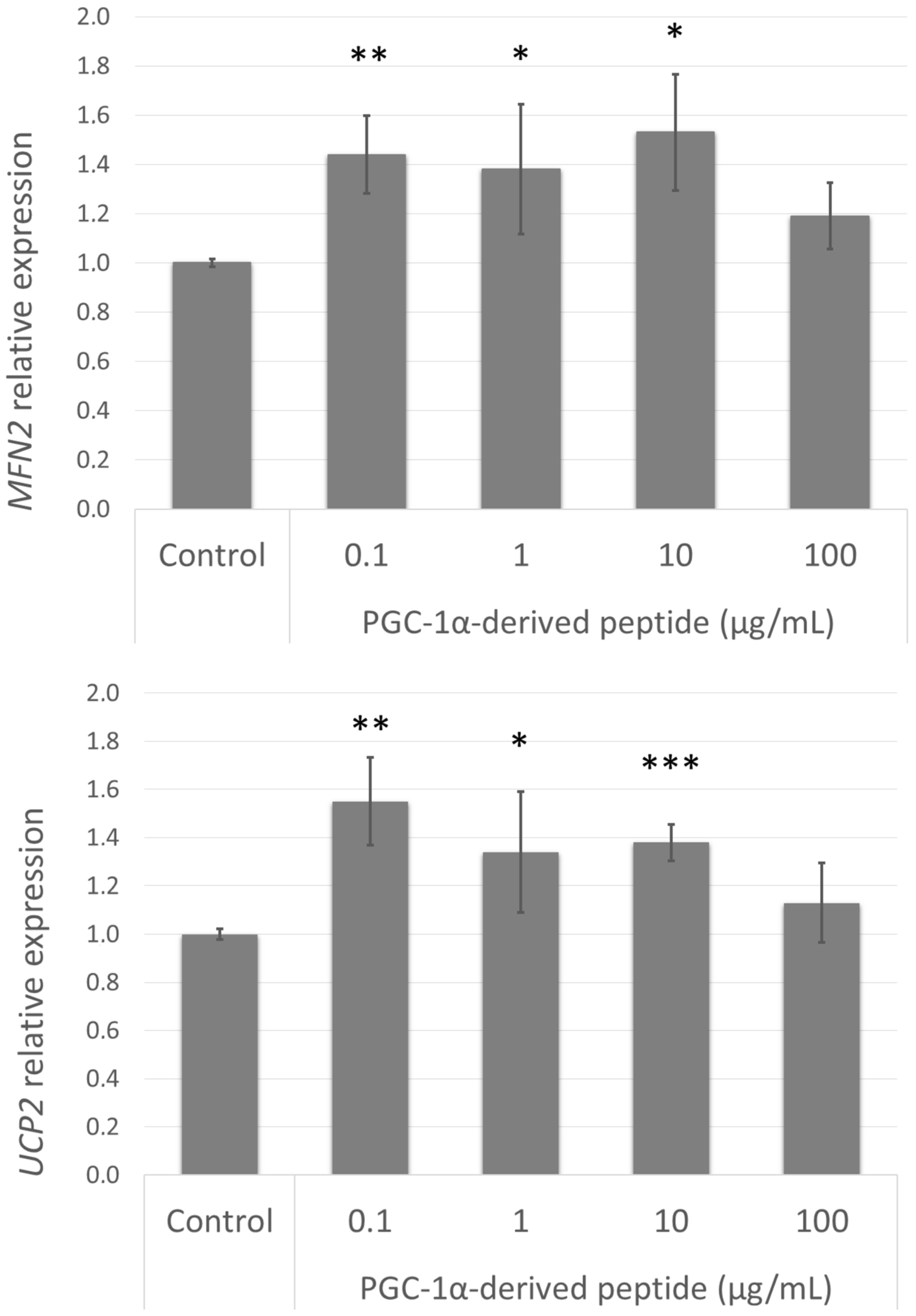
3.8. Effect of the PGC-1α-Derived Peptide on PGC-1α Downstream Gene Expression
The elevated expression of PPARGC1A caused an increase in mitochondrial membrane potential and a decrease in ROS levels in UVA/B treated cells; this improvement could be caused by the upregulation of certain genes involved in these processes. Therefore, we assessed the gene expression of MFN2 and UCP2 (Figure 7) in cells treated with the PGC-1α-derived peptide. The elevated expression of these genes correlated with the elevated expression of PPARGC1A.
