Light as a Modulator of Non-Image-Forming Brain Functions—Positive and Negative Impacts of Increasing Light Availability
Abstract
:1. Introduction
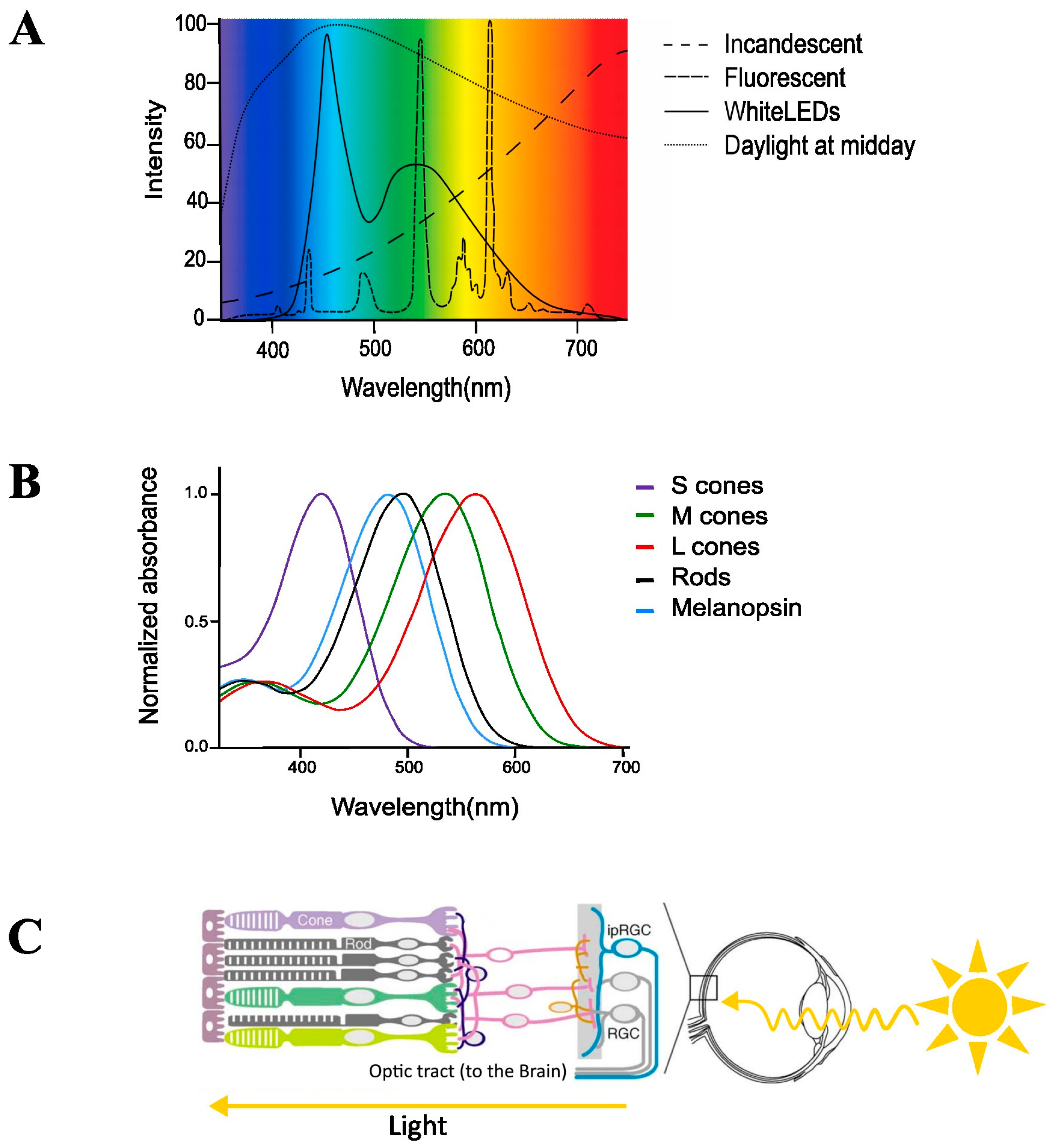
2. Current ‘Modern’ Lighting
3. Classical Light-Sensitive Pathways of the Brain for the Visual Systems
4. Non-Image-Forming System
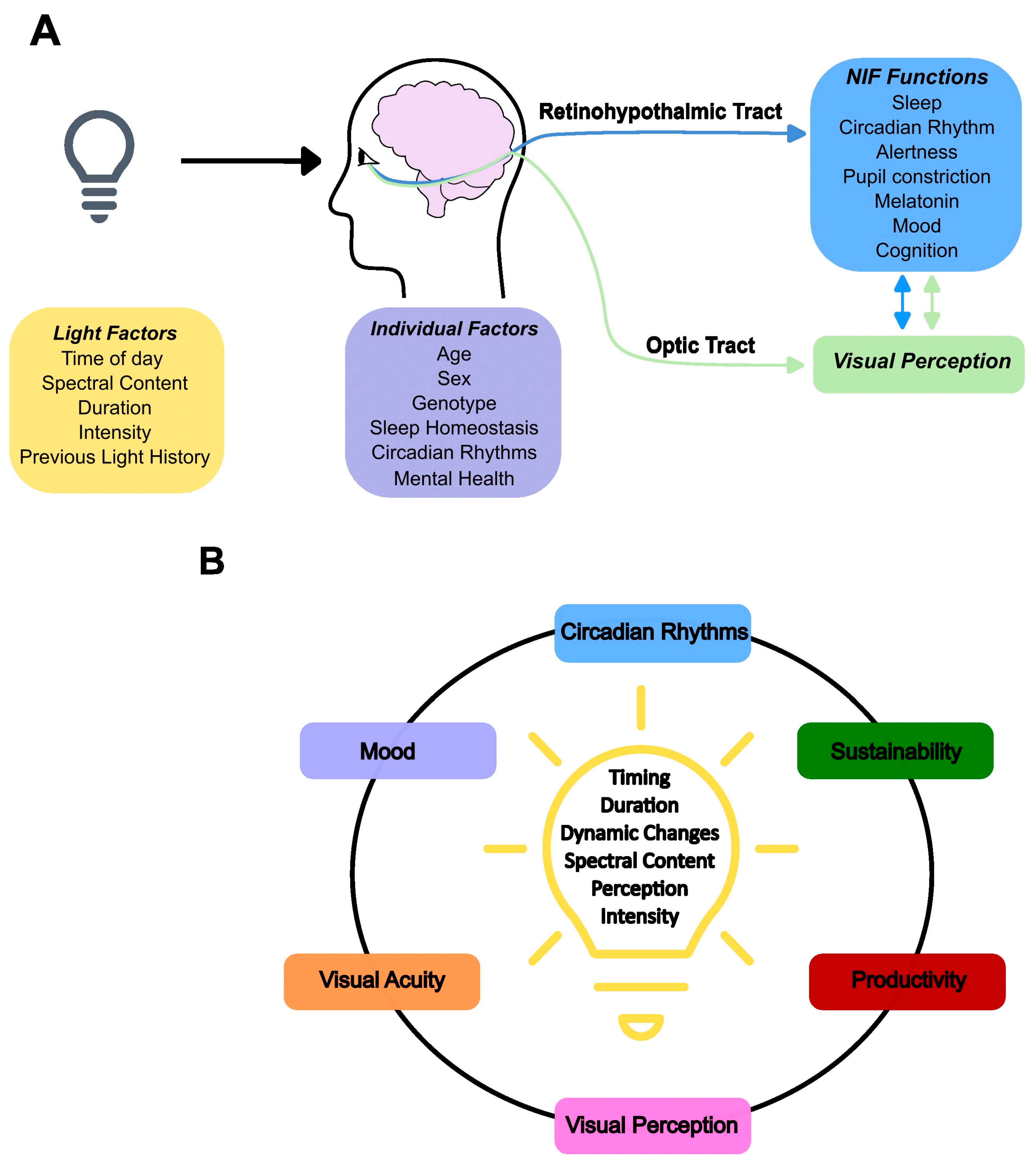
5. Light: Circadian and Acute Impacts
6. NIF Brain Circuits of Light, Impact on Cognition and Inter-Individual Variations
7. Light’s Influence on Human Cognition Is Mediated through Melanopsin Photoreception
8. Emotional Processing and Mood
9. Adverse Impacts on Sleep and the Particular Case of Teenagers
10. Health and Lighting
11. Light Environments
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLH | blue light hazard |
| CCT | correlated colour temperature |
| DOC | disorders of consciousness |
| EEG | electroencephalogram |
| fMRI | functional Magnetic Resonance Imaging |
| ipRGCs | intrinsically photosensitive retinal ganglion cells |
| LED | Light-emitting diode |
| LGN | lateral geniculate nucleus |
| NIF | non-image forming |
| OPN | olivary pretectal nuclei |
| PET | Positron Emission Tomography |
| PFC | prefrontal cortex |
| PLR | pupil light reflex |
| RGC | retinal ganglion cells |
| S-cones | short wavelength-cones |
| SAD | seasonal associative disorder |
| SCN | suprachiasmatic nuclei |
| VLPO | ventro-lateral preoptic nucleus |
| UHF | ultra-high field |
References
- Wässle, H. Parallel Processing in the Mammalian Retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and Using Light in the Melanopsin Age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A Novel Human Opsin in the Inner Retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandewalle, G.; Maquet, P.; Dijk, D.J. Light as a Modulator of Cognitive Brain Function. Trends Cogn. Sci. 2009, 13, 429–438. [Google Scholar] [CrossRef]
- Brainard, G.C.; Hanifin, J.R.; Greeson, J.M.; Byrne, B.; Glickman, G.; Gerner, E.; Rollag, M.D. Action Spectrum for Melatonin Regulation in Humans: Evidence for a Novel Circadian Photoreceptor. J. Neurosci. 2001, 21, 6405–6412. [Google Scholar] [CrossRef] [Green Version]
- Gooley, J.J.; Mien, I.H.; Hilaire, M.A.S.; Yeo, S.-C.; Chua, E.C.-P.; van Reen, E.; Hanley, C.J.; Hull, J.T.; Czeisler, C.A.; Lockley, S.W. Melanopsin and Rod-Cone Photoreceptors Play Different Roles in Mediating Pupillary Light Responses during Exposure to Continuous Light in Humans. J. Neurosci. 2012, 32, 14242–14253. [Google Scholar] [CrossRef] [Green Version]
- Wirz-Justice, A.; Skene, D.J.; Münch, M. The Relevance of Daylight for Humans. Biochem. Pharmacol. 2020, 191, 114304. [Google Scholar] [CrossRef]
- Legates, T.A.; Altimus, C.M.; Wang, H.; Lee, H.K.; Yang, S.; Zhao, H.; Kirkwood, A.; Weber, E.T.; Hattar, S. Aberrant Light Directly Impairs Mood and Learning through Melanopsin-Expressing Neurons. Nature 2012, 491, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Boyce, P.R. Light, Lighting and Human Health. Light. Res. Technol. 2022, 54, 101–144. [Google Scholar] [CrossRef]
- Von Dollen, P.; Pimputkar, S.; Speck, J.S. Let There Be Light-With Gallium Nitride: The 2014 Nobel Prize in Physics. Angew. Chem. Int. Ed. 2014, 53, 13978–13980. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhao, J.; Li, Y.; Zhang, Z.; Zheng, Y. Ubiquitous Light-Emitting Diodes: Potential Threats to Retinal Circadian Rhythms and Refractive Development. Sci. Total Environ. 2023, 862, 160809. [Google Scholar] [CrossRef] [PubMed]
- Pimputkar, S.; Speck, J.S.; Denbaars, S.P.; Nakamura, S. Prospects for LED Lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Hatori, M.; Panda, S. The Emerging Roles of Melanopsin in Behavioral Adaptation to Light. Trends Mol. Med. 2010, 16, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The Biological Impacts of Artificial Light at Night: The Research Challenge. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140133. [Google Scholar] [CrossRef] [Green Version]
- Houser, K.W.; Boyce, P.R.; Zeitzer, J.M.; Herf, M. Human-Centric Lighting: Myth, Magic or Metaphor? Light. Res. Technol. 2020, 53, 97–118. [Google Scholar] [CrossRef]
- Stockman, A.; Sharpe, L.T. Into the Twilight Zone: The Complexities of Mesopic Vision and Luminous Efficiency. Ophthalmic Physiol. Opt. 2006, 26, 225–239. [Google Scholar] [CrossRef]
- DeSimone, K.; Viviano, J.D.; Schneider, K.A. Population Receptive Field Estimation Reveals New Retinotopic Maps in Human Subcortex. J. Neurosci. 2015, 35, 9836–9847. [Google Scholar] [CrossRef] [PubMed]
- Keeler, C.E. Iris movements in blind mice. Am. J. Physiol. -Leg. Content 1927, 81, 107–112. [Google Scholar] [CrossRef]
- Takahashi, J.S.; DeCoursey, P.J.; Bauman, L.; Menaker, M. Spectral Sensitivity of a Novel Photoreceptive System Mediating Entrainment of Mammalian Circadian Rhythms. Nature 1984, 308, 186–188. [Google Scholar] [CrossRef]
- Klein, D.C.; Weller, J.L. Rapid Light-Induced Decrease in Pineal Serotonin N-Acetyltransferase Activity. Science 1972, 177, 532–533. [Google Scholar] [CrossRef]
