Neurotropin® Accelerates the Differentiation of Schwann Cells and Remyelination in a Rat Lysophosphatidylcholine-Induced Demyelination Model
Abstract
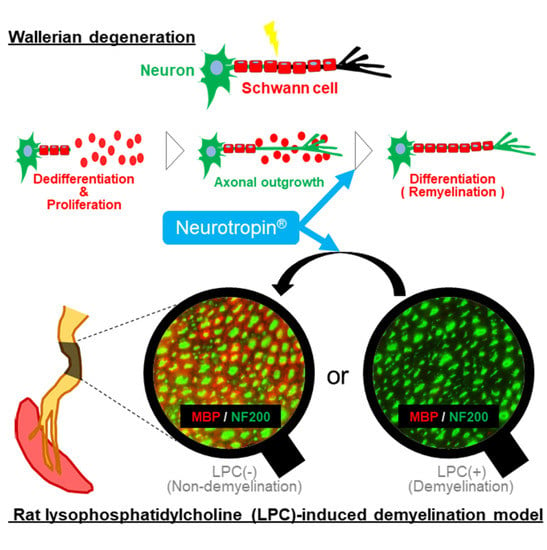
:1. Introduction
2. Results
2.1. NTP Increases AKT Activity and Reduces ERK1/2 Activity in SCs under Growth Conditions
2.2. NTP Increases AKT Activity and Reduces ERK1/2 Activity in SCs under Differentiation Conditions
2.3. NTP Does Not Affect SCs Proliferation
2.4. NTP Accelerates the Differentiation of SCs In Vitro
2.5. NTP Promotes Remyelination and Functional Recovery in a Rat Lysophosphatidylcholine (LPC)-Induced Demyelination Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Primary SCs Culture
4.4. Western Blotting
4.5. BrdU Uptake Assay
4.6. Cell Proliferation Assay
4.7. Assay for SCs Differentiation In Vitro
4.8. Dorsal Root Ganglion Neuron and Schwann Cell Co-Culture
4.9. Immunocytochemistry
4.10. Surgical Procedures
4.11. Histological Analysis
4.12. Sciatic Functional Index
4.13. Von Frey Filament Test
4.14. Hot Plate Test
4.15. Electrophysiological Analysis
4.16. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BrdU | 5-bromo-2-deoxyuridine |
| CMAPs | compound muscle action potentials |
| CNS | central nervous system |
| db-cAMP | dibutyryl cyclic AMP |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DRG | dorsal root ganglion |
| ERK | extracellular signal-regulated kinase |
| FBS | fetal bovine serum |
| ITS | intermediary toe spread |
| JNK | c-Jun-N-terminal kinase |
| LPC | lysophosphatidylcholine |
| MAG | myelin-associated glycoprotein |
| MAPK | mitogen activated protein kinase |
| MBP | myelin basic protein |
| MKK | MAPK kinase |
| NCV | nerve conduction velocity |
| NF200 | neurofilament 200 |
| NRG | neuregulin |
| NTP | Neurotropin® |
| NU | Neurotropin Unit |
| P0 | protein zero |
| PBS | phosphate-buffered saline |
| PCNA | proliferating cell nuclear antigen |
| PL | print length |
| PNI | peripheral nerve injury |
| PNS | peripheral nervous system |
| SCs | Schwann cells |
| SFI | sciatic functional index |
| TL | terminal latency |
| TS | toe spread |
References
- Noble, J.; Munro, C.A.; Prasad, V.S.; Midha, R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, O.A.; Gordon, T. Role of chronic schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery 2009, 65, A105–A114. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.W.; Brosius Lutz, A.; Cheng, Y.C.; Latremoliere, A.; Duong, K.; Miller, C.M.; Posada, S.; Cobos, E.J.; Zhang, A.X.; Wagers, A.J.; et al. Diminished schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 2014, 83, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Heinen, A.; Beyer, F.; Tzekova, N.; Hartung, H.P.; Kury, P. Fingolimod induces the transition to a nerve regeneration promoting schwann cell phenotype. Exp. Neurol. 2015, 271, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Hoyng, S.A.; de Winter, F.; Tannemaat, M.R.; Blits, B.; Malessy, M.J.; Verhaagen, J. Gene therapy and peripheral nerve repair: A perspective. Front. Mol. Neurosci. 2015, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A. Myelination and support of axonal integrity by glia. Nature 2010, 468, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kury, P.; Stoll, G.; Muller, H.W. Molecular mechanisms of cellular interactions in peripheral nerve regeneration. Curr. Opin. Neurol. 