1. Introduction
In 1982, Borbély described sleep regulation as a two-process model, relying on a reciprocal interaction between a process controlled by the circadian clock and a homeostatic process, and this model has been prominent in the sleep field in the past decades [
1,
2,
3]. Light also exerts powerful effects on sleep and waking in different ways. First of all, the light/dark alternation is a key Zeitgeber that drives the circadian timing system, which in turns acts to time sleep and wakefulness to the 24 h day [
4]. Moreover, several papers have more recently highlighted the importance of light direct effects, independent of the circadian system, on sleep, waking and behavior [
5,
6,
7]. These data obtained in rodents led us to propose a three-process model including the direct photic influence in addition to the homeostatic and circadian drives [
6,
8] and ongoing studies are underway in order to validate this model in humans.
Ultradian light–dark (LD) cycles, consisting of the alternation of short light and dark exposures in rodents, are a precious tool to study the influence of light and dark exposure, according to circadian time, on the distribution and quality of sleep and waking. Whereas it is now clearly demonstrated that light or dark exposure influence sleep and waking [
6,
9,
10,
11,
12], the effects of such ultradian LD cycles on the distribution of the different vigilance states remain controversial [
5,
13,
14,
15,
16,
17,
18] and their influence on sleep and wake quality has been poorly explored [
5,
13]. The three-process model suggests that the direct effects of light interact with the circadian and homeostatic regulations of sleep under such ultradian cycles. Therefore, the aims of this study are (1) to precisely evaluate the effects of light and dark exposures, according to circadian time, on sleep and waking distribution and quality, and (2) to determine if these effects depend on the duration of LD cycles. To do this, mice were exposed during 24 h to two ultradian LD cycles of different durations: T2 cycle, consisting of the alternation of 1 h of light/1 h of dark, and T7 cycle, consisting of 3.5 h of light/3.5 h of darkness. Exposure to light promotes both NREM and REM sleep and induces a decrease in wake amounts and alertness. These effects are modulated by circadian time, appearing especially during early subjective night, and their kinetics is highly dependent on the duration of pulses.
3. Discussion
Our study presents major findings concerning the direct effects of alternating light and dark exposures on sleep and waking. We showed that light has major influence on both organization and quality of sleep and wakefulness, which appears especially during early subjective night, with a clear interaction between the direct photic regulation and the circadian regulation. Moreover, the time-course of the amounts and quality of NREM sleep and waking clearly depends on pulses duration, suggesting an interaction of the photic regulation of sleep with the sleep homeostatic process.
The acute sleep-promoting effect of light and wake-promoting effect of dark have already been demonstrated in mice and rats both when applied as a single pulse [
5,
9,
13,
15] or in short ultradian LD cycles ([
5,
14,
16,
17] but see [
18,
19]). As suggested in some of these previous studies [
13,
14,
16], our results obtained under the T2 cycle confirm that the most important effect of LD alternation is observed during early subjective night. Importantly, exposure to longer pulses under the T7 cycle (3.5 h vs. 1 h pulse for the T2 cycle) shows the same circadian evolution of light effects, suggesting a strong interaction between direct effects of light and circadian regulation of sleep and waking, independent of the duration of light and dark pulses. This circadian gating of the direct effects of light suggests that it facilitates the photic influence during subjective night, a period during which nocturnal animals spend more time awake, interacting with their environment, than during subjective day. Additionally, the different pathways of photoreception and phototransduction seem to have time of day-dependent sensitivity. Indeed, melanopsin, the photopigment present in intrinsic photosensitive retinal ganglion cells, seems to be particularly involved in light direct effects observed during the early subjective night [
5,
6,
9], which could also explain the circadian modulation of light direct influence.
Contrary to the large light-induced NREM sleep promotion, REM sleep is affected to a far lesser extent by light–dark alternation under short-duration ultradian LD cycle. Given that REM sleep appears only after a consistent period of NREM sleep, short light pulses could be sufficient to promote NREM sleep but longer exposures might be necessary to significantly promote REM sleep. This hypothesis is supported by our results obtained under the T7 cycle, where REM sleep is significantly increased during light pulses compared to dark exposures. These data suggest that REM sleep induction by light might be secondary to the NREM sleep induction.