- Lucas, R.J.; Freedman, M.S.; Muñoz, M.; Garcia-Fernández, J.M.; Foster, R.G. Regulation of the Mammalian Pineal by Non-Rod, Non-Cone, Ocular Photoreceptors. Science 1999, 284, 505–507. [Google Scholar] [CrossRef]
- Freedman, M.S.; Lucas, R.J.; Soni, B.; von Schantz, M.; Muñoz, M.; David-Gray, Z.; Foster, R. Regulation of Mammalian Circadian Behavior by Non-Rod, Non-Cone, Ocular Photoreceptors. Science 1999, 284, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Douglas, R.H.; Foster, R.G. Characterization of an Ocular Photopigment Capable of Driving Pupillary Constriction in Mice. Nat. Neurosci. 2001, 4, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Cooper, H.M.; Foster, R.G. Retinal Projections in Mice with Inherited Retinal Degeneration: Implications for Circadian Photoentrainment. J. Comp. Neurol. 1998, 395, 417–439. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Shanahan, T.L.; Klerman, E.B.; Martens, H.; Brotman, D.J.; Emens, J.S.; Klein, T.; Rizzo, J.F. Suppression of Melatonin Secretion in Some Blind Patients by Exposure to Bright Light. N. Engl. J. Med. 1995, 332, 6–11. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Skene, D.J.; Hanifin, J.P.; Rollag, M.D.; English, J.; Arendt, J.; Brainard, G.C. Melatonin Regulation in Humans with Color Vision Deficiencies. J. Clin. Endocrinol. Metab. 1996, 81, 2980–2985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapan, K.; Arendt, J.; Skene, D.J. An Action Spectrum for Melatonin Suppression: Evidence for a Novel Non-Rod, Non-Cone Photoreceptor System in Humans. J. Physiol. 2001, 535, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Jiang, G.; de Grip, W.J.; Pär Hayes, W.; Rollag, M.D. Melanopsin: An Opsin in Melanophores, Brain, and Eye. Proc. Natl. Acad. Sci. USA 1998, 95, 340–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.; Sato, T.K.; Castrucci, A.M.; Rollag, M.D.; DeGrip, W.J.; Hogenesch, J.B.; Provencio, I.; Kay, S.A. Melanopsin (Opn4) Requirement for Normal Light-Induced Circadian Phase Shifting. Science 2002, 298, 2213–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mure, L.S.; Cornut, P.-L.; Rieux, C.; Drouyer, E.; Denis, P.; Gronfier, C.; Cooper, H.M. Melanopsin Bistability: A Fly’s Eye Technology in the Human Retina. PLoS ONE 2009, 4, e5991. [Google Scholar] [CrossRef] [PubMed]
- Mure, L.S.; Rieux, C.; Hattar, S.; Cooper, H.M. Melanopsin-Dependent Nonvisual Responses: Evidence for Photopigment Bistability in Vivo. J. Biol. Rhythm. 2007, 22, 411–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuyama, T.; Yamashita, T.; Imamoto, Y.; Shichida, Y. Photochemical Properties of Mammalian Melanopsin. Biochemistry 2012, 51, 5454–5462. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, C.; Skene, D.J.; Revell, V.L. Human Nonvisual Responses to Simultaneous Presentation of Blue and Red Monochromatic Light. J. Biol. Rhythm. 2012, 27, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.M.; Gias, C.; Hatori, M.; Keding, S.R.; Semo, M.; Coffey, P.J.; Gigg, J.; Piggins, H.D.; Panda, S.; Lucas, R.J. Melanopsin Contributions to Irradiance Coding in the Thalamo-Cortical Visual System. PLoS Biol. 2010, 8, e1000558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecker, J.L.; Dumitrescu, O.N.; Wong, K.Y.; Alam, N.M.; Chen, S.K.; LeGates, T.; Renna, J.M.; Prusky, G.T.; Berson, D.M.; Hattar, S. Melanopsin-Expressing Retinal Ganglion-Cell Photoreceptors: Cellular Diversity and Role in Pattern Vision. Neuron 2010, 67, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.E.; Martial, F.P.; Lucas, R.J. Form Vision from Melanopsin in Humans. Nat. Commun. 2019, 10, 2274. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.M.; Tsujimura, S.I.; Allen, A.E.; Wynne, J.; Bedford, R.; Vickery, G.; Vugler, A.; Lucas, R.J. Melanopsin-Based Brightness Discrimination in Mice and Humans. Curr. Biol. 2012, 22, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Storchi, R.; Bedford, R.A.; Martial, F.P.; Allen, A.E.; Wynne, J.; Montemurro, M.A.; Petersen, R.S.; Lucas, R.J. Modulation of Fast Narrowband Oscillations in the Mouse Retina and DLGN According to Background Light Intensity. Neuron 2017, 93, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Milosavljevic, N.; Storchi, R.; Eleftheriou, C.G.; Colins, A.; Petersen, R.S.; Lucas, R.J. Photoreceptive Retinal Ganglion Cells Control the Information Rate of the Optic Nerve. Proc. Natl. Acad. Sci. USA 2018, 115, E11817–E11826. [Google Scholar] [CrossRef] [Green Version]
- Storchi, R.; Milosavljevic, N.; Eleftheriou, C.G.; Martial, F.P.; Orlowska-Feuer, P.; Bedford, R.A.; Brown, T.M.; Montemurro, M.A.; Petersen, R.S.; Lucas, R.J. Melanopsin-Driven Increases in Maintained Activity Enhance Thalamic Visual Response Reliability across a Simulated Dawn. Proc. Natl. Acad. Sci. USA 2015, 112, E5734–E5743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güler, A.D.; Ecker, J.L.; Lall, G.S.; Haq, S.; Altimus, C.M.; Liao, H.W.; Barnard, A.R.; Cahill, H.; Badea, T.C.; Zhao, H.; et al. Melanopsin Cells Are the Principal Conduits for Rod-Cone Input to Non-Image-Forming Vision. Nature 2008, 453, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kankipati, L.; Girkin, C.A.; Gamlin, P.D. Post-Illumination Pupil Response in Subjects without Ocular Disease. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2764–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tri, M.; Do, H. Melanopsin and the Intrinsically Photosensitive Retinal Ganglion Cells: Biophysics to Behavior. Neuron 2019, 104, 205–226. [Google Scholar] [CrossRef]
