Stability of Anthocyanins and Their Degradation Products from Cabernet Sauvignon Red Wine under Gastrointestinal pH and Temperature Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anthocyanins and Phenolic Acids in Red Wine
2.2. Evolution of Anthocyanins and Phenolic Acids under Simulated Gastrointestinal pH and Temperature Conditions
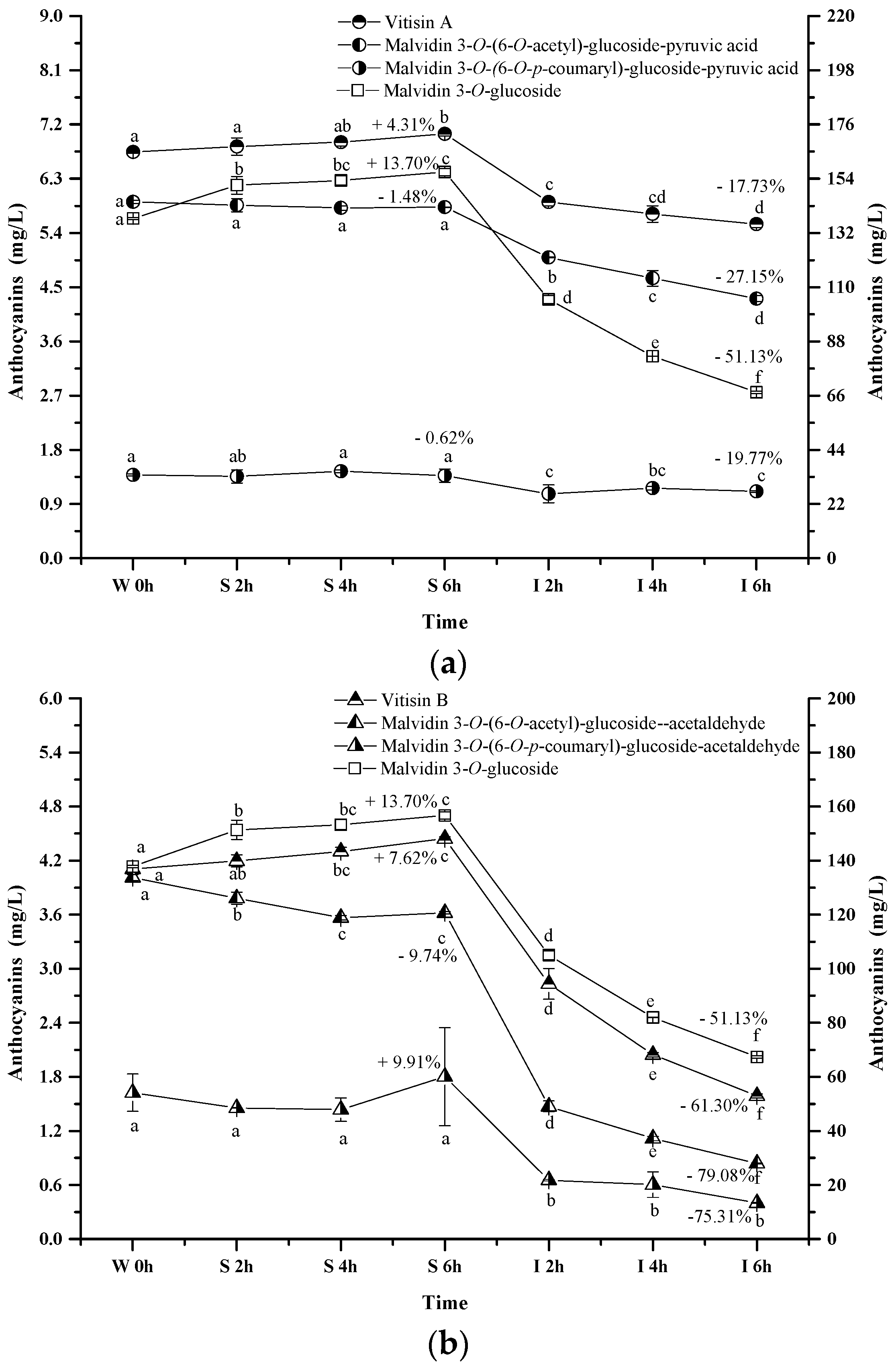
2.2.1. Total Anthocyanins
2.2.2. Monomeric Anthocyanins
2.2.3. Acylated Anthocyanins
2.2.4. Pyranoanthocyanins
2.2.5. Polymeric Anthocyanins
2.2.6. Phenolic Acids
2.3. Evolution of Anthocyanin and Phenolic Acid Standards in Simulated Gastrointestinal pH and Temperature Conditions
3. Materials and Methods
3.1. Evolution of Anthocyanin and Phenolic Acid Standards in Simulated Gastrointestinal pH Conditions
3.2. Chemicals
3.3. Grape Harvest and Wine-Making
3.4. Digestion of Wine Sample, Anthocyanin Standards, and Phenolic Acid Standards in Simulated Gastrointestinal pH and Temperature Contidions
3.5. Anthocyanins and Phenolic Acids HPLC Analyses
3.6. UPLC-ESI-MS/MS
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A


References
- Han, F.L.; Li, Z.; Xu, Y. Contribution of monomeric anthocyanins to the color of young red wine: Statistical and experimental approaches. J. Food Sci. 2015, 80, C2751–C2758. [Google Scholar] [PubMed]
- Han, F.L.; Zhang, W.N.; Pan, Q.H.; Zheng, C.R.; Chen, H.Y.; Duan, C.Q. Principal component regression analysis of the relation between CIELAB color and monomeric anthocyanins in young cabernet sauvignon wines. Molecules 2008, 13, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; De Gianni, A.; Mentana, A.; Quinto, M.; Centonze, D.; Del Nobile, M.A. Colour-related phenolics, volatile composition, and sensory profile of Nero di Troia wines treated with oak chips or by micro-oxygenation. Eur. Food Res. Technol. 2016, 242, 1631–1646. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Ferreira, V.; Dizy, M.; Fernández-Zurbano, P. Characterization of taste-active fractions in red wine combining HPLC fractionation, sensory analysis and ultra performance liquid chromatography coupled with mass spectrometry detection. Anal. Chim. Acta 2010, 673, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Guo, A.Q.; Zhang, Y.L.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Dan, J.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.L.; Simon, J.E.; Lila, M.A.; Rochet, J.C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry (Poly)phenols and cardiovascular health. J. Agric. Food Chem. 2014, 62, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P. Red wine and diabetes health: Getting skin in the game. Diabetes 2014, 63, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, J.; Tanabe, S.; Bergeron, C.; Gafner, S.; Grenier, D. Anthocyanin-rich black currant extract and cyanidin-3-O-glucoside have cytoprotective and anti-inflammatory properties. J. Med. Food 2012, 15, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Kunz, C.; Rudloff, S. Inhibition of pancreatic cancer cell migration by plasma anthocyanins isolated from healthy volunteers receiving an anthocyanin-rich berry juice. Eur. J. Nutr. 2017, 56, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Curtis, A.D.; Bracone, F.; Digesù, C.; Morganti, A.G.; Iacoviello, L.; de Gaetano, G.; Donati, M.B. Dietary anthocyanins and health: Data from FLORA and ATHENA EU projects. Br. J. Clin. Pharmacol. 