Circadian and Sleep Modulation of Dreaming in Women with Major Depression
Abstract
:1. Introduction
2. Results
2.1. Baseline Dream Recall, Number of Dreams and Dream Emotional Load
2.2. Circadian Modulation of Dream Recall, Number of Dreams and Dream Emotional Load
2.3. NREM/REM Modulation of Dream Recall, Number of Dreams and Dream Emotional Load
2.4. Association of Dream Emotional Load and Subjective Perception of Mood
3. Discussion
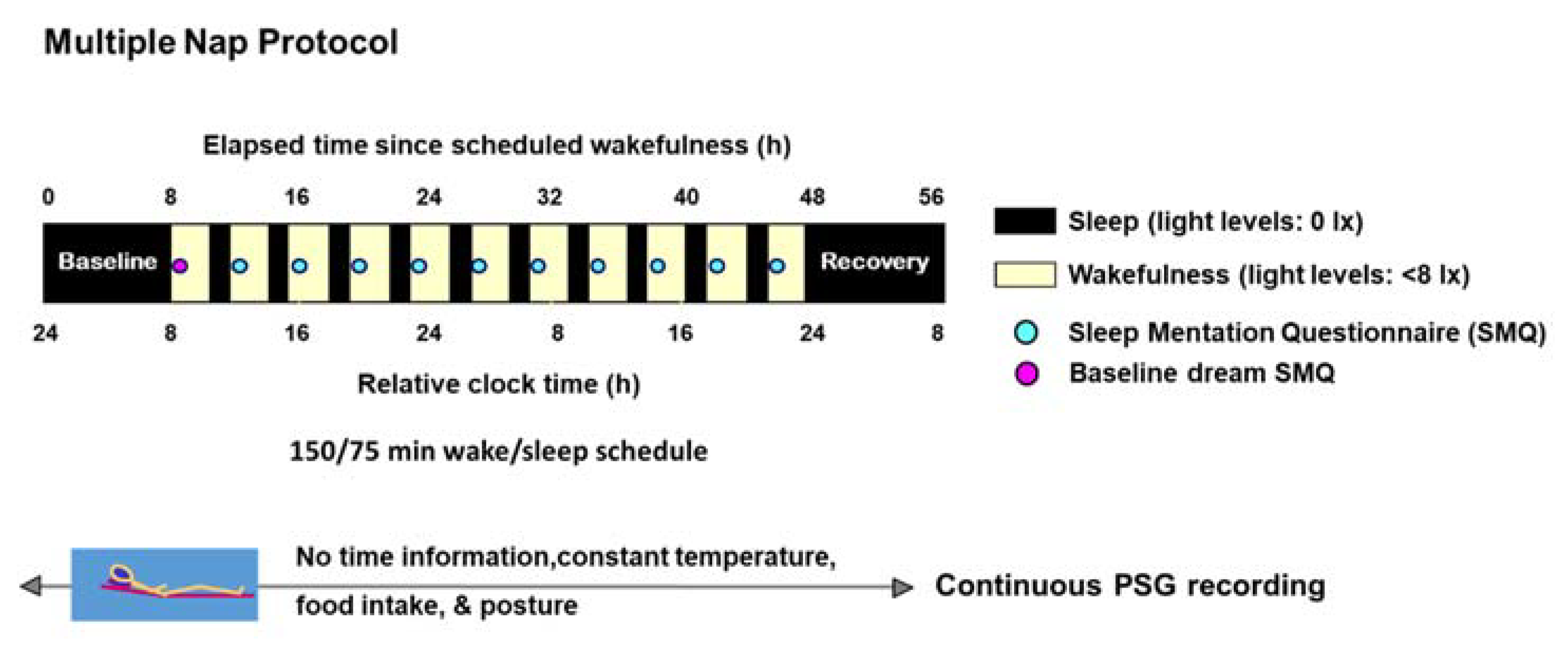
4. Materials and Methods
4.1. Study Participants
4.2. Study Design
4.3. Salivary Melatonin and Classification of Circadian Day and Night
4.4. Sleep Polysomnographic Measures
4.5. Dream Recall and Dream Emotional Load
4.6. Mood Ratings
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; pp. 1–18. [Google Scholar]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Riemann, D.; Krone, L.B.; Wulff, K.; Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology 2020, 45, 74–89. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Araujo, J.F. Sleep disorders and suicidal ideation in patients with depressive disorder. Psychiatry Res. 2007, 153, 131–136. [Google Scholar] [CrossRef]
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19. [Google Scholar] [CrossRef]
- Hertenstein, E.; Feige, B.; Gmeiner, T.; Kienzler, C.; Spiegelhalder, K.; Johann, A.; Jansson-Fröjmark, M.; Palagini, L.; Rucker, G.; Riemann, D.; et al. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 43, 96–105. [Google Scholar] [CrossRef]
- Benz, F.; Knoop, T.; Ballesio, A.; Bacaro, V.; Johann, A.F.; Rücker, G.; Feige, B.; Riemann, D.; Baglioni, C. The efficacy of cognitive and behavior therapies for insomnia on daytime symptoms: A systematic review and network meta-analysis. Clin. Psychol. Rev. 2020, 80, 101873. [Google Scholar] [CrossRef]
- Cheng, P.; Kalmbach, D.A.; Tallent, G.; Joseph, C.L.; Espie, C.A.; Drake, C.L. Depression prevention via digital cognitive behavioral therapy for insomnia: A randomized controlled trial. Sleep 2019, 42, zsz150. [Google Scholar] [CrossRef]
- Boivin, D.B.; Czeisler, C.A.; Dijk, D.-J.; Duffy, J.F.; Folkard, S.; Minors, D.S.; Totterdell, P.; Waterhouse, J.M. Complex Interaction of the Sleep-Wake Cycle and Circadian Phase Modulates Mood in Healthy Subjects. Arch. Gen. Psychiatry 1997, 54, 145–152. [Google Scholar] [CrossRef]
- Birchler-Pedross, A.; Schröder, C.M.; Münch, M.; Knoblauch, V.; Blatter, K.; Schnitzler-Sack, C.; Wirz-Justice, A.; Cajochen, C. Subjective Well-Being Is Modulated by Circadian Phase, Sleep Pressure, Age, and Gender. J. Biol. Rhythm. 2009, 24, 232–242. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Morris, C.J.; Scheer, F.A.J.L. Circadian misalignment increases mood vulnerability in simulated shift work. Sci. Rep. 2020, 10, 18614. [Google Scholar] [CrossRef]
- Chellappa, S.L. Circadian misalignment: A biological basis for mood vulnerability in shift work. Eur. J. Neurosci. 2020, 52, 3846–3850. [Google Scholar] [CrossRef]