- Baver, S.B.; Pickard, G.E.; Sollars, P.J.; Pickard, G.E. Two Types of Melanopsin Retinal Ganglion Cell Differentially Innervate the Hypothalamic Suprachiasmatic Nucleus and the Olivary Pretectal Nucleus. Eur. J. Neurosci. 2008, 27, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Stafford, B.K.; Godin, A.L.; King, W.M.; Wong, K.Y. Photoresponse Diversity among the Five Types of Intrinsically Photosensitive Retinal Ganglion Cells. J. Physiol. 2014, 592, 1619–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delwig, A.; Larsen, D.D.; Yasumura, D.; Yang, C.F.; Shah, N.M.; Copenhagen, D.R. Retinofugal Projections from Melanopsin-Expressing Retinal Ganglion Cells Revealed by Intraocular Injections of Cre-Dependent Virus. PLoS ONE 2016, 11, e0149501. [Google Scholar] [CrossRef]
- Hattar, S.; Kumar, M.; Park, A.; Tong, P.; Tung, J.; Yau, K.W.; Berson, D.M. Central Projections of Melanopsin-Expressing Retinal Ganglion Cells in the Mouse. J. Comp. Neurol. 2006, 497, 326–349. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Xi, Y.; Peng, Y.; Yang, Y.; Huang, X.; Fu, Y.; Tao, Q.; Xiao, J.; Yuan, T.; An, K.; et al. A Visual Circuit Related to Habenula Underlies the Antidepressive Effects of Light Therapy. Neuron 2019, 102, 128–142.e8. [Google Scholar] [CrossRef] [Green Version]
- Sonoda, T.; Schmidt, T.M. Re-Evaluating the Role of Intrinsically Photosensitive Retinal Ganglion Cells: New Roles in Image-Forming Functions. Integr. Comp. Biol. 2016, 56, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Sonoda, T.; Li, J.Y.; Hayes, N.W.; Chan, J.C.; Okabe, Y.; Belin, S.; Nawabi, H.; Schmidt, T.M. A Non-Canonical Inhibitory Circuit Dampens Behavioral Sensitivity to Light. Science 2020, 368, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Mure, L.S. Intrinsically Photosensitive Retinal Ganglion Cells of the Human Retina. Front. Neurol. 2021, 12, 636330. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.M.; Huberman, A.D. Neuroscience: A Chromatic Retinal Circuit Encodes Sunrise and Sunset for the Brain. Curr. Biol. 2020, 30, R316–R318. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, L.; Hanna, L.; Mouland, J.; Martial, F.; West, A.; Smedley, A.R.; Bechtold, D.A.; Webb, A.R.; Lucas, R.J.; Brown, T.M. Colour As a Signal for Entraining the Mammalian Circadian Clock. PLoS Biol. 2015, 13, e1002127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouland, J.W.; Martial, F.; Watson, A.; Lucas, R.J.; Brown, T.M. Cones Support Alignment to an Inconsistent World by Suppressing Mouse Circadian Responses to the Blue Colors Associated with Twilight. Curr. Biol. 2019, 29, 4260–4267.e4. [Google Scholar] [CrossRef] [Green Version]
- Mouland, J.W.; Martial, F.P.; Lucas, R.J.; Brown, T.M. Modulations in Irradiance Directed at Melanopsin, but Not Cone Photoreceptors, Reliably Alter Electrophysiological Activity in the Suprachiasmatic Nucleus and Circadian Behaviour in Mice. J. Pineal Res. 2021, 70, e12735. [Google Scholar] [CrossRef]
- Van Oosterhout, F.; Fisher, S.P.; Van Diepen, H.C.; Watson, T.S.; Houben, T.; Vanderleest, H.T.; Thompson, S.; Peirson, S.N.; Foster, R.G.; Meijer, J.H. Ultraviolet Light Provides a Major Input to Non-Image-Forming Light Detection in Mice. Curr. Biol. 2012, 22, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.E.; Brown, T.M.; Lucas, R.J. A Distinct Contribution of Short-Wavelength-Sensitive Cones to Light-Evoked Activity in the Mouse Pretectal Olivary Nucleus. J. Neurosci. 2011, 31, 16833–16843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitschan, M.; Lazar, R.; Yetik, E.; Cajochen, C. No Evidence for an S Cone Contribution to Acute Neuroendocrine and Alerting Responses to Light. Curr. Biol. 2019, 29, R1297–R1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.M.; Thapan, K.; Arendt, J.; Revell, V.L.; Skene, D.J. S-cone Contribution to the Acute Melatonin Suppression Response in Humans. J. Pineal Res. 2021, 71, e12719. [Google Scholar] [CrossRef]
- Gaggioni, G.; Maquet, P.; Schmidt, C.; Dijk, D.; Vandewalle, G. Neuroimaging, Cognition, Light and Circadian Rhythms. Front. Syst. Neurosci. 2014, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, J.F.; Kronauer, R.E.; Czeisler, C.A. Phase-Shifting Human Circadian Rhythms: Influence of Sleep Timing, Social Contact and Light Exposure. J. Physiol. 1996, 495, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lockley, S.W.; Brainard, G.C.; Czeisler, C.A. High Sensitivity of the Human Circadian Melatonin Rhythm to Resetting by Short Wavelength Light. J. Clin. Endocrinol. Metab. 2003, 88, 4502–4505. [Google Scholar] [CrossRef] [PubMed]