2001, 14, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Masuguchi, K.; Watanabe, H.; Kawashiri, T.; Ushio, S.; Ozawa, N.; Morita, H.; Oishi, R.; Egashira, N. Neurotropin(r) relieves oxaliplatin-induced neuropathy via gi protein-coupled receptors in the monoaminergic descending pain inhibitory system. Life Sci. 2014, 98, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kushikata, T.; Kudo, M.; Kudo, T.; Hirota, K. Antinociceptive effects of neurotropin in a rat model of central neuropathic pain: Dsp-4 induced noradrenergic lesion. Neurosci. Lett. 2011, 503, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Namba, H.; Yoshida, H.; Okai, H.; Miura, T.; Kawamura, M. The antiallodynic effect of neurotropin is mediated via activation of descending pain inhibitory systems in rats with spinal nerve ligation. Anesth. Analg. 2008, 107, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Ohara, H.; Go, K.; Koga, Y.; Ienaga, K. Neurotropin induces antinociceptive effect by enhancing descending pain inhibitory systems involving 5-HT3 and noradrenergic α2 receptors in spinal dorsal horn. Life Sci. 1998, 62, 2181–2190. [Google Scholar] [CrossRef]
- Okazaki, R.; Namba, H.; Yoshida, H.; Okai, H.; Taguchi, K.; Kawamura, M. Combined antiallodynic effect of neurotropin(r) and pregabalin in rats with l5-spinal nerve ligation. Life Sci. 2013, 92, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Namimatsu, A.; Fukuhara, K.; Yago, H.; Yoneda, R.; Saito, K.; Inoki, R. Release of inflammatory mediators by noxious stimuli; effect of neurotropin on the release. Eur. J. Pharmacol. 1988, 157, 93–99. [Google Scholar] [CrossRef]
- Nishimoto, S.; Okada, K.; Tanaka, H.; Okamoto, M.; Fujisawa, H.; Okada, T.; Naiki, M.; Murase, T.; Yoshikawa, H. Neurotropin attenuates local inflammatory response and inhibits demyelination induced by chronic constriction injury of the mouse sciatic nerve. Biologicals 2016, 44, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Iijima, S.; Hoshikawa, S.; Miura, T.; Yamamoto, S.; Oda, H.; Nakamura, K.; Tanaka, S. Opposing extracellular signal-regulated kinase and akt pathways control schwann cell myelination. J. Neurosci. 2004, 24, 6724–6732. [Google Scholar] [CrossRef] [PubMed]
- Harrisingh, M.C.; Perez-Nadales, E.; Parkinson, D.B.; Malcolm, D.S.; Mudge, A.W.; Lloyd, A.C. The ras/raf/erk signalling pathway drives schwann cell dedifferentiation. EMBO J. 2004, 23, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Shin, Y.K.; Park, H.T. Mitogen activated protein kinase family proteins and c-jun signaling in injury-induced schwann cell plasticity. Exp. Neurobiol. 2014, 23, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V.; Bartlett Bunge, M.; Wood, P.M. Cyclic amp synergistically enhances neuregulin-dependent erk and akt activation and cell cycle progression in schwann cells. Glia 2006, 53, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Svaren, J.; Meijer, D. The molecular machinery of myelin gene transcription in schwann cells. Glia 2008, 56, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Newbern, J.; Birchmeier, C. Nrg1/ErbB signaling networks in schwann cell development and myelination. Semin. Cell Dev. Biol. 2010, 21, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Garratt, A.N.; Britsch, S.; Birchmeier, C. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays 2000, 22, 987–996. [Google Scholar] [CrossRef]
- Vartanian, T.; Goodearl, A.; Viehover, A.; Fischbach, G. Axonal neuregulin signals cells of the oligodendrocyte lineage through activation of HER4 and schwann cells through HER2 and HER3. J. Cell Biol. 1997, 137, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bacallao, K.; Monje, P.V. Requirement of camp signaling for schwann cell differentiation restricts the onset of myelination. PLoS ONE 2015, 10, e0116948. [Google Scholar] [CrossRef] [PubMed]