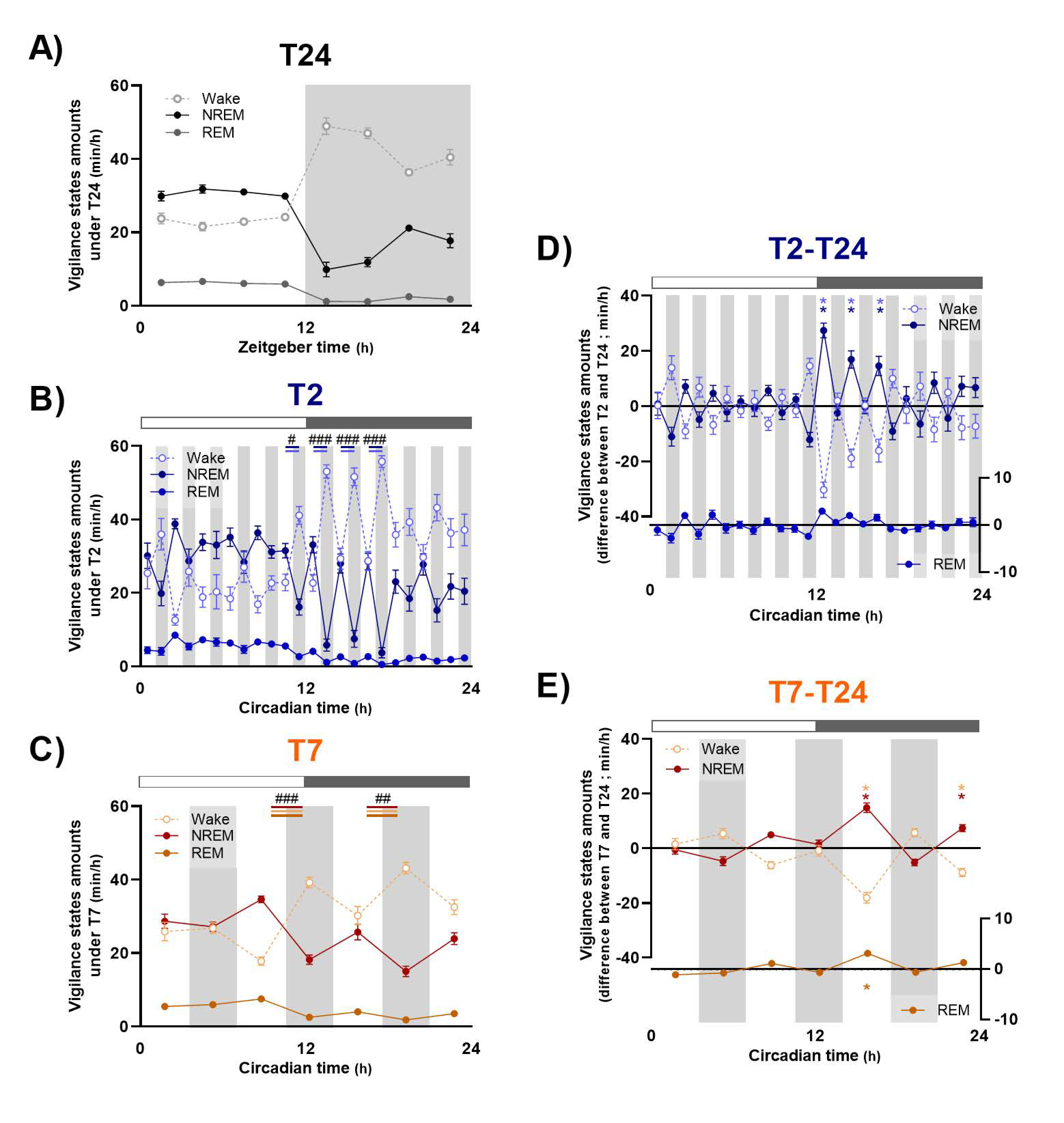
Even if repeated light and dark pulses modulate sleep and waking amounts in an ultradian way, a circadian variation of sleep–wake distribution remains when mice are exposed to ultradian cycles during 24 h. These results confirm previous observations [
5,
14,
15,
19] and suggest that such an exposure limited to 24 h allows circadian clock-related mechanisms to remain expressed, even if circadian photoentrainment is masked by the ultradian LD alternation. On the contrary, more prolonged exposures can lead to a total disappearance of circadian rhythmicity [
17,
18].