- Revell, V.L.; Molina, T.A.; Eastman, C.I. Human Phase Response Curve to Intermittent Blue Light Using a Commercially Available Device. J. Physiol. 2012, 590, 4859–4868. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Eastman, C.I. Phase Delaying the Human Circadian Clock with Blue-Enriched Polychromatic Light. Chronobiol. Int. 2009, 26, 709–725. [Google Scholar] [CrossRef]
- Smith, M.R.; Revell, V.L.; Eastman, C.I. Phase Advancing the Human Circadian Clock with Blue-Enriched Polychromatic Light. Sleep Med. 2009, 10, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.A.; Flynn-Evans, E.E.; Aeschbach, D.; Brainard, G.C.; Czeisler, C.A.; Lockley, S.W. Diurnal Spectral Sensitivity of the Acute Alerting Effects of Light. Sleep 2014, 37, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High Sensitivity of Human Melatonin, Alertness, Thermoregulation, and Heart Rate to Short Wavelength Light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef] [Green Version]
- Lockley, S.W.; Evans, E.E.; Scheer, F.A.J.L.; Brainard, G.C.; Czeisler, C.A.; Aeschbach, D. Short-Wavelength Sensitivity for the Direct Effects of Light on Alertness, Vigilance, and the Waking Electroencephalogram in Humans. Sleep 2006, 29, 161–168. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Lang, D.; Götz, T.; Krebs, J.; Cajochen, C. Acute Exposure to Evening Blue-Enriched Light Impacts on Human Sleep. J. Sleep Res. 2013, 22, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Santhi, N.; Thorne, H.C.; Van Der Veen, D.R.; Johnsen, S.; Mills, S.L.; Hommes, V.; Schlangen, L.J.M.; Archer, S.N.; Dijk, D.J. The Spectral Composition of Evening Light and Individual Differences in the Suppression of Melatonin and Delay of Sleep in Humans. J. Pineal Res. 2012, 53, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Niedernhuber, M.; Spitschan, M.; Slawik, H.C.; Meyer, M.P.; Bekinschtein, T.A.; Cajochen, C. Melatonin Suppression Does Not Automatically Alter Sleepiness, Vigilance, Sensory Processing, or Sleep. Sleep 2022, 45, zsac199. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Zeitzer, J.M.; Czeisler, C.A.; Dijk, D.J. Dose-Response Relationship for Light Intensity and Ocular and Electroencephalographic Correlates of Human Alertness. Behav. Brain Res. 2000, 115, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; Hazelhoff, E.M.; Martial, F.P.; Cajochen, C.; Lucas, R.J. Exploiting Metamerism to Regulate the Impact of a Visual Display on Alertness and Melatonin Suppression Independent of Visual Appearance. Sleep 2018, 41, zsy100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, A.Y.; Sletten, T.L.; Flynn-Evans, E.E.; Lockley, S.W.; Rajaratnam, S.M.W. Daytime Exposure to Short- and Medium-Wavelength Light Did Not Improve Alertness and Neurobehavioral Performance. J. Biol. Rhythm. 2016, 31, 470–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, M.; Carrier, J. Daytime Sleep Propensity after Moderate Circadian Phase Shifts Induced with Bright Light Exposure. Sleep 1997, 20, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolders, K.C.H.J.; Peeters, S.T.; Vogels, I.M.L.C.; de Kort, Y.A.W. Investigation of Dose-Response Relationships for Effects of White Light Exposure on Correlates of Alertness and Executive Control during Regular Daytime Working Hours. J. Biol. Rhythm. 2018, 33, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Lok, R.; Smolders, K.C.H.J.; Beersma, D.G.M.; de Kort, Y.A.W. Light, Alertness, and Alerting Effects of White Light: A Literature Overview. J. Biol. Rhythm. 2018, 33, 589–601. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.M.; Huang, X.D.; Zhu, S.; Hu, Z.F.; So, K.F.; Ren, C.R.; Tao, Q. Alerting Effects of Light in Healthy Individuals: A Systematic Review and Meta-Analysis. Neural Regen. Res. 2022, 17, 1929–1936. [Google Scholar] [CrossRef]
- Siraji, M.A.; Kalavally, V.; Schaefer, A.; Haque, S. Effects of Daytime Electric Light Exposure on Human Alertness and Higher Cognitive Functions: A Systematic Review. Front. Psychol. 2022, 12, 6079. [Google Scholar] [CrossRef]
- Pilorz, V.; Tam, S.K.E.; Hughes, S.; Pothecary, C.A.; Jagannath, A.; Hankins, M.W.; Bannerman, D.M.; Lightman, S.L.; Vyazovskiy, V.V.; Nolan, P.M.; et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016, 14, e1002482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, J.W.; Hannibal, J.; Hagiwara, G.; Colas, D.; Ruppert, E.; Ruby, N.F.; Heller, H.C.; Franken, P.; Bourgin, P. Melanopsin as a Sleep Modulator: Circadian Gating of the Direct Effects of Light on Sleep and Altered Sleep Homeostasis in Opn4−/− Mice. PLoS Biol. 2009, 7, e1000125. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meijden, W.P.; Te Lindert, B.H.W.; Ramauta, J.R.; Wei, Y.; Coppens, J.E.; Kamermans, M.; Cajochen, C.; Bourgin, P.; Van Someren, E.J.W. Sustained Effects of Prior Red Light on Pupil Diameter and Vigilance during Subsequent Darkness. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daurat, A.; Aguirre, A.; Foret, J.; Gonnet, P.; Keromes, A.; Benoit, O. Bright Light Affects Alertness and Performance Rhythms during a 24-h Constant Routine. Physiol. Behav. 1993, 53, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Phipps-Nelson, J.; Redman, J.R.; Dijk, D.J.; Rajaratnam, S.M.W. Daytime Exposure to Bright Light, as Compared to Dim Light, Decreases Sleepiness and Improves Psychomotor Vigilance Performance. Sleep 2003, 26, 695–700. [Google Scholar] [CrossRef]
