Effect of Modified Yukmijihwang-Tang on Sleep Quality in the Rat
Abstract
:1. Introduction
2. Results
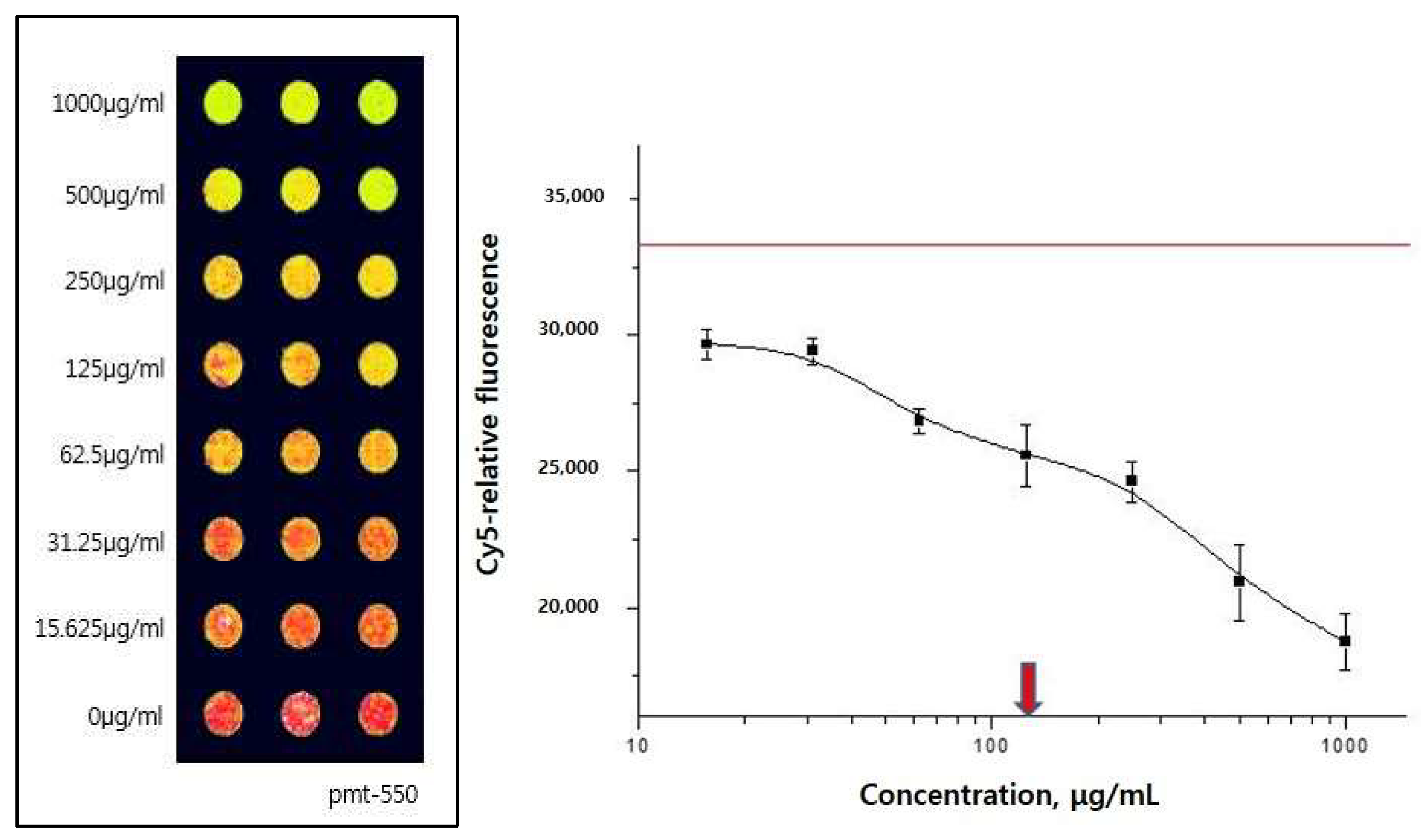
2.1. 5-HT2c Receptor Binding Assay
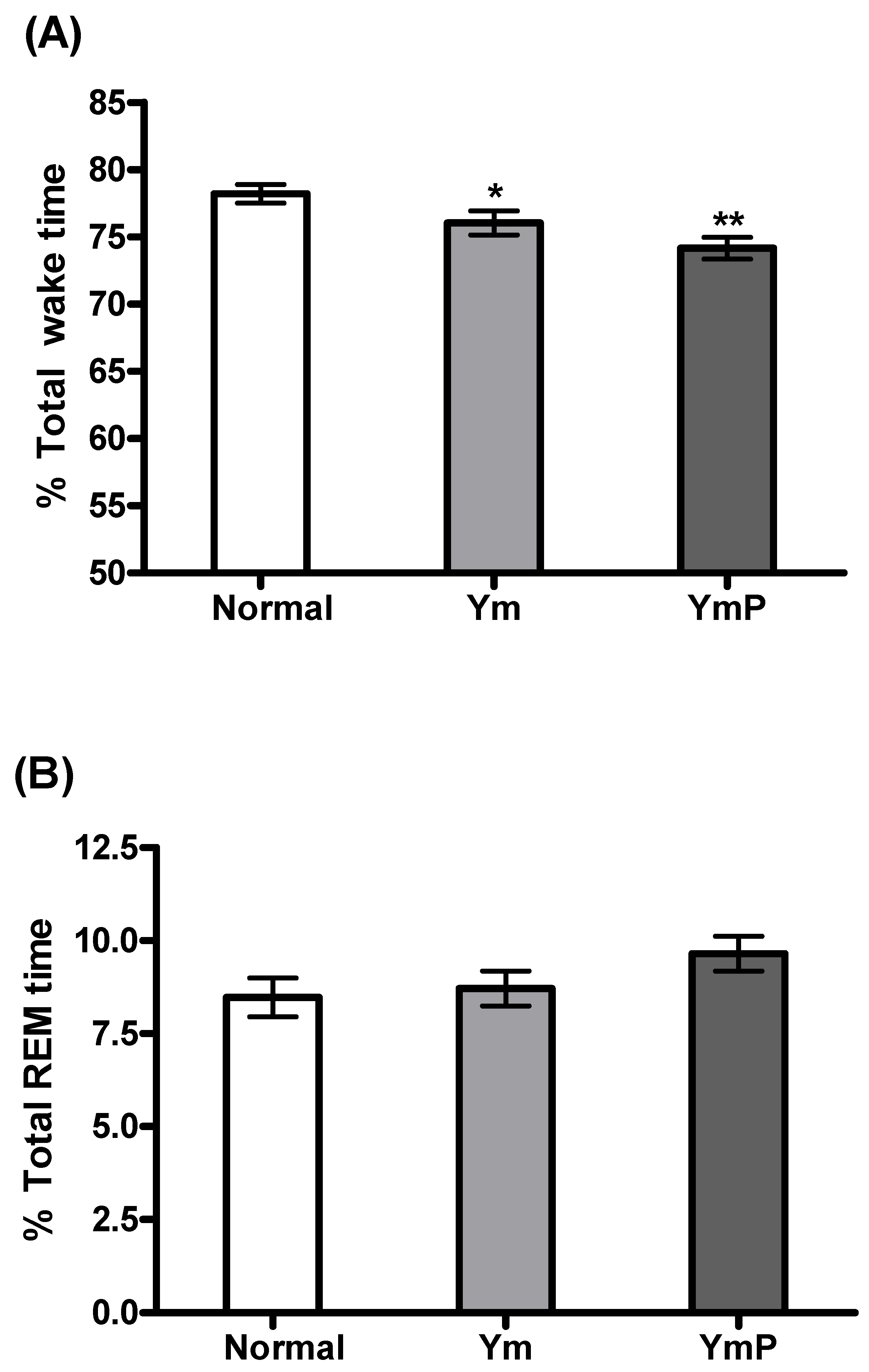
2.2. Effect of YmP on EEG
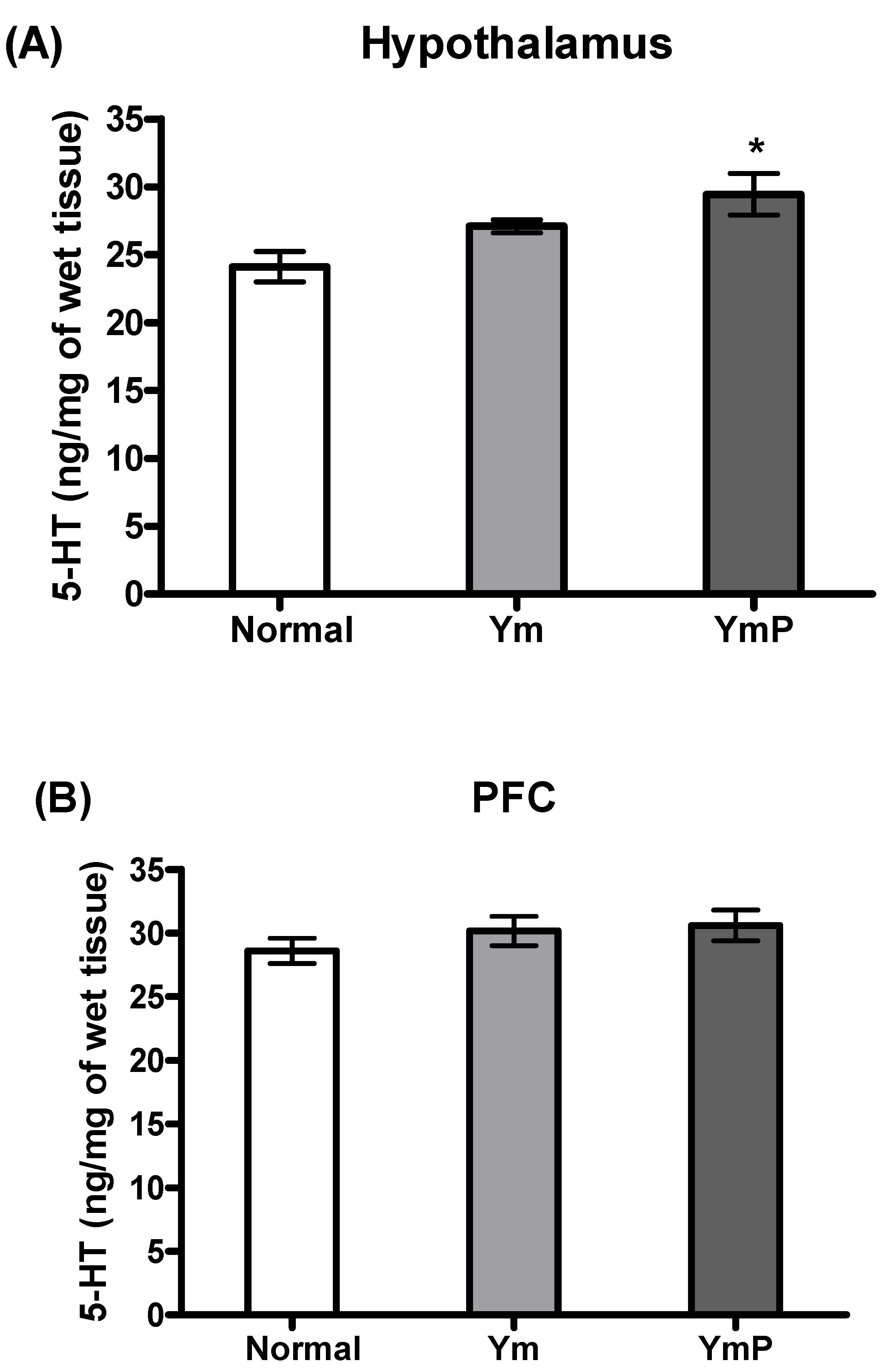
2.3. Effect of YmP on 5-HT Level in the Hypothalamus and PFC
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Ym and YmP
4.3. 5-HT2c Receptor Binding Assay
4.4. Protein Chip Analysis
4.5. Animals
4.6. EEG Surgery
4.7. Methodology of EEG Recording
4.8. EEG Data Analysis
4.9. 5-HT Measurement
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, M.G.; Bergamo Francisco, P.M.; de Azevedo Barros, M.B. Sleep duration pattern and chronic diseases in Brazilian adults (ISACAMP, 2008/09). Sleep Med. 2012, 13, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; Vgontzas, A.N. Insomnia and its impact on physical and mental health. Curr. Psychiatry Rep. 2013, 15, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariente, A.; de Gage, S.B.; Moore, N.; Begaud, B. The Benzodiazepine-Dementia Disorders Link: Current State of Knowledge. CNS Drugs 2016, 30, 1–7. [Google Scholar] [CrossRef]
- Borja, N.L.; Daniel, K.L. Ramelteon for the treatment of insomnia. Clin. Ther. 2006, 28, 1540–1555. [Google Scholar] [CrossRef]
- Ramar, K.; Olson, E.J. Management of common sleep disorders. Am. Fam. Physician 2013, 88, 231–238. [Google Scholar]
- Atkin, T.; Comai, S.; Gobbi, G. Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery. Pharmacol. Rev. 2018, 70, 197–245. [Google Scholar] [CrossRef]
- Min, S.K. Korean traditional medicines as a source of novel drugs for neuropsychiatric disorders. Int. J. Neuropsychoph 2006, 9, S67. [Google Scholar]