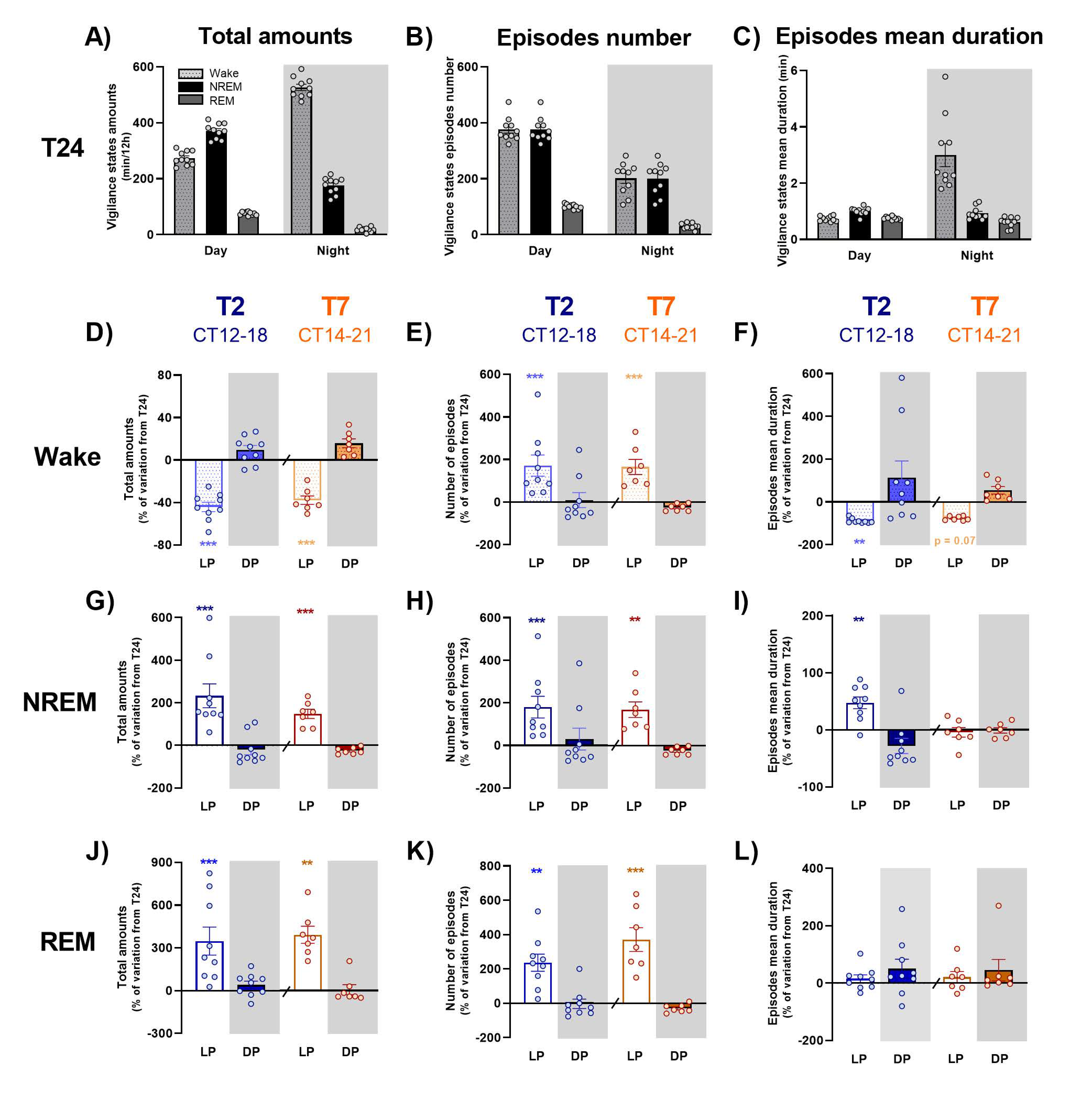
In order to go further, dissecting the effects of the T2 and T7 cycles on sleep and waking organization, we evaluated the variation from T24 of each vigilance state amount, their episodes’ number and mean duration, averaged for light and dark exposures, respectively. The time course of these variations was also evaluated across light and dark exposures, along with the ECoG spectral composition of wake and NREM sleep, as a reflection of their quality. The effect of LD alternation is distinct according to vigilance state.