- Wessig, C.; Bendszus, M.; Stoll, G. In vivo visualization of focal demyelination in peripheral nerves by gadofluorine M-enhanced magnetic resonance imaging. Exp. Neurol. 2007, 204, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, Y.; Zhang, Y.P.; Shields, L.B.; Hu, X.; Yu, P.; Burke, D.A.; Wang, H.; Jun, C.; Byers, J.; et al. Enhanced adenoviral gene delivery to motor and dorsal root ganglion neurons following injection into demyelinated peripheral nerves. J. Neurosci. Res. 2010, 88, 2374–2384. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Tanaka, H.; Okamoto, M.; Okada, K.; Murase, T.; Yoshikawa, H. Methylcobalamin promotes the differentiation of schwann cells and remyelination in lysophosphatidylcholine-induced demyelination of the rat sciatic nerve. Front. Cell. Neurosci. 2015, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Church, J.S.; Milich, L.M.; Lerch, J.K.; Popovich, P.G.; McTigue, D.M. E6020, a synthetic TLR4 agonist, accelerates myelin debris clearance, schwann cell infiltration, and remyelination in the rat spinal cord. Glia 2017, 65, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Takeoka, Y.; Imai, H.; Yamamoto, H.; Suehiro, S.; Ueda, S.; Katoh, S. Differential action of nerve growth factor, cyclic amp and neurotropin on PC12h cells. Cell Struct. Funct. 1988, 13, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Fukui, T.; Hikichi, C.; Ishikawa, T.; Murate, K.; Adachi, T.; Imai, H.; Fukuhara, K.; Ueda, A.; Kaplan, A.P.; et al. Neurotropin promotes ngf signaling through interaction of GM1 ganglioside with TRK neurotrophin receptor in PC12 cells. Brain Res. 2015, 1596, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, T.; Egashira, N.; Itoh, Y.; Shimazoe, T.; Ikegami, Y.; Yano, T.; Yoshimura, M.; Oishi, R. Neurotropin reverses paclitaxel-induced neuropathy without affecting anti-tumour efficacy. Eur. J. Cancer 2009, 45, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, T.; Egashira, N.; Watanabe, H.; Ikegami, Y.; Hirakawa, S.; Mihara, Y.; Yano, T.; Ikesue, H.; Oishi, R. Prevention of oxaliplatin-induced mechanical allodynia and neurodegeneration by neurotropin in the rat model. Eur. J. Pain 2011, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.P.; Kim, J.; Syed, N.; Tung, Y.J.; Bhaskaran, A.; Mindos, T.; Mirsky, R.; Jessen, K.R.; Maurel, P.; Parkinson, D.B.; et al. P38 mapk activation promotes denervated schwann cell phenotype and functions as a negative regulator of schwann cell differentiation and myelination. J. Neurosci. 2012, 32, 7158–7168. [Google Scholar] [CrossRef] [PubMed]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular mechanisms involved in schwann cell plasticity. Front. Mol. Neurosci. 2017, 10, 38. [Google Scholar] [CrossRef]
- Shin, Y.K.; Jang, S.Y.; Park, J.Y.; Park, S.Y.; Lee, H.J.; Suh, D.J.; Park, H.T. The Neuregulin-Rac-MKK7 pathway regulates antagonistic c-jun/Krox20 expression in Schwann cell dedifferentiation. Glia 2013, 61, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.J.; Yang, M.; Gustin, J.A.; Chang, L.W.; Freimuth, R.R.; Nagarajan, R.; Milbrandt, J. Analysis of peripheral nerve expression profiles identifies a novel myelin glycoprotein, MP11. J. Neurosci. 2008, 28, 7563–7573. [Google Scholar] [CrossRef] [PubMed]