- Chung, I.W.; Kim, Y.S.; Ahn, J.S.; Lee, H.S.; Chen, G.; Manji, H.K.; Potter, W.Z.; Pickar, D. Pharmacological Profile of Natural-Products Used to Treat Psychotic Illnesses. Psychopharmacol. Bull. 1995, 31, 139–145. [Google Scholar]
- Attele, A.S.; Xie, J.T.; Yuan, C.S. Treatment of insomnia: An alternative approach. Altern. Med. Rev. A J. Clin. Ther. 2000, 5, 249–259. [Google Scholar]
- Johnston, G.A. GABA(A) receptor channel pharmacology. Curr. Pharm. Des. 2005, 11, 1867–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abourashed, E.A.; Koetter, U.; Brattstrom, A. In vitro binding experiments with a Valerian, hops and their fixed combination extract (Ze91019) to selected central nervous system receptors. Phytomedicine 2004, 11, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Jerman, J.C.; Brough, S.J.; Gager, T.; Wood, M.; Coldwell, M.C.; Smart, D.; Middlemiss, D.N. Pharmacological characterisation of human 5-HT2 receptor subtypes. Eur. J. Pharmacol. 2001, 414, 23–30. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.M.; Shimizu, M.; Lee, C.J.; Han, D.S.; Jung, C.K.; Jo, J.H.; Kim, Y.M. Hypnotic effects and binding studies for GABA(A) and 5-HT(2C) receptors of traditional medicinal plants used in Asia for insomnia. J. Ethnopharmacol. 2010, 132, 225–232. [Google Scholar] [CrossRef]
- Viola, A.U.; Brandenberger, G.; Toussaint, M.; Bouhours, P.; Paul Macher, J.; Luthringer, R. Ritanserin, a serotonin-2 receptor antagonist, improves ultradian sleep rhythmicity in young poor sleepers. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 429–434. [Google Scholar] [CrossRef]