- Birchler-Pedross, A.; Frey, S.; Götz, T.; Brunner, P.; Knoblauch, V.; Wirz-Justice, A.; Chellappa, S.; Cajochen, C. Subjective Mood in Young Unmedicated Depressed Women under High and Low Sleep Pressure Conditions. Biology 2016, 5, 52. [Google Scholar] [CrossRef] [Green Version]
- Spanò, G.; Pizzamiglio, G.; McCormick, C.; Clark, I.A.; De Felice, S.; Miller, T.D.; Edgin, J.O.; Rosenthal, C.R.; Maguire, E.A. Dreaming with hippocampal damage. eLife 2020, 9, 9. [Google Scholar] [CrossRef]
- Zhang, J.; Wamsley, E.J. EEG predictors of dreaming outside of REM sleep. Psychophysiology 2019, 56, e13368. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Cajochen, C. Ultradian and circadian modulation of dream recall: EEG correlates and age effects. Int. J. Psychophysiol. 2013, 89, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Agargun, M.Y.; Cartwright, R. REM sleep, dream variables and suicidality in depressed patients. Psychiatry Res. 2003, 119, 33–39. [Google Scholar] [CrossRef]
- Palagini, L.; Rosenlicht, N. Sleep, dreaming, and mental health: A review of historical and neurobiological perspectives. Sleep Med. Rev. 2011, 15, 179–186. [Google Scholar] [CrossRef]
- Chellappa, S.; Frey, S.; Knoblauch, V.; Cajochen, C. Cortical activation patterns herald successful dream recall after NREM and REM sleep. Biol. Psychol. 2011, 87, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Chellappa, S.; Münch, M.; Knoblauch, V.; Cajochen, C. Age effects on spectral electroencephalogram activity prior to dream recall. J. Sleep Res. 2011, 21, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Siclari, F.; Baird, B.; Perogamvros, L.; Bernardi, G.; LaRocque, J.J.; Riedner, B.; Boly, M.; Postle, B.R.; Tononi, G. The neural correlates of dreaming. Nat. Neurosci. 2017, 20, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Laxhmi, C.S.; Münch, M.; Blatter, K.; Knoblauch, V.; Cajochen, C. Does the Circadian Modulation of Dream Recall Modify with Age? Sleep 2009, 32, 1201–1209. [Google Scholar] [CrossRef] [Green Version]
- Scarpelli, S.; Bartolacci, C.; D’Atri, A.; Gorgoni, M.; De Gennaro, L. The Functional Role of Dreaming in Emotional Processes. Front. Psychol. 2019, 10, 459. [Google Scholar] [CrossRef] [Green Version]
- Robbins, P.R.; Tanck, R.H. Depressed Mood, Dream Recall and Contentless Dreams. Imagin. Cogn. Pers. 1988, 8, 165–174. [Google Scholar] [CrossRef]
- Schredl, M. Dream recall in depressed patients. Psychother. Psychosom. Med. Psychol. 1995, 45, 414–417. [Google Scholar]
- Armitage, R.; Rochlen, A.; Fitch, T.; Trivedi, M.; Rush, A.J. Dream recall and major depression: A preliminary report. Dreaming 1995, 5, 189–198. [Google Scholar] [CrossRef]
- Barrett, D.; Loeffler, M. Comparison of dream content of depressed vs. nondepressed dreamers. Psychol. Rep. 1992, 70, 403–406. [Google Scholar] [CrossRef]
- Emens, J.; Lewy, A.; Kinzie, J.M.; Arntz, D.; Rough, J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009, 168, 259–261. [Google Scholar] [CrossRef]