Under short ultradian T2 cycle, inappropriate exposure to light during subjective night induces a dramatic fragmentation of waking, with a clear decrease in the mean duration of episodes, as shown earlier [
9], in spite of an increase of almost 100% of their number. Episodes longer than 1 h are particularly affected and split into shorter ones. This fragmentation is accompanied by a decrease in alertness, characterized by a decline of theta and gamma band activities during the whole hour of light exposure. Opposite, but weaker, effects appear when mice are exposed to dark during subjective day, particularly due to the effects of first and last exposures to darkness. These results confirm our previous ones showing a rapid and prolonged increase in theta and gamma activities during wake when mice are exposed to dark in the beginning of the day [
5]. In addition, this study is, to our knowledge, the first one to demonstrate that inappropriate light exposure during subjective night not only promotes sleep, at the expense of wakefulness, but also drastically alters alertness, an effect that could reflect a direct effect of light or an indirect consequence of the very significant fragmentation of wake episodes. Contrary to waking, NREM and REM sleep episodes’ mean duration is not dramatically modified under the T2 cycle, in spite of a slight increase in the one of NREM sleep, but the light-induced increase in their total amount relies mostly on an increase in the number of their episodes, which is consistent with a previous report [
13]. The time course of NREM sleep amounts modulation by light exposure is consistent with a primary alerting effect of light, with a 10 min delay before sleep induction [
10,
17]. These modifications of sleep episodes organization are accompanied by a decline of delta power during light exposure and a subsequent increase during dark exposure of subjective night, as a perfect reflection of NREM sleep amounts modulation at the same time points. Therefore, these results suggest an ultradian variation of sleep depth under LD alternation.
Longer light pulses under the T7 cycle induce similar modifications of sleep and waking organization, with an important fragmentation of waking episodes whereas NREM and REM sleep episodes are of the same duration but much more numerous. In the same way as under the T2 cycle, light induces a decrease in alertness, with a reduction in ECoG gamma activity, whereas dark promotes it. Finally, the important decrease in NREM sleep amounts observed during the beginning of dark pulses during subjective night induces a significant rebound of delta activity, in accordance with data published by Szalontai et al. [
17], who showed that wake-inducing effect of darkness temporarily blocks the dissipation of the homeostatic sleep drive induced by 6 h of sleep deprivation. Importantly, even if light and dark exposures under the T7 cycle show similar global effects on sleep and waking than under the T2 cycle, the time course of these modulations across light and dark exposures is completely different according to the pulses’ duration. Indeed, while these modulations are maintained along 1 h pulses of light and dark under both the T2 and T7 cycles, light-induced effects are progressively attenuated after one hour of exposure under the T7 cycles, and, more importantly, dark-induced modifications are completely reversed for the second part of the pulse. This time course explains that changes in vigilance states amounts during light and dark pulses in comparison with baseline T24 is of similar level for the T2 and T7 cycles, in spite of the difference of pulses duration. Therefore, when deciphering the acute photic effects under a 1 h of light/1 h of dark schedule, we can assume that within the time frame of 1 h there is not enough time for the animal to build up sleep homeostatic pressure importantly. On the contrary, the duration of 3.5 h pulses is long enough for the dynamics of the homeostatic process to accumulate sleep pressure. Finally, these data suggest that the homeostatic process gets the upper hand over the direct effects of light under ultradian LD cycle with longer light and dark pulses.
Altogether, results obtained under T2 and T7 ultradian LD cycles show that the influence of light and dark on sleep and waking is highly dependent on circadian time and on the duration of pulses. Then, inappropriate lighting exposure according to circadian timing, i.e., light during subjective night or darkness during subjective day, not only modify sleep and wake distribution, but also has a dramatic effect on alertness and sleep depth. Such alterations of vigilance and quality of sleep can have multiple consequences on daily life, among them attention problems, cognitive deficits, car accidents, etc., and these effects are observed even in absence of sleep deprivation. Finally, homeostatic process could, when ultradian light and dark exposures are longer, overtake direct effects of light in the regulation of sleep and waking organization and quality. These data have immediate applications in human, in order to adapt lighting for shift-workers and more generally to optimize societal lighting.
4. Materials and Methods
Experimental protocols and animal care were in compliance with European Community Council Directive 2010/63/EU and the current project was approved by the Regional Ethical Committee of Strasbourg for Animal Experimentation and the French Ministry of Higher Education and Research (approval no. #8426-20161129164229760 v6, 19 February 2019).
4.1. Subjects and Housing Conditions
C57BL/6 adult male mice were obtained from our animal facility, the Chronobiotron (CNRS UMS 3415). They were housed in transparent Makrolon cages (33 cm × 17 cm × 14 cm) enriched with nesting material, in groups of 2 to 3, in a room with controlled temperature (24 ± 1 °C) and humidity (40 ± 5%) under a 12 h–12 h white polychromatic LD cycle (light on at 7:00 a.m.; 5000 K; 150–200 lux). They had ad libitum access to food and water.