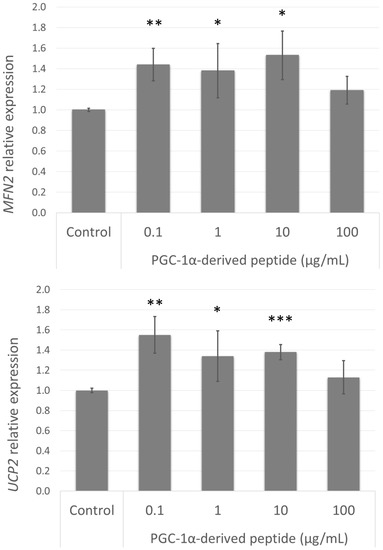
Figure 7.
MFN2 and UCP2 gene expression in NHDF cells. NHDF cells were treated with the PGC-1α-derived peptide at various concentrations for 24 h, then the genes expressions were determined by quantitative RT-PCR. The values represent means ± SEM of at least 3 independent experiments (* p < 0.05, ** p < 0.01, *** p < 0.001 compared with untreated control group).
4. Discussion
During skin aging, there is a decrease in mitochondria, by number and function. The main reason for this decrease is that the mitochondria are damaged by ROS and the damage causes protein modifications and mitochondrial DNA mutations leading to a decrease in mitochondrial biogenesis, oxidative phosphorylation and a decline in ATP production. Because mitochondria play a central role in energy metabolism the compounds stimulating the mitochondria biogenesis could bring many benefits to skin aging, such as oxidative defence, cell survival, improved skin barrier and protection from pollution [30,31].
Mitochondria biogenesis is a process controlled mainly by the PGC-1α [8]. However, the influence of PGC-1α expression in aged skin has not been studied yet. We found a decrease in PPARGC1A gene expression in normal human dermal fibroblast cells from old donors compared with young donors; this could contribute to the decreased mitochondrial number and function observed in aged skin cells.
In this work, we tried to increase PPARGC1A gene expression with a PGC-1α-derived peptide. We suggested that a fragment of PGC-1α protein could stimulate the gene expression of this protein. Indeed, the PGC-1α-derived peptide increased PPARGC1A gene expression at the tested concentrations.
The increase in the PGC-1α level has certain benefits, such as the elevation of mitochondrial mass and ATP production in cells. The elevation of mitochondrial biogenesis is probably caused by the elevation of NRFs, which is regulated by PGC-1α and stimulates the expression of numerous mitochondrial genes. The increase in mitochondrial biogenesis results in the elevation of ATP production [2]. Therefore, we assessed the effect of the PGC-1α-derived peptide on ATP production. The PGC-1α-derived peptide caused significant elevation of ATP production in NHDF cells. Further, the effect of the PGC-1α-derived peptide on the mitochondrial function was tested in cells after UVA/B treatment. The PGC-1α-derived peptide increased the mitochondrial membrane potential in UVA/B treated NHDF cells. It had been assessed earlier by Denning et al. that UV irradiation caused a decrease in mitochondrial membrane potential in human keratinocytes [29]. PGC-1α also affects the expression of ROS-detoxifying enzymes and uncoupling proteins (UCPs) and thus it decreases the ROS levels in cells [32]. The PGC-1α-derived peptide also decreased ROS levels in NHDF cells treated with UVA/B irradiation and increased the expression of UCP2. We also proved elevation of the MFN2 gene encoding mitofusin. Mitofusin is involved in maintaining mitochondrial morphology and enhances mitochondrial membrane potential [33]. The levels of UCP2 and MFN2 genes correlates with the PPARGC1A gene expression. However, there is a difference between the PPARGC1A gene expression and the influence of ATP production, mitochondrial membrane potential and ROS level in the cells treated with 100 µg/mLof PGC-1α-derived peptide. The protein level of PGC-1α could bring the better results, but we demonstrated the influence of indirect evidence (mitochondrial membrane potential, ATP, ROS). Because the PGC-1α influence these cellular processes, we could predict also the influencing of PGC-1α level in the cells.