- Kripke, D.F.; Nievergelt, C.M.; Joo, E.; Shekhtman, T.; Kelsoe, J.R. Circadian polymorphisms associated with affective disorders. J. Circadian Rhythm. 2009, 7, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Sasabayashi, D.; Yücel, M.; Whittle, S.; Lorenzetti, V.; Walterfang, M.; Suzuki, M.; Pantelis, C.; Malhi, G.S.; Allen, N.B. Pineal Gland Volume in Major Depressive and Bipolar Disorders. Front. Psychiatry 2020, 11, 450. [Google Scholar] [CrossRef]
- Frey, S.; Birchler-Pedross, A.; Hofstetter, M.; Brunner, P.; Götz, T.; Münch, M.; Blatter, K.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Young Women With Major Depression Live on Higher Homeostatic Sleep Pressure Than Healthy Controls. Chronobiol. Int. 2012, 29, 278–294. [Google Scholar] [CrossRef]
- Watson, C.J.; Baghdoyan, H.A.; Lydic, R. Neuropharmacology of Sleep and Wakefulness. Sleep Med. Clin. 2010, 5, 513–528. [Google Scholar] [CrossRef] [Green Version]
- King, D.B.; DeCicco, T.L. The relationships between dream content and physical health, mood, and self-construal. Dreaming 2007, 17, 127–139. [Google Scholar] [CrossRef]
- Miller, N.J.; DeCicco, T.L.; Dale, A.L.; Murkar, A. Assessing the effects of meditation on dream imagery, depression and anxiety. Int. J. Dream Res. 2015, 8, 99–104. [Google Scholar]
- Schredl, M.; Berger, M.; Riemann, D. The effect of trimipramine on dream recall and dream emotions in depressive outpatients. Psychiatry Res. 2009, 167, 279–286. [Google Scholar] [CrossRef]
- Pesant, N.; Zadra, A. Dream content and psychological well-being: A longitudinal study of the continuity hypothesis. J. Clin. Psychol. 2006, 62, 111–121. [Google Scholar] [CrossRef]
- Serpe, A.; De Cicco, T.L. An investigation into anxiety and depression in dream imagery: The issue of co-morbidity. Int. J. Dream Res. 2020, 13, 82–89. [Google Scholar]
- Schredl, M.; Schäfer, G.; Weber, B.; Heuser, I. Dreaming and insomnia: Dream recall and dream content of patients with insomnia. J. Sleep Res. 1998, 7, 191–198. [Google Scholar] [CrossRef]
- Tribl, G.G.; Wetter, T.C.; Schredl, M. Dreaming under antidepressants: A systematic review on evidence in depressive patients and healthy volunteers. Sleep Med. Rev. 2013, 17, 133–142. [Google Scholar] [CrossRef]
- Nicolas, A.; Ruby, P.M. Dreams, Sleep, and Psychotropic Drugs. Front. Neurol. 2020, 11, 507495. [Google Scholar] [CrossRef]
- Torsvall, L.; Åkerstedt, T. A diurnal type scale. Construction, consistency and validation in shift work. Scand. J. Work. Environ. Health 1980, 6, 283–290. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Aeschbach, D. Sleep and anxiety: From mechanisms to interventions. Sleep Med. Rev. 2021, 61, 101583. [Google Scholar] [CrossRef]
- Saeidi, M.; Komasi, S.; Soroush, A.; Khazaie, H.; Zakiei, A. Dreams content and emotional load in cardiac rehabilitation patients and their relation to anxiety and depression. Ann. Card. Anaesth. 2018, 21, 388–392. [Google Scholar] [CrossRef]