- Demireva, E.Y.; Suri, D.; Morelli, E.; Mahadevia, D.; Chuhma, N.; Teixeira, C.M.; Ziolkowski, A.; Hersh, M.; Fifer, J.; Bagchi, S.; et al. 5-HT2C receptor blockade reverses SSRI-associated basal ganglia dysfunction and potentiates therapeutic efficacy. Mol. Psychiatry 2020, 25, 3304–3321. [Google Scholar] [CrossRef]
- Papp, N.; Koncz, S.; Kostyalik, D.; Kitka, T.; Petschner, P.; Vas, S.; Bagdy, G. Acute 5-HT2C Receptor Antagonist SB-242084 Treatment Affects EEG Gamma Band Activity Similarly to Chronic Escitalopram. Front. Pharmacol. 2019, 10, 1636. [Google Scholar] [CrossRef]
- Palacios, J.M.; Pazos, A.; Hoyer, D. A short history of the 5-HT2C receptor: From the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology 2017, 234, 1395–1418. [Google Scholar] [CrossRef] [Green Version]
- Ferre, S. An update on the mechanisms of the psychostimulant effects of caffeine. J. Neurochem. 2008, 105, 1067–1079. [Google Scholar] [CrossRef]
- Sangare, A.; Dubourget, R.; Geoffroy, H.; Gallopin, T.; Rancillac, A. Serotonin differentially modulates excitatory and inhibitory synaptic inputs to putative sleep-promoting neurons of the ventrolateral preoptic nucleus. Neuropharmacology 2016, 109, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.O.; Lorrain, D.; Dionne, I.J. Exercise and sleep in aging: Emphasis on serotonin. Pathol.-Biol. 2014, 62, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R. Why Sleep Is Important for Health: A Psychoneuroimmunology Perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solarewicz, J.Z.; Angoa-Perez, M.; Kuhn, D.M.; Mateika, J.H. The sleep-wake cycle and motor activity, but not temperature, are disrupted over the light-dark cycle in mice genetically depleted of serotonin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R10–R17. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.H.; Koon, C.M.; Yu, S.L.; Lee, K.Y.; Lau, C.B.; Chan, E.H.; Wing, Y.K.; Fung, K.P.; Leung, P.C. Hypnotic effects of a novel anti-insomnia formula on Drosophila insomnia model. Chin. J. Integr. Med. 2016, 22, 335–343. [Google Scholar] [CrossRef]