In conclusion, PPARGC1A gene expression in aged fibroblasts decreased–this probably contributes to decreased metabolism, and a reduction in mitochondria, by number and function often observed in aged skin. The PGC-1α-derived peptide caused the elevation of PPARGC1A gene expression in NHDF cells. Further, it also influenced ATP production and mitochondrial membrane potential in NHDF cells. The elevation of PGC-1α also caused a reduction in ROS levels. These results show that the increase in the PGC-1α level could benefit skin fitness, and the effect of the PGC-1α-derived peptide emerges as a candidate for further in vivo studies to confirm such assumptions.
Acknowledgments
The authors thank to Martina Teplá for her technical assistance.
Author Contributions
Lenka Suchá and Iva Dolečková conceived and designed the experiments, Lenka Suchá performed the experiments, Romana Šuláková synthesis of the peptide, Roman Fryčák purification and identification of the peptide, Lenka Suchá and Iva Dolečková and Romana Šuláková wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DCM | dichloromethane |
| DIPEA | N,N-Diisopropylethylamine |
| Fmoc | 9-fluorenylmethyloxycarbonyl |
| HBTU | O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate |
| mtTFA | mitochondrial transcription factor A |
| NHDF | normal human dermal fibroblasts |
| NRF | nuclear respiratory factor |
| PBS | phosphate buffer saline |
| PGC-1α | peroxisome proliferator-activated receptor-γ coactivator 1 |
| ROS | reactive oxygen species |
References
- Brown, K.; Liu, Y.; Chen, D. Aging: The Mitochondrial Connection. J. Clin. Exp. Pathol. 2012, 4. [Google Scholar] [CrossRef]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism—Emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- Bratic, I.; Trifunovic, A. Mitochondrial energy metabolism and ageing. Biochim. Biophys. Acta 2010, 1797, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Prolla, T. PGC-1α in aging and anti-aging interventions. Biochim. Biophys. Acta 2009, 1790, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, J.D. PGC-1 coactivators in the control of energy metabolism. Acta Biochim. Biophys. Sin. 2011, 43, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Wenz, T. PGC-1α activation as a therapeutic approach in mitochondrial disease. IUBMB Life 2009, 61, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1α: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N.; Kelly, D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 2006, 116, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Jeong, H.; Borovecki, F.; Parkhurst, C.N.; Tanese, N.; Krainc, D. Transcriptional Repression of PGC-1α by Mutant Huntingtin Leads to Mitochondrial Dysfunction and Neurodegeneration. Cell 2006, 127, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Liao, Z.; Locascio, J.J.; Lesniak, K.A.; Roderick, S.S.; Watt, M.L.; Eklund, A.C.; Zhang-James, Y.; Kim, P.D.; Hauser, M.A.; et al. PGC-1α, A Potential Therapeutic Target for Early Intervention in Parkinson’s Disease. Sci. Transl. Med. 2010, 2, 52ra73. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Gomez-Cabrera, M.C.; Borras, C.; Froio, T.; Sanchis-Gomar, F.; Martinez-Bello, V.E.; Pallardo, F.V. Mitochondrial biogenesis in exercise and in ageing. Adv. Drug Deliv. Rev. 2009, 61, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Chung, E.; Diffee, G.; Ji, L.L. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: Role of PGC-1α. Exp. Gerontol 2013, 48, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Poulsen, P.; Carlsson, E.; Ridderstråle, M.; Almgren, P.; Wojtaszewski, J.; Beck-Nielsen, H.; Groop, L.; Vaag, A. Multiple environmental and genetic factors influence skeletal muscle PGC-1α and PGC-1β gene expression in twins. J. Clin. Investig. 2004, 114, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Bai, Y.; Li, Y.; Richardson, A.; Ward, W.F. PGC-1α-Induced Mitochondrial Alterations in 3T3 Fibroblast Cells. Ann. N. Y. Acad. Sci. 2007, 1100, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T.; Jones, D.L.; Walker, D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Dillon, L.M.; Rebelo, A.P.; Moraes, C.T. The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life 2012, 64, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.; Noe, N.; Tischner, C.; Kladt, N.; Lellek, V.; Schauß, A.; Wenz, T. Defining the action spectrum of potential PGC-1α activators on a mitochondrial and cellular level in vivo. Hum. Mol. Genet. 2014, 23, 2400–2415. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, K. Exercise-induced PGC-1α transcriptional factors in skeletal muscle. Integr. Med. Res. 2014, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Q.; Zhang, L.; Fang, Z.; Zhao, F.; Lv, Z.; Gu, Z.; Zhang, J.; Wang, J.; Zen, K.; et al. Hypoxia induces PGC-1α expression and mitochondrial biogenesis in the myocardium of TOF patients. Cell Res. 2010, 20, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Meske, V.; Albert, F.; Ohm, T.G. Cell Cultures of Autopsy-Derived Fibroblasts. In Human Cell Culture and Protocols; Methods in Molecular MedicineTM; Humana Press: New York, NY, USA, 2005; Volume 107, pp. 111–123. ISBN 978-1-59259-861-8. [Google Scholar]
- Hašová, M.; Crhák, T.; Šafránková, B.; Dvořáková, J.; Muthný, T.; Velebný, V.; Kubala, L. Hyaluronan minimizes effects of UV irradiation on human keratinocytes. Arch. Dermatol. Res. 2011, 303, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Grohm, J.; Kim, S.-W.; Mamrak, U.; Tobaben, S.; Cassidy-Stone, A.; Nunnari, J.; Plesnila, N.; Culmsee, C. Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ. 2012, 19, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. In Advanced Protocols in Oxidative Stress II; Humana Press: Totowa, NJ, USA, 2010; pp. 57–72. [Google Scholar]
- Katayama, K.; Armendariz-Borunda, J.; Raghow, R.; Kang, A.H.; Seyer, J.M. A pentapeptide from type I procollagen promotes extracellular matrix production. J. Biol. Chem. 1993, 268, 9941–9944. [Google Scholar] [PubMed]
- Tsai, W.-C.; Hsu, C.-C.; Chung, C.-Y.; Lin, M.-S.; Li, S.-L.; Pang, J.-H.S. The pentapeptide KTTKS promoting the expressions of type I collagen and transforming growth factor-β of tendon cells. J. Orthop. Res. 2007, 25, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009.
- Denning, M.F.; Wang, Y.; Tibudan, S.; Alkan, S.; Nickoloff, B.J.; Qin, J.-Z. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ. 2002, 9, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Dal Farra, C.; Botto, J.-M.; Domloge, N. Mitochondria: A new focus as an anti-aging target in skin care. J. Cosmet. Dermatol. 2010, 9, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Tranah, G.J. Mitochondrial-nuclear epistasis: Implications for human aging and longevity. Ageing Res. Rev. 2011, 10, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zorzano, A.; Pich, S. What is the biological significance of the two mitofusin proteins present in the outer mitochondrial membrane of mammalian cells? IUBMB Life 2006, 58, 441–443. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).