- Rüger, M.; Gordijn, M.C.M.; Beersma, D.G.M.; De Vries, B.; Daan, S. Time-of-Day-Dependent Effects of Bright Light Exposure on Human Psychophysiology: Comparison of Daytime and Nighttime Exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1413–R1420. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Nakagawa, S. Effects of Daytime Light Exposure on Cognitive Brain Activity as Measured by the ERP P300. Physiol. Behav. 2015, 138, 313–318. [Google Scholar] [CrossRef]
- Tam, S.K.E.; Hasan, S.; Hughes, S.; Hankins, M.W.; Foster, R.G.; Bannerman, D.M.; Peirson, S.N. Modulation of Recognition Memory Performance by Light Requires Both Melanopsin and Classical Photoreceptors. Proc. R. Soc. B Biol. Sci. 2016, 283, 20162275. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, D.C.; Fogerson, P.M.; Lazzerini Ospri, L.; Thomsen, M.B.; Layne, R.M.; Severin, D.; Zhan, J.; Singer, J.H.; Kirkwood, A.; Zhao, H.; et al. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell 2018, 175, 71–84.e18. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Huang, P.; Huang, L.; Hu, Z.; Liu, X.; Shen, J.; Xi, Y.; Yang, Y.; Fu, Y.; Tao, Q.; et al. A Visual Circuit Related to the Nucleus Reuniens for the Spatial-Memory-Promoting Effects of Light Treatment. Neuron 2021, 109, 347–362.e7. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Alkozei, A.; Vanuk, J.R.; Reign, D.; Grandner, M.A.; Dailey, N.S. Blue Light Exposure Increases Functional Connectivity between Dorsolateral Prefrontal Cortex and Multiple Cortical Regions. Neuroreport 2022, 33, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.K.; Kent, B.A.; Mayer, M.D.; Stickgold, R.; Lockley, S.W.; Rahman, S.A. Daytime Exposure to Short Wavelength-Enriched Light Improves Cognitive Performance in Sleep-Restricted College-Aged Adults. Front. Neurol. 2021, 12, 624217. [Google Scholar] [CrossRef] [PubMed]
- Lok, R.; Joyce, D.S.; Zeitzer, J.M. Impact of Daytime Spectral Tuning on Cognitive Function. J. Photochem. Photobiol. B 2022, 230, 112439. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Tam, S.K.E.; Foster, R.G.; Vyazovskiy, V.V.; Bannerman, D.M.; Peirson, S.N. Modulation of Recognition Memory Performance by Light and Its Relationship with Cortical EEG Theta and Gamma Activities. Biochem. Pharmacol. 2021, 191, 114404. [Google Scholar] [CrossRef]
- Zhang, Z.; Beier, C.; Weil, T.; Hattar, S. The Retinal IpRGC-Preoptic Circuit Mediates the Acute Effect of Light on Sleep. Nat. Commun. 2021, 12, 5115. [Google Scholar] [CrossRef]
- Lyon, D.C.; Nassi, J.J.; Callaway, E.M. A Disynaptic Relay from Superior Colliculus to Dorsal Stream Visual Cortex in Macaque Monkey. Neuron 2010, 65, 270–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saalmann, Y.B.; Pinsk, M.A.; Wang, L.; Li, X.; Kastner, S. The Pulvinar Regulates Information Transmission between Cortical Areas Based on Attention Demands. Science 2012, 337, 753–756. [Google Scholar] [CrossRef] [Green Version]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, F.; Peigneux, P.; Fuchs, S.; Verhaeghe, S.; Laureys, S.; Middleton, B.; Degueldre, C.; del Fiore, G.; Vandewalle, G.; Balteau, E.; et al. Nonvisual Responses to Light Exposure in the Human Brain during the Circadian Night. Curr. Biol. 2004, 14, 1842–1846. [Google Scholar] [CrossRef]
- Vandewalle, G.; Balteau, E.; Phillips, C.; Degueldre, C.; Moreau, V.; Sterpenich, V.; Albouy, G.; Darsaud, A.; Desseilles, M.; Dang-Vu, T.T.; et al. Daytime Light Exposure Dynamically Enhances Brain Responses. Curr. Biol. 2006, 16, 1616–1621. [Google Scholar] [CrossRef]
- Vandewalle, G.; Schmidt, C.; Albouy, G.; Sterpenich, V.; Darsaud, A.; Rauchs, G.; Berken, P.Y.; Balteau, E.; Dagueldre, C.; Luxen, A.; et al. Brain Responses to Violet, Blue, and Green Monochromatic Light Exposures in Humans: Prominent Role of Blue Light and the Brainstem. PLoS ONE 2007, 2, e1247. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, G.; Gais, S.; Schabus, M.; Balteau, E.; Carrier, J.; Darsaud, A.; Sterpenich, V.; Albouy, G.; Dijk, D.J.; Maquet, P. Wavelength-Dependent Modulation of Brain Responses to a Working Memory Task by Daytime Light Exposure. Cereb. Cortex 2007, 17, 2788–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandewalle, G.; Archer, S.N.; Wuillaume, C.; Balteau, E.; Degueldre, C.; Luxen, A.; Dijk, D.J.; Maquet, P. Effects of Light on Cognitive Brain Responses Depend on Circadian Phase and Sleep Homeostasis. J. Biol. Rhythm. 2011, 26, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandewalle, G.; Schwartz, S.; Grandjean, D.; Wuillaume, C.; Balteau, E.; Degueldre, C.; Schabus, M.; Phillips, C.; Luxen, A.; Dijk, D.J.; et al. Spectral Quality of Light Modulates Emotional Brain Responses in Humans. Proc. Natl. Acad. Sci. USA 2010, 107, 19549–19554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandewalle, G.; van Ackeren, M.J.; Daneault, V.; Hull, J.T.; Albouy, G.; Lepore, F.; Doyon, J.; Czeisler, C.A.; Dumont, M.; Carrier, J.; et al. Light Modulates Oscillatory Alpha Activity in the Occipital Cortex of Totally Visually Blind Individuals with Intact Non-Image-Forming Photoreception. Sci. Rep. 2018, 8, 16968. [Google Scholar] [CrossRef] [PubMed]