- Abdollahnejad, F.; Mosaddegh, M.; Kamalinejad, M.; Mirnajafi-Zadeh, J.; Najafi, F.; Faizi, M. Investigation of sedative and hypnotic effects of Amygdalus communis L. extract: Behavioral assessments and EEG studies on rat. J. Nat. Med. 2016, 70, 190–197. [Google Scholar]
- Monti, J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef]
- Oikonomou, G.; Altermatt, M.; Zhang, R.W.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The Serotonergic Raphe Promote Sleep in Zebrafish and Mice. Neuron 2019, 103, 686–701.e8. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rodriguez, F.; Wilson, C.L.; Maidment, N.T.; Poland, R.E.; Engel, J. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience 2003, 121, 523–530. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, A.; Rubio-Arias, J.A.; Ramos-Campo, D.J.; Reche-Garcia, C.; Leyva-Vela, B.; Nadal-Nicolas, Y. Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia. Int. J. Environ. Res. Public Health 2020, 17, 2227. [Google Scholar] [CrossRef] [Green Version]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markov, D.; Goldman, M. Normal sleep and circadian rhythms: Neurobiologic mechanisms underlying sleep and wakefulness. Psychiatr. Clin. N. Am. 2006, 29, 841–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monti, J.M.; Jantos, H. Differential effects of the 5-HT1A receptor agonist flesinoxan given locally or systemically on REM sleep in the rat. Eur. J. Pharmacol. 2003, 478, 121–130. [Google Scholar] [CrossRef]