4.2. Surgery
At the age of 9 weeks (8.94 ± 1.3), mice were subjected to surgery protocol for electrodes implantation. Under deep anesthesia with intraperitoneal injection of zoletil (40 mg/kg) and xylazine (10 mg/kg), animals were implanted with three gold-plated screws allowing ECoG frontal-parietal derivation and one ground electrode. Two EMG electrodes were inserted into the neck muscles along the back of the skull. All electrodes were then soldered to a connector and cemented to the skull before the skin was sutured. Animals were isolated from the day of surgery until the end of experiments. In total, 13 to 14 days were given to recover from surgery and to habituate to the baseline conditions before any recording began.
4.3. Sleep Recordings
ECoG and EMG signals were recorded during (1) 24 h of undisturbed baseline under 12 h–12 h LD cycle called “T24 cycle” (
n = 10), (2) 24 h of ultradian 1 h light/1 h dark cycle called “T2 cycle” (
n = 9) and then (3) 24 h of ultradian 3.5 h light/3.5 h dark cycle called “T7 cycle” (
n = 7). A 12-day period between the two ultradian protocols allowed mice to entirely recover from sleep alterations. Recordings were supported by commercial Compumedics hardware (Neuvo 64–512 Channel EEG HD-LTM, Charlotte, NC, USA) and software (Profusion PSG4 Software, Abbotsford, Australia). ECoG and EMG signals were amplified, filtered, and analog-to-digital converted at 500 Hz for ECoG and 250 Hz for EMG. Sleep stages were manually classified in 4 s epochs according to criteria classically used in mice [
20]: non-rapid eye movement (NREM) sleep was distinguished by high-amplitude ECoG dominated by synchronized delta activity (0.75–4 Hz) and a low EMG signal; REM sleep was defined by a regular low-amplitude theta rhythm (4–12 Hz) and a low EMG signal; wake was characterized by a higher and variable EMG and a low-amplitude ECoG with both slower and faster components.
4.4. Quantitative Analyses
The amounts of each vigilance state were measured across time within T24 (3 h bins), T2 (1 h bins) and T7 (3.5 h bins) cycles. In order to directly compare ultradian T2 and T7 cycles to T24 one, we calculated the difference between each vigilance state amount during each light and dark pulse of T2 and T7 and corresponding time periods of T24 cycle.
In order to better understand the effects of T2 and T7 cycles on sleep and waking organization, we calculated total amounts, number of episodes and episodes mean duration of wake, NREM and REM sleep under each light and dark exposures. Values were averaged for light vs. dark pulses during subjective day, early (CT12-18) and late (CT17-24) subjective night under T2 or subjective night (CT14-21) under T7, and were represented as percentages of variation of the ones obtained during corresponding time points of T24 cycle [(data under T2 or T7/data under T24)*100-100]. A time-course analysis of sleep and wake distribution variation across light and dark pulses was also performed: the difference of each vigilance state amounts between ultradian LD cycles and T24 was calculated per 10 min (T2 cycle) or 30 min (T7 cycle) bins and averaged for light and dark pulses.
4.5. Spectral Analyses
Before processing, the data were cleaned by removing artefacts (values exceeding 3 SD from the mean, together with a 1 s window around selected artefacts). ECoG power spectra were calculated using Chronux signal processing toolbox [
21] with a time-frequency product of three and five tapers. Fast Fourier transform was performed on a moving window (window 4 s; overlap 1 s) and power of delta (0.75–4 Hz), theta (4–12 Hz) and gamma (30–70 Hz) frequency bands was analyzed.
The time course of theta and gamma band power during wake episodes was averaged per bins of 10—(T2) or 30—(T7) min and represented as percentages of variation from the one under T24 at corresponding time points (per bins of 1 h for comparison to T2 or 3.5 h for comparison to T7 cycle). Delta band activity was also measured per 20—(T2) or 30—(T7) min bins during NREM sleep episodes. These data were averaged for subjective day, early (CT12-18) and late (CT17-24) subjective night under T2 or subjective night (CT14-21) under T7.
4.6. Statistical Analysis
Statistical analysis was realized using Statistica (Statsoft, Tulsa, OK, USA; version 13). Repeated-measures analysis of variance (ANOVA) were used to measure time-course changes in vigilance states amounts and in frequency bands power according to cycle and light condition. The effect of cycle was evaluated on the total amounts, episodes number and mean duration of each vigilance state. HSD Tukey multiple range tests were then performed to determine post hoc significance. The threshold for rejecting the null hypothesis was 0.05 throughout.