2017, 83, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.H.; Liu, Y.; Sun, R.F.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M. Biosynthesis of anthocyanins in shiraz grape berries. J. Biol. Chem. 2004, 640, 353–360. [Google Scholar] [CrossRef]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.H.; Lauvergeat, V.; Gomès, E.; Li, S.H.; Godoy, F.; Arcejohnson, P.; Delrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2013, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed]
- Han, F.L.; Jiang, S.M.; He, J.J.; Pan, Q.H.; Duan, C.Q.; Zhang, M.X. Anthocyanins in ‘Cabernet Gernischet’ (Vitis vinifera L. Cv.) aged red wine and their color in aqueous solution analyzed by partial least square regression. Food Sci. Biotechnol. 2009, 18, 724–731. [Google Scholar]
- Avizcuri, J.M.; Sáenz-Navajas, M.P.; Echávarri, J.F.; Ferreira, V.; Fernández-Zurbano, P. Evaluation of the impact of initial red wine composition on changes in color and anthocyanin content during bottle storage. Food Chem. 2016, 213, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vanzela, E.S.; Rebello, L.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutierrez, I. Chromatic characteristics and color-related phenolic composition of Brazilian young red wines made from the hybrid grape cultivar BRS Violeta (“BRS Rúbea” × “IAC 1398-21”). Food Res. Int. 2013, 54, 33–43. [Google Scholar] [CrossRef]
- Dipalmo, T.; Crupi, P.; Pati, S.; Clodoveo, M.L.; Di Luccia, A. Studying the evolution of anthocyanin-derived pigments in a typical red wine of Southern Italy to assess its resistance to aging. LWT-Food Sci. Technol. 2016, 71, 1–9. [Google Scholar] [CrossRef]
- Federico Casassa, L.; Bolcato, E.A.; Sari, S.E.; Fanzone, M.L.; Jofré, V.P. Combined effect of prefermentative cold soak and SO2 additions in Barbera D’Asti and Malbec wines: Anthocyanin composition, chromatic and sensory properties. LWT-Food Sci. Technol. 2016, 66, 134–142. [Google Scholar] [CrossRef]
- Sivilotti, P.; Herrera, J.C.; Lisjak, K.; Česnik, H.B.; Sabbatini, P.; Peterlunger, E.; Castellarin, S.D. Impact of leaf removal, applied before and after flowering, on anthocyanin, tannin, and methoxypyrazine concentrations in ‘Merlot’ (Vitis vinifera L.) grapes and wines. J. Agric. Food Chem. 2016, 64, 4487–4496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orduña, R.M.D. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Rodríguez-Morgado, B.; Jara-Palacios, M.J.; Rivas-Gonzalo, J.C.; Parrado, J.; Heredia, F.J. Pre-fermentative addition of an enzymatic grape seed hydrolysate in warm climate winemaking. Effect on the differential colorimetry, copigmentation and polyphenolic profiles. Food Chem. 2016, 209, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Ju, Y.; Ruan, X.; Zhao, X.; Yue, X.; Zhuang, X.; Qin, M.; Fang, Y. Color, anthocyanin, and antioxidant characteristics of young wines produced from spine grapes (Vitis davidii Foex) in China. Food Nutr. Res. 2017, 61, 1339552. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ilárduya, M.B.; Sánchez-Fernández, C.; Viloria-Bernal, M.; López-Márquez, D.M.; Berrueta, L.Á.; Gallo, B.; Vicente, F. Mass spectrometry fragmentation pattern of coloured flavanol-anthocyanin and anthocyanin-flavanol derivatives in aged red wines of Rioja. Aust. J. Grape Wine Res. 2012, 18, 203–214. [Google Scholar] [CrossRef]
- Oliveira, J.; Azevedo, J.; Silva, A.M.S.; Teixeira, N.; Cruz, L.; Mateus, N.; de Freitas, V. Pyranoanthocyanin dimers: A new family of turquoise blue anthocyanin-derived pigments found in port wine. J. Agric. Food Chem. 2010, 58, 5154–5159. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Alberts, P.; Stander, M.A.; de Villiers, A. Advanced ultra high pressure liquid chromatography-tandem mass spectrometric methods for the screening of red wine anthocyanins and derived pigments. J. Chromatogr. A 2012, 1235, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Vega, D.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Identification, content and distribution of anthocyanins and low molecular weight anthocyanin-derived pigments in Spanish commercial red wines. Food Chem. 2014, 158, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Mateus, N.; Silva, A.M.S.; González-Paramás, A.M.; Santos-Buelga, C.; Freitas, V.D. Structural and chromatic characterization of a new malvidin 3-glucoside-vanillyl-catechin pigment. Food Chem. 2007, 102, 1344–1351. [Google Scholar] [CrossRef]
- Pissarra, J.; Lourenço, S.; González-Paramás, A.M.; Mateus, N.; Buelga, C.S.; Silva, A.M.S.; Freitas, V.D. Structural characterization of new malvidin 3-glucoside-catechin aryl/alkyl-linked pigments. J. Agric. Food Chem. 2004, 52, 5519–5526. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.; Serratosa, M.P.; Merida, J. Influence of bottle storage time on colour, phenolic composition and sensory properties of sweet red wines. Food Chem. 2014, 146, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Quaglieri, C.; Jourdes, M.; Waffo-Teguo, P.; Teissedre, P.L. Updated knowledge about pyranoanthocyanins: Impact of oxygen on their contents, and contribution in the winemaking process to overall wine color. Trends Food Sci. Technol. 2017, 67, 139–149. [Google Scholar] [CrossRef]