- Daneault, V.; Hébert, M.; Albouy, G.; Doyon, J.; Dumont, M.; Carrier, J.; Vandewalle, G. Aging Reduces the Stimulating Effect of Blue Light on Cognitive Brain Functions. Sleep 2014, 37, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Alkozei, A.; Smith, R.; Pisner, D.A.; Vanuk, J.R.; Berryhill, S.M.; Fridman, A.; Shane, B.R.; Knight, S.A.; Killgore, W.D.S. Exposure to Blue Light Increases Subsequent Functional Activation of the Prefrontal Cortex during Performance of a Working Memory Task. Sleep 2016, 39, 1671–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkozei, A.; Smith, R.; Killgore, W.D.S. Exposure to Blue Wavelength Light Modulates Anterior Cingulate Cortex Activation in Response to “uncertain” versus “Certain” Anticipation of Positive Stimuli. Neurosci. Lett. 2016, 616, 5–10. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Dailey, N.S.; Raikes, A.C.; Vanuk, J.R.; Taylor, E.; Alkozei, A. Blue Light Exposure Enhances Neural Efficiency of the Task Positive Network during a Cognitive Interference Task. Neurosci. Lett. 2020, 735, 135242. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, E.M.; Poudel, G.R.; Jamadar, S.D.; Phillips, A.J.K.; Cain, S.W. Afraid of the Dark: Light Acutely Suppresses Activity in the Human Amygdala. PLoS ONE 2021, 16, e0252350. [Google Scholar] [CrossRef]
- Cajochen, C.; Frey, S.; Anders, D.; Späti, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening Exposure to a Light-Emitting Diodes (LED)-Backlit Computer Screen Affects Circadian Physiology and Cognitive Performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef] [Green Version]
- Vandewalle, G.; Archer, S.N.; Wuillaume, C.; Balteau, E.; Degueldre, C.; Luxen, A.; Maquet, P.; Dijk, D.J. Functional Magnetic Resonance Imaging-Assessed Brain Responses during an Executive Task Depend on Interaction of Sleep Homeostasis, Circadian Phase, and PER3 Genotype. J. Neurosci. 2009, 29, 7948–7956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chellappa, S.L.; Bromundt, V.; Frey, S.; Cajochen, C. Age-Related Neuroendocrine and Alerting Responses to Light. Geroscience 2021, 43, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Bromundt, V.; Frey, S.; Steinemann, A.; Schmidt, C.; Schlote, T.; Goldblum, D.; Cajochen, C. Association of Intraocular Cataract Lens Replacement With Circadian Rhythms, Cognitive Function, and Sleep in Older Adults. JAMA Ophthalmol. 2019, 137, 878–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobczak, A.M.; Bohaterewicz, B.; Fafrowicz, M.; Domagalik, A.; Beldzik, E.; Oginska, H.; Golonka, N.; Rekas, M.; Bronicki, D.; Romanowska-Dixon, B.; et al. The Influence of Intraocular Lens Implantation and Alterations in Blue Light Transmittance Level on the Brain Functional Network Architecture Reorganization in Cataract Patients. Brain Sci. 2021, 11, 1400. [Google Scholar] [CrossRef] [PubMed]
- Daneault, V.; Dumont, M.; Massé, É.; Forcier, P.; Boré, A.; Lina, J.M.; Doyon, J.; Vandewalle, G.; Carrier, J. Plasticity in the Sensitivity to Light in Aging: Decreased Non-Visual Impact of Light on Cognitive Brain Activity in Older Individuals but No Impact of Lens Replacement. Front. Physiol. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Chellappa, S.L. Individual Differences in Light Sensitivity Affect Sleep and Circadian Rhythms. Sleep 2021, 44, zsaa214. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M.; Santhi, N. Individual Differences and Diversity in Human Physiological Responses to Light. EBioMedicine 2022, 75, 103640. [Google Scholar] [CrossRef]
- Edwards, L.J.; Kirilina, E.; Mohammadi, S.; Weiskopf, N. Microstructural Imaging of Human Neocortex in Vivo. Neuroimage 2018, 182, 184–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in Vivo Neurite Orientation Dispersion and Density Imaging of the Human Brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Schoonderwoerd, R.A.; de Rover, M.; Janse, J.A.; Hirschler, L.; Willemse, C.R.; Scholten, L.; Klop, I.; van Berloo, S.; van Osch, M.J.; Swaab, D.F.; et al. The Photobiology of the Human Circadian Clock. Proc. Natl. Acad. Sci. USA 2022, 119, e2118803119. [Google Scholar] [CrossRef]
- Sharifpour, R.; Campbell, I.; Beckers, E.; Balda, F.; Mortazavi, N.; Koshmanova, E.; Paparella, I.; Sherif, S.; Phillips, C.; Vandewalle, G. Pitfalls in Recording Bold Signal Responses to Light in Small Hypothalamic Nuclei Using Ultra-High-Field 7 Tesla MRI. SSRN Electron. J. 2022, 119, e2212123119. [Google Scholar] [CrossRef]