- Fenik, V.B.; Kubin, L. Differential localization of carbachol- and bicuculline-sensitive pontine sites for eliciting REM sleep-like effects in anesthetized rats. J. Sleep Res. 2009, 18, 99–112. [Google Scholar] [CrossRef] [PubMed]


| Galenic Name | Amount (g) |
|---|---|
| Rehmannia glutinosa | 12.00 |
| Cornus officinalis | 8.00 |
| Dioscorea batatas | 8.00 |
| Paeonia suffruticosa | 6.00 |
| Poria cocos | 6.00 |
| Alisma orientale | 6.00 |
| Total | 46.00 |
| Galenic Name | Amount (g) |
|---|---|
| Paeonia suffructicosa | 6.00 |
| Poria cocos | 6.00 |
| Alisma orentale | 6.00 |
| Polygonum multifluorum | 12.00 |
| Total | 30.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, H.-S.; Ye, M.; Kim, M.-A.; Kang, H.; Rhie, S.J.; Lee, M.Y.; Jung, I.C.; Kang, I.-C.; Shim, I. Effect of Modified Yukmijihwang-Tang on Sleep Quality in the Rat. Clocks & Sleep 2022, 4, 277-286. https://doi.org/10.3390/clockssleep4020024
Lee S, Lee H-S, Ye M, Kim M-A, Kang H, Rhie SJ, Lee MY, Jung IC, Kang I-C, Shim I. Effect of Modified Yukmijihwang-Tang on Sleep Quality in the Rat. Clocks & Sleep. 2022; 4(2):277-286. https://doi.org/10.3390/clockssleep4020024
Chicago/Turabian StyleLee, SunYoung, Hun-Soo Lee, Minsook Ye, Min-A Kim, Hwajung Kang, Sung Ja Rhie, Mi Young Lee, In Chul Jung, In-Cheol Kang, and Insop Shim. 2022. "Effect of Modified Yukmijihwang-Tang on Sleep Quality in the Rat" Clocks & Sleep 4, no. 2: 277-286. https://doi.org/10.3390/clockssleep4020024
APA StyleLee, S., Lee, H.-S., Ye, M., Kim, M.-A., Kang, H., Rhie, S. J., Lee, M. Y., Jung, I. C., Kang, I.-C., & Shim, I. (2022). Effect of Modified Yukmijihwang-Tang on Sleep Quality in the Rat. Clocks & Sleep, 4(2), 277-286. https://doi.org/10.3390/clockssleep4020024