- Schredl, M.; Reinhard, I. Gender differences in dream recall: A meta-analysis. J. Sleep Res. 2008, 17, 125–131. [Google Scholar] [CrossRef]
- Cain, S.W.; Dennison, C.F.; Zeitzer, J.M.; Guzik, A.M.; Khalsa, S.B.; Santhi, N.; Schoen, M.; Czeisler, C.A.; Duffy, J.F. Sex Differences in Phase Angle of Entrainment and Melatonin Amplitude in Humans. J. Biol. Rhythm. 2010, 25, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Frey, S.; Birchler-Pedross, A.; Hofstetter, M.; Brunner, P.; Götz, T.; Münch, M.; Blatter, K.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Challenging the sleep homeostat: Sleep in depression is not premature aging. Sleep Med. 2012, 13, 933–945. [Google Scholar] [CrossRef]
- Birchler-Pedross, A.; Frey, S.; Chellappa, S.; Götz, T.; Brunner, P.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Higher Frontal EEG Synchronization in Young Women with Major Depression: A Marker for Increased Homeostatic Sleep Pressure? Sleep 2011, 34, 1699–1706. [Google Scholar] [CrossRef]
- Wittchen, H.U.; Wunderlich, U.; Gruschwitz, S.; Zaudig, M. Strukturiertes Klinisches Interview für DSM-IV (SKID); Beltz-Test: Göttingen, Germany, 1996. [Google Scholar]
- Williams, J.B.; Terman, M. Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS); New York State Psychiatric Institute: New York, NY, USA, 2003. [Google Scholar]
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Knoblauch, V.; Martens, W.L.; Wirz-Justice, A.; Cajochen, C. Human sleep spindle characteristics after sleep deprivation. Clin. Neurophysiol. 2003, 114, 2258–2267. [Google Scholar] [CrossRef]
- Duffy, J.F.; Dijk, D.-J. Getting Through to Circadian Oscillators: Why Use Constant Routines? J. Biol. Rhythm. 2002, 17, 4–13. [Google Scholar] [CrossRef]
- Weber, J.M.; Schwander, J.C.; Unger, I.; Meier, D. A direct ultrasensitive RIA for the melatonin in human saliva: Comparison with serum levels. J. Sleep Res. 1997, 26, 75. [Google Scholar]
- Rechtschaffen, A.; Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects; US Department of Health, Education and Welfare, Public Health Service: Bethesda, MD, USA, 1968.





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birchler-Pedross, A.; Frey, S.; Cajochen, C.; Chellappa, S.L. Circadian and Sleep Modulation of Dreaming in Women with Major Depression. Clocks & Sleep 2022, 4, 114-128. https://doi.org/10.3390/clockssleep4010012
Birchler-Pedross A, Frey S, Cajochen C, Chellappa SL. Circadian and Sleep Modulation of Dreaming in Women with Major Depression. Clocks & Sleep. 2022; 4(1):114-128. https://doi.org/10.3390/clockssleep4010012
Chicago/Turabian StyleBirchler-Pedross, Angelina, Sylvia Frey, Christian Cajochen, and Sarah L. Chellappa. 2022. "Circadian and Sleep Modulation of Dreaming in Women with Major Depression" Clocks & Sleep 4, no. 1: 114-128. https://doi.org/10.3390/clockssleep4010012