- Milosavljevic, N.; Cehajic-Kapetanovic, J.; Procyk, C.A.; Lucas, R.J. Chemogenetic Activation of Melanopsin Retinal Ganglion Cells Induces Signatures of Arousal and/or Anxiety in Mice. Curr. Biol. 2016, 26, 2358–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandewalle, G.; Collignon, O.; Hull, J.T.; Daneault, V.; Albouy, G.; Lepore, F.; Phillips, C.; Doyon, J.; Czeisler, C.A.; Dumont, M.; et al. Blue Light Stimulates Cognitive Brain Activity in Visually Blind Individuals HHS Public Access. J. Cogn. Neurosci. 2013, 25, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, F.H.; Hull, J.T.; Peirson, S.N.N.; Wulff, K.; Aeschbach, D.; Gooley, J.J.; Brainard, G.C.C.; Gregory-Evans, K.; Rizzo, J.F.F.; Czeisler, C.A.; et al. Short-Wavelength Light Sensitivity of Circadian, Pupillary, and Visual Awareness in Humans Lacking an Outer Retina. Curr. Biol. 2007, 17, 2122–2128. [Google Scholar] [CrossRef] [Green Version]
- Evangelisti, S.; La Morgia, C.; Testa, C.; Manners, D.N.; Brizi, L.; Bianchini, C.; Carbonelli, M.; Barboni, P.; Sadun, A.A.; Tonon, C.; et al. Brain Functional MRI Responses to Blue Light Stimulation in Leber’s Hereditary Optic Neuropathy. Biochem. Pharmacol. 2021, 19, 114488. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Ly, J.Q.M.; Meyer, C.; Balteau, E.; Degueldre, C.; Luxen, A.; Phillips, C.; Cooper, H.M.; Vandewalle, G. Photic Memory for Executive Brain Responses. Proc. Natl. Acad. Sci. USA 2014, 111, 6087–6091. [Google Scholar] [CrossRef] [Green Version]
- Viénot, F.; Brettel, H.; Dang, T.-V.; le Rohellec, J. Domain of Metamers Exciting Intrinsically Photosensitive Retinal Ganglion Cells (IpRGCs) and Rods. J. Opt. Soc. Am. A 2012, 29, A366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tow, S.L.C.; Dubail, M.; Tow, S.L.C.; Aung, T.; Tow, S.L.C.; Aung, T.; Gooley, J.J.; Hsieh, P.J. Cerebral Neural Correlates of Differential Melanopic Photic Stimulation in Humans. Neuroimage 2017, 146, 763–769. [Google Scholar] [CrossRef]
- Spitschan, M.; Bock, A.S.; Ryan, J.; Frazzetta, G.; Brainard, D.H.; Aguirre, G.K. The Human Visual Cortex Response to Melanopsin-Directed Stimulation Is Accompanied by a Distinct Perceptual Experience. Proc. Natl. Acad. Sci. USA 2017, 114, 12291–12296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitschan, M.; Datta, R.; Stern, A.M.; Brainard, D.H.; Aguirre, G.K. Human Visual Cortex Responses to Rapid Cone and Melanopsin-Directed Flicker. J. Neurosci. 2016, 36, 1471–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.H.; Tu, Y.C.; Yeh, S.L. In Search of Blue-Light Effects on Cognitive Control. Sci. Rep. 2021, 11, 15505. [Google Scholar] [CrossRef] [PubMed]
- Stefani, O.; Freyburger, M.; Veitz, S.; Basishvili, T.; Meyer, M.; Weibel, J.; Kobayashi, K.; Shirakawa, Y.; Cajochen, C. Changing Color and Intensity of LED Lighting across the Day Impacts on Circadian Melatonin Rhythms and Sleep in Healthy Men. J. Pineal Res. 2021, 70, e12714. [Google Scholar] [CrossRef] [PubMed]
- Geerdinck, L.M.; Bikker, J.W.; Meekes, G.J.B.M.; de Ruyter, B.; Leffers, P.; Versteylen, M.; Kuijpers, P.M.J.C.; Giménez, M.C.; Schlangen, L.J.M.; Herremans, H. Patient Room Lighting Influences on Sleep, Appraisal and Mood in Hospitalized People. J. Sleep Res. 2016, 26, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, M.; Maierová, L.; Cajochen, C.; Scartezzini, J.L.; Münch, M. Optimized Office Lighting Advances Melatonin Phase and Peripheral Heat Loss Prior Bedtime. Sci. Rep. 2022, 12, 4267. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; St. Hilaire, M.A.; Grant, L.K.; Barger, L.K.; Brainard, G.C.; Czeisler, C.A.; Klerman, E.B.; Lockley, S.W. Dynamic Lighting Schedules to Facilitate Circadian Adaptation to Shifted Timing of Sleep and Wake. J. Pineal Res. 2022, 73, e12805. [Google Scholar] [CrossRef]
- Brown, T.M. Melanopic Illuminance Defines the Magnitude of Human Circadian Light Responses under a Wide Range of Conditions. J. Pineal Res. 2020, 69, e12655. [Google Scholar] [CrossRef] [Green Version]
- Vetter, C.; Pattison, P.M.; Houser, K.; Herf, M.; Phillips, A.J.K.; Wright, K.P.; Skene, D.J.; Brainard, G.C.; Boivin, D.B.; Glickman, G. A Review of Human Physiological Responses to Light: Implications for the Development of Integrative Lighting Solutions. LEUKOS J. Illum. Eng. Soc. N. Am. 2021, 18, 1–28. [Google Scholar] [CrossRef]
- Even, C.; Schröder, C.M.; Friedman, S.; Rouillon, F. Efficacy of Light Therapy in Nonseasonal Depression: A Systematic Review. J. Affect. Disord. 2008, 108, 11–23. [Google Scholar] [CrossRef]