- Leitman, E.M.; Tewari, A.; Horn, M.; Urbanski, M.; Damanakis, E.; Einheber, S.; Salzer, J.L.; de Lanerolle, P.; Melendez-Vasquez, C.V. Mlck regulates schwann cell cytoskeletal organization, differentiation and myelination. J. Cell Sci. 2011, 124, 3784–3796. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.; Aixi, Y.; Danmou, X.; Wusheng, K.; Zhengren, P.; Ting, R. The mood stabilizer valproic acid induces proliferation and myelination of rat schwann cells. Neurosci. Res. 2011, 70, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, D.B.; Bhaskaran, A.; Arthur-Farraj, P.; Noon, L.A.; Woodhoo, A.; Lloyd, A.C.; Feltri, M.L.; Wrabetz, L.; Behrens, A.; Mirsky, R.; et al. C-jun is a negative regulator of myelination. J. Cell Biol. 2008, 181, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Garratt, A.N.; Voiculescu, O.; Topilko, P.; Charnay, P.; Birchmeier, C. A dual role of ERBB2 in myelination and in expansion of the schwann cell precursor pool. J. Cell Biol. 2000, 148, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.P.; Zhang, D.P.; Mak, K.S.; Bonder, D.E.; Pomeroy, S.L.; Kim, H.A. Schwann cell proliferation during wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: Axon-dependent removal of newly generated schwann cells by apoptosis. Mol. Cell. Neurosci. 2008, 38, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.C.; Cottrell, D.F.; Brophy, P.J.; Fleetwood-Walker, S.M. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J. Neurosci. 2003, 23, 3221–3233. [Google Scholar] [PubMed]
- Inoue, M.; Xie, W.; Matsushita, Y.; Chun, J.; Aoki, J.; Ueda, H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience 2008, 152, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Nagai, J.; Uchida, H.; Matsushita, Y.; Yano, R.; Ueda, M.; Niwa, M.; Aoki, J.; Chun, J.; Ueda, H. Autotaxin and lysophosphatidic acid1 receptor-mediated demyelination of dorsal root fibers by sciatic nerve injury and intrathecal lysophosphatidylcholine. Mol. Pain 2010, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Tsai, Y.J.; Chen, S.H.; Lin, C.T.; Lue, J.H. Lysophosphatidylcholine causes neuropathic pain via the increase of neuronal nitric oxide synthase in the dorsal root ganglion and cuneate nucleus. Pharmacol. Biochem. Behav. 2013, 106, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Askari, N.; Yaghoobi, M.M.; Shamsara, M.; Esmaeili-Mahani, S. Human dental pulp stem cells differentiate into oligodendrocyte progenitors using the expression of Olig2 transcription factor. Cells Tissues Organs 2014, 200, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Askari, N.; Yaghoobi, M.M.; Shamsara, M.; Esmaeili-Mahani, S. Tetracycline-regulated expression of Olig2 gene in human dental pulp stem cells lead to mouse sciatic nerve regeneration upon transplantation. Neuroscience 2015, 305, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Hata, T.; Iida, J.; Yoneda, R.; Isida, S. Decrease in pain threshold in sart stressed mice. Jpn. J. Pharmacol. 1979, 29, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Miyamoto, Y.; Hamasaki, H.; Sanbe, A.; Kusakawa, S.; Nakamura, A.; Tsumura, H.; Maeda, M.; Nemoto, N.; Kawahara, K.; et al. The atypical guanine-nucleotide exchange factor, dock7, negatively regulates schwann cell differentiation and myelination. J. Neurosci. 2011, 31, 12579–12592. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rey, M.; Iruarrizaga-Lejarreta, M.; Lozano, J.J.; Aransay, A.M.; Fernandez, A.F.; Lavin, J.L.; Mosen-Ansorena, D.; Berdasco, M.; Turmaine, M.; Luka, Z.; et al. S-adenosylmethionine levels regulate the schwann cell DNA methylome. Neuron 2014, 81, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Callizot, N.; Combes, M.; Steinschneider, R.; Poindron, P. A new long term in vitro model of myelination. Exp. Cell Res. 2011, 317, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Targ, E.F.; Kocsis, J.D. Action potential characteristics of demyelinated rat sciatic nerve following application of 4-aminopyridine. Brain Res. 1986, 363, 1–9. [Google Scholar] [CrossRef]