- Garcia-Alonso, M.; Minihane, A.M.; Rimbach, G.; Rivas-Gonzalo, J.C.; de Pascual-Teresa, S. Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma. J. Nutr. Biochem. 2009, 20, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Krisa, S.; Mauray, A.; Besson, C.; Lamaison, J.L.; Scalbert, A.; Merillon, J.M.; Texier, O. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse. Br. J. Nutr. 2010, 103, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wallace, T.C.; Keatley, K.E.; Failla, M.L.; Giusti, M.M. Stability of black raspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. J. Agric. Food Chem. 2009, 57, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Roowi, S.; Rouanet, J.M.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. The bioavallability of raspberry anthocyanins and ellagitannins in rats. Mol. Nutr. Food Res. 2007, 51, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Gastrointestinal stability and bioavailability of (poly)phenolic compounds following ingestion of Concord grape juice by humans. Mol. Nutr. Food Res. 2012, 56, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009, 531, S92–S101. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M.; Mattila, I.; Hyötyläinen, T.; Gopalacharyulu, P.; Cheynier, V.; Souquet, J.M.; Bes, M.; Le Bourvellec, C.; Guyot, S.; Orešič, M. Characterization of microbial metabolism of Syrah grape products in an in vitro colon model using targeted and non-targeted analytical approaches. Eur. J. Nutr. 2013, 52, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Março, P.H.; Scarminio, I.S. Q-mode curve resolution of UV-vis spectra for structural transformation studies of anthocyanins in acidic solutions. Anal. Chim. Acta 2007, 583, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Waterhouse, A.L. Identification of cabernet sauvignon anthocyanin gut microflora metabolites. J. Agric. Food Chem. 2008, 56, 9299–9304. [Google Scholar] [CrossRef] [PubMed]
- Keppler, K.; Humpf, H. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorgan. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilua, M.J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar] [CrossRef]
- He, J.; Carvalho, A.R.; Mateus, N.; De, F.V. Spectral features and stability of oligomeric pyranoanthocyanin-flavanol pigments isolated from red wines. J. Agric. Food Chem. 2010, 58, 9249–9258. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G.; Kroon, P.; Cassidy, A.; Kay, C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef] [PubMed]
- Kamonpatana, K.; Failla, M.L.; Kumar, P.S.; Giusti, M.M. Anthocyanin structure determines susceptibility to microbial degradation and bioavailability to the buccal mucosa. J. Agric. Food Chem. 2014, 62, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Bailón, T.; Alvarez-García, M.; Rivas-Gonzalo, J.C.; Heredia, F.J.; Santos-Buelga, C. Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin 3-O-glucoside and (+)-catechin. J. Agric. Food Chem. 2001, 49, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Bravo-Haro, S.; Rivas-Gonzalo, J.C. Interactions between catechin and malvidin-3-monoglucoside in model solutions. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1995, 201, 269–274. [Google Scholar] [CrossRef]
- Jiménez-Girón, A.; Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Muñoz-González, I.; Sánchez-Patán, F.; Monagas, M.; Martín-Álvarez, P.J.; Murri, M.; Tinahones, F.J.; Andrés-Lacueva, C.; et al. Moreno-Arribas, M.V. Comparative study of microbial-derived phenolic metabolites in human feces after intake of gin, red wine, and dealcoholized red wine. J. Agric. Food Chem. 2013, 61, 3909–3915. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Fulcrand, H.; Cheynier, V. Formation of anthocyanin-flavanol adducts in model solutions. Anal. Chim. Acta 2006, 563, 15–25. [Google Scholar] [CrossRef]
- Zhao, M.; Luo, Y.; Li, Y.; Liu, X.; Wu, J.; Liao, X.; Chen, F. The identification of degradation products and degradation pathway of malvidin-3-glucoside and malvidin-3,5-diglucoside under microwave treatment. Food Chem. 2013, 141, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.; Richard, T.; Saucier, C.; Teissedre, P.L.; Monti, J.P.; Glories, Y. Anthocyanone a: A quinone methide derivative resulting from malvidin 3-O-glucoside degradation. J. Agric. Food Chem. 2007, 55, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Li, Y.; Han, Y.; Han, F.; Li, J.; Nie, Y.; Xu, Y. Studies on the preparative isolation and stability of seven main anthocyanins from Yan 73 grape. J. Sci. Food Agric. 2014, 94, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Lim, B.O.; Decker, E.A.; Mcclements, D.J. In vitro human digestion models for food applications. Food Chem. 2010, 125, 1–12. [Google Scholar]
- Yang, P.; Li, H.Q.; Wang, H.; Han, F.L.; Jing, S.Y.; Yuan, C.L.; Guo, A.Q.; Zhang, Y.L.; Xu, Z.M. Dispersive Liquid-Liquid microextraction method for HPLC determination of phenolic compounds in wine. Food Anal. Method. 2017, 10, 2383–2397. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Anthocyanins | R1 | R2 |
|---|---|---|
| Delphinidin 3-O-glucoside | OH | OH |
| Cyanidin 3-O-glucoside | OH | H |
| Petunidin 3-O-glucoside | OCH3 | OH |
| Peonidin 3-O-glucoside | OCH3 | H |
| Malvidin 3-O-glucoside | OCH3 | OCH3 |








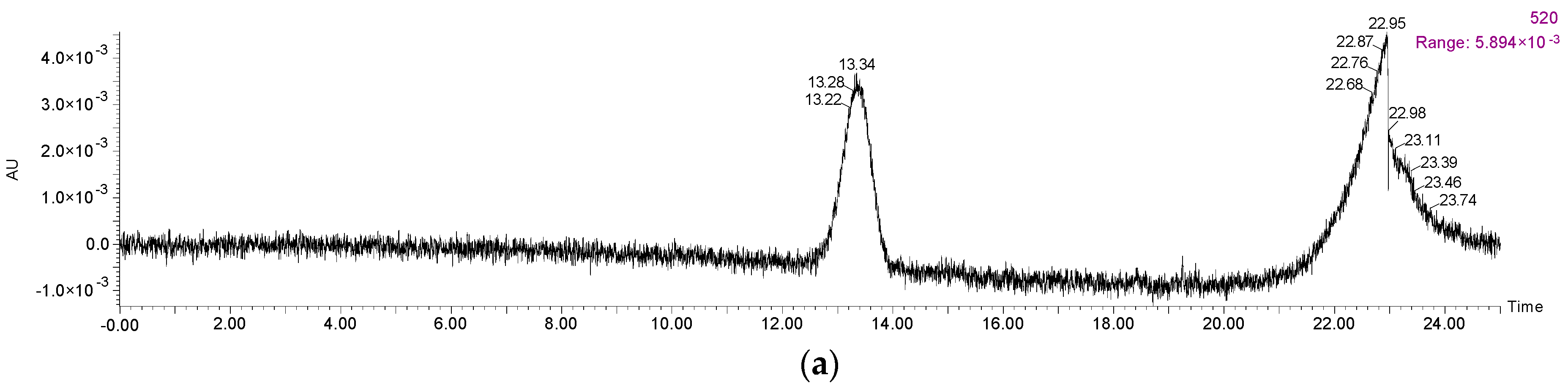
| Peak No. | Compounds | Rt (min) | λmax | Precursor Ion | Product Ion | Concentration (mg/L) |
|---|---|---|---|---|---|---|
| 1 | Delphinidin 3-O-glucoside | 7.978 | 524 | 465 | 303 | 2.801 ± 0.011 |
| 2 | Cyanidin 3-O-glucoside | 11.297 | 524 | 449 | 287 | 0.814 ± 0.010 |
| 3 | Petunidin 3-O-glucoside | 13.226 | 524 | 479 | 317 | 5.047 ± 0.014 |
| 4 | Peonidin 3-O-glucoside | 17.137 | 523 | 463 | 301 | 2.295 ± 0.001 |
| 5 | Malvidin 3-O-glucoside | 18.921 | 276, 525 | 493 | 331 | 137.815 ± 0.396 |
| 6 | Vitisin A | 22.183 | 510 | 561 | 399 | 6.743 ± 0.015 |
| 7 | Vitisin B | 24.669 | - | 517 | 355 | 4.106 ± 0.032 |
| 8 | Malvidin 3-O-(6-O-acetyl)-glucoside-pyruvic acid | 26.168 | 514 | 603 | 399 | 5.916 ± 0.026 |
| 9 | Malvidin 3-O-glucoside-ethyl-catechin (1) | 29.29 | 525 | 809 | 357 | 1.999 ± 0.357 |
| 10 | Malvidin 3-O-(6-O-acetyl)-glucoside-acetaldehyde | 29.926 | 495 | 559 | 355 | 4.012 ± 0.032 |
| 11 | Malvidin 3-O-glucoside-ethyl-catechin (2) | 30.847 | 532 | 809 | 357 | 0.337 ± 0.008 |
| 12 | Malvidin 3-O-glucoside-ethyl-catechin (3) | 32.762 | 532 | 809 | 357 | 0.404 ± 0.008 |
| 13 | Peonidin 3-O-(6-O-acetyl)-glucoside | 34.396 | 523 | 505 | 301 | 3.530 ± 0.464 |
| 14 | Malvidin 3-O-(6-O-acetyl)-glucoside | 35.799 | 528 | 535 | 331 | 80.068 ± 0.419 |
| 15 | Malvidin 3-O-(6-O-p-coumaryl)-glucoside-pyruvic acid | 36.989 | 517 | 707 | 399 | 1.382 ± 0.018 |
| 16 | Malvidin 3-O-(6-O-caffeoyl)-glucoside | 40.239 | 531 | 655 | 331 | 1.510 ± 0.382 |
| 17 | Malvidin 3-O-(6-O-p-coumaryl)-glucoside-acetaldehyde | 42.046 | - | 663 | 355 | 1.624 ± 0.208 |
| 18 | Malvidin 3-O-(6-O-cis-p-coumaryl)-glucoside | 42.615 | 532 | 639 | 331 | 1.354 ± 0.034 |
| 19 | Peonidin 3-O-(6-O-trans-p-coumaryl)-glucoside | 45.814 | 524 | 609 | 301 | 1.175 ± 0.433 |
| 20 | Malvidin 3-O-(6-O-trans-p-coumaryl)-glucoside | 46.45 | 531 | 639 | 331 | 16.081 ± 0.570 |
| 21 | Malvidin 3-O-glucoside-4-vinylphenol adduct | - | - | 609 | 447 | - |
| 22 | Malvidin 3-O-(6-O-acetyl)-glucoside-4-vinylphenol adduct | - | - | 651 | 447 | - |
| Total anthocyanins | - | - | - | - | 299.752 ± 2.352 |
| Peak No. | Compounds | Rt (min) | λmax | Concentration (mg/L) |
|---|---|---|---|---|
| 1 | Gallic acid | 4.243 | 271 | 11.676 ± 0.321 |
| 2 | Protocatechuic acid | 7.319 | 259, 293 | 4.771 ± 0.208 |
| 3 | Vanillic acid | 15.363 | 291 | 8.156 ± 0.853 |
| 4 | Syringic acid | 16.671 | 274 | 21.350 ± 0.412 |
| 5 | p-Coumaric acid | 26.818 | 309 | 2.846 ± 0.162 |
| Compound | λmax | Precursor Ion | Product Ion |
|---|---|---|---|
| Syringic acid | 274, 279 | 197 | 153 |
| Peonidin 3-O-glucoside * | - | 463 | 301 |
| Malvidin 3-O-glucoside * | 525 | 493 | 331 |
| Malvidin 3-O-glucoside chalcone * | 343 | 511 | 349, 223 |
| Malvidin 3-O-glucoside chalcone | 343 | 509 | 347, 221 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Yuan, C.; Wang, H.; Han, F.; Liu, Y.; Wang, L.; Liu, Y. Stability of Anthocyanins and Their Degradation Products from Cabernet Sauvignon Red Wine under Gastrointestinal pH and Temperature Conditions. Molecules 2018, 23, 354. https://doi.org/10.3390/molecules23020354
Yang P, Yuan C, Wang H, Han F, Liu Y, Wang L, Liu Y. Stability of Anthocyanins and Their Degradation Products from Cabernet Sauvignon Red Wine under Gastrointestinal pH and Temperature Conditions. Molecules. 2018; 23(2):354. https://doi.org/10.3390/molecules23020354
Chicago/Turabian StyleYang, Ping, Chunlong Yuan, Hua Wang, Fuliang Han, Yangjie Liu, Lin Wang, and Yang Liu. 2018. "Stability of Anthocyanins and Their Degradation Products from Cabernet Sauvignon Red Wine under Gastrointestinal pH and Temperature Conditions" Molecules 23, no. 2: 354. https://doi.org/10.3390/molecules23020354
APA StyleYang, P., Yuan, C., Wang, H., Han, F., Liu, Y., Wang, L., & Liu, Y. (2018). Stability of Anthocyanins and Their Degradation Products from Cabernet Sauvignon Red Wine under Gastrointestinal pH and Temperature Conditions. Molecules, 23(2), 354. https://doi.org/10.3390/molecules23020354