- Terman, M.; Terman, J.S. Light Therapy for Seasonal and Nonseasonal Depression: Efficacy, Protocol, Safety, and Side Effects. CNS Spectr. 2005, 10, 647–663. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, A.; Partonen, T. The Diagnosis, Symptomatology, and Epidemiology of Seasonal Affective Disorder. CNS Spectr. 2005, 10, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandewalle, G.; Hébert, M.; Beaulieu, C.; Richard, L.; Daneault, V.; Garon, M.L.; Leblanc, J.; Grandjean, D.; Maquet, P.; Schwartz, S.; et al. Abnormal Hypothalamic Response to Light in Seasonal Affective Disorder. Biol. Psychiatry 2011, 70, 954–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavoie, M.P.; Lam, R.W.; Bouchard, G.; Sasseville, A.; Charron, M.C.; Gagné, A.M.; Tremblay, P.; Filteau, M.J.; Hébert, M. Evidence of a Biological Effect of Light Therapy on the Retina of Patients with Seasonal Affective Disorder. Biol. Psychiatry 2009, 66, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Lefler, B.J.; Emens, J.S.; Bauer, V.K. The Circadian Basis of Winter Depression. Proc. Natl. Acad. Sci. USA 2006, 103, 7414–7419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, C.; Muto, V.; Jaspar, M.; Kussé, C.; Lambot, E.; Chellappa, S.L.; Degueldre, C.; Balteau, E.; Luxen, A.; Middleton, B.; et al. Seasonality in Human Cognitive Brain Responses. Proc. Natl. Acad. Sci. USA 2016, 113, 3066–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbini, B.; Di Molfetta, D.; Gasperini, M.; Manfredonia, M.G.; Smeraldi, E. Seasonal Concordance of Recurrence in Mood Disorder Patients. Eur. Psychiatry 1995, 10, 171–174. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Duffy, J.F.; Shanahan, T.L.; Brown, E.N.; Mitchell, J.F.; Rimmer, D.W.; Ronda, J.M.; Silva, E.J.; Allan, J.S.; Emens, J.S.; et al. Stability, Precision, and near-24-Hour Period of the Human Circadian Pacemaker. Science 1999, 284, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Udry, M.; El Wardani, M.; Münch, M. Can Extra Daytime Light Exposure Improve Well-Being and Sleep? A Pilot Study of Patients With Glaucoma. Front. Neurol. 2021, 11, 1839. [Google Scholar] [CrossRef]
- Thorne, H.C.; Jones, K.H.; Peters, S.P.; Archer, S.N.; Dijk, D.J. Daily and Seasonal Variation in the Spectral Composition of Light Exposure in Humans. Chronobiol. Int. 2009, 26, 854–866. [Google Scholar] [CrossRef] [Green Version]
- Glickman, G.; Byrne, B.; Pineda, C.; Hauck, W.W.; Brainard, G.C. Light Therapy for Seasonal Affective Disorder with Blue Narrow-Band Light-Emitting Diodes (LEDs). Biol. Psychiatry 2006, 59, 502–507. [Google Scholar] [CrossRef] [Green Version]
- Strong, R.E.; Marchant, B.K.; Reimherr, F.W.; Williams, E.; Soni, P.; Mestas, R. Narrow-Band Blue-Light Treatment of Seasonal Affective Disorder in Adults and the Influence of Additional Nonseasonal Symptoms. Depress. Anxiety 2009, 26, 273–278. [Google Scholar] [CrossRef]
- Do, A.; Li, V.W.; Huang, S.; Michalak, E.E.; Tam, E.M.; Chakrabarty, T.; Yatham, L.N.; Lam, R.W. Blue-Light Therapy for Seasonal and Non-Seasonal Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can. J. Psychiatry 2022, 67, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Roecklein, K.A.; Wong, P.M.; Miller, M.A.; Donofry, S.D.; Kamarck, M.L.; Brainard, G.C. Melanopsin, Photosensitive Ganglion Cells, and Seasonal Affective Disorder. Neurosci. Biobehav. Rev. 2013, 37, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruani, J.; Geoffroy, P.A. Clinical Medicine Multi-Level Processes and Retina-Brain Pathways of Photic Regulation of Mood. J. Clin. Med. 2022, 2022, 448. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.; Kaufmann, C.; Leutritz, T.; Arnold, Y.L.; Speck, O.; Ullsperger, M. The Human Habenula Is Responsive to Changes in Luminance and Circadian Rhythm. Neuroimage 2019, 189, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, T.; Cai, X. Light-Sensitive Circuits Related to Emotional Processing Underlie the Antidepressant Neural Targets of Light Therapy. Behav. Brain Res. 2021, 396, 112862. [Google Scholar] [CrossRef]
- Koenigs, M.; Grafman, J. The Functional Neuroanatomy of Depression: Distinct Roles for Ventromedial and Dorsolateral Prefrontal Cortex. Behav. Brain Res. 2009, 201, 239–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drevets, W.C.; Bogers, W.; Raichle, M.E. Functional Anatomical Correlates of Antidepressant Drug Treatment Assessed Using PET Measures of Regional Glucose Metabolism. Eur. Neuropsychopharmacol. 2002, 12, 527–544. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Liotti, M.; Brannan, S.K.; McGinnis, S.; Mahurin, R.K.; Jerabek, P.A.; Silva, J.A.; Tekell, J.L.; Martin, C.C.; Lancaster, J.L.; et al. Reciprocal Limbic-Cortical Function and Negative Mood: Converging PET Findings in Depression and Normal Sadness. Am. J. Psychiatry 1999, 156, 245–253. [Google Scholar] [CrossRef]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Reiss, A.L.; Schatzberg, A.F. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol. Psychiatry 2007, 62, 429. [Google Scholar] [CrossRef] [Green Version]
- Sabbah, S.; Worden, M.S.; Laniado, D.D.; Berson, D.M.; Sanes, J.N. Luxotonic Signals in Human Prefrontal Cortex as a Possible Substrate for Effects of Light on Mood and Cognition. Proc. Natl. Acad. Sci. USA 2022, 119, e2118192119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ru, T.; Chen, Q.; Qian, L.; Luo, X.; Zhou, G. Effects of Illuminance and Correlated Color Temperature of Indoor Light on Emotion Perception. Sci. Rep. 2021, 11, 14351. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Liu, Q.; Li, X.; Hou, K.; Xing, Q. Indoor Lighting Effects on Subjective Impressions and Mood States: A Critical Review. Build. Environ. 2022, 224, 109591. [Google Scholar] [CrossRef]
- Spitschan, M