- Okada, K.; Tanaka, H.; Temporin, K.; Okamoto, M.; Kuroda, Y.; Moritomo, H.; Murase, T.; Yoshikawa, H. Methylcobalamin increases ERK1/2 and akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp. Neurol. 2010, 222, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Varejao, A.S.; Meek, M.F.; Ferreira, A.J.; Patricio, J.A.; Cabrita, A.M. Functional evaluation of peripheral nerve regeneration in the rat: Walking track analysis. J. Neurosci. Methods 2001, 108, 1–9. [Google Scholar] [CrossRef]
- Pereira Lopes, F.R.; Martin, P.K.; Frattini, F.; Biancalana, A.; Almeida, F.M.; Tomaz, M.A.; Melo, P.A.; Borojevic, R.; Han, S.W.; Martinez, A.M. Double gene therapy with granulocyte colony-stimulating factor and vascular endothelial growth factor acts synergistically to improve nerve regeneration and functional outcome after sciatic nerve injury in mice. Neuroscience 2013, 230, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Goulart, C.O.; Jurgensen, S.; Souto, A.; Oliveira, J.T.; de Lima, S.; Tonda-Turo, C.; Marques, S.A.; de Almeida, F.M.; Martinez, A.M. A combination of schwann-cell grafts and aerobic exercise enhances sciatic nerve regeneration. PLoS ONE 2014, 9, e110090. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, G.M.; Ritchie, J.; Henry, J.L. Paw withdrawal threshold in the von frey hair test is influenced by the surface on which the rat stands. J. Neurosci. Methods 1999, 87, 185–193. [Google Scholar] [CrossRef]
- Noda, K.; Akita, H.; Ogata, M.; Saji, M. Paclitaxel-induced hyperalgesia modulates negative affective component of pain and nr1 receptor expression in the frontal cortex in rats. Neurosci. Res. 2014, 80, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Bartolini, A.; Ghelardini, C. The phospholipase C-IP3 pathway is involved in muscarinic antinociception. Neuropsychopharmacology 2003, 28, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Quattrone, A.; Galeotti, N. Silencing of the RNA-binding protein hur attenuates hyperalgesia and motor disability in experimental autoimmune encephalomyelitis. Neuropharmacology 2017, 123, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Guevara, C.; Fernandez, A.C.; Cardenas, R.; Suarez-Roca, H. Reduction of spinal PGE2 concentrations prevents swim stress-induced thermal hyperalgesia. Neurosci. Lett. 2015, 591, 110–114. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, H.; Tanaka, H.; Sayanagi, J.; Iwahashi, T.; Suzuki, K.; Nishimoto, S.; Okada, K.; Murase, T.; Yoshikawa, H. Neurotropin® Accelerates the Differentiation of Schwann Cells and Remyelination in a Rat Lysophosphatidylcholine-Induced Demyelination Model. Int. J. Mol. Sci. 2018, 19, 516. https://doi.org/10.3390/ijms19020516
Matsuoka H, Tanaka H, Sayanagi J, Iwahashi T, Suzuki K, Nishimoto S, Okada K, Murase T, Yoshikawa H. Neurotropin® Accelerates the Differentiation of Schwann Cells and Remyelination in a Rat Lysophosphatidylcholine-Induced Demyelination Model. International Journal of Molecular Sciences. 2018; 19(2):516. https://doi.org/10.3390/ijms19020516
Chicago/Turabian StyleMatsuoka, Hozo, Hiroyuki Tanaka, Junichi Sayanagi, Toru Iwahashi, Koji Suzuki, Shunsuke Nishimoto, Kiyoshi Okada, Tsuyoshi Murase, and Hideki Yoshikawa. 2018. "Neurotropin® Accelerates the Differentiation of Schwann Cells and Remyelination in a Rat Lysophosphatidylcholine-Induced Demyelination Model" International Journal of Molecular Sciences 19, no. 2: 516. https://doi.org/10.3390/ijms19020516
APA StyleMatsuoka, H., Tanaka, H., Sayanagi, J., Iwahashi, T., Suzuki, K., Nishimoto, S., Okada, K., Murase, T., & Yoshikawa, H. (2018). Neurotropin® Accelerates the Differentiation of Schwann Cells and Remyelination in a Rat Lysophosphatidylcholine-Induced Demyelination Model. International Journal of Molecular Sciences, 19(2), 516. https://doi.org/10.3390/ijms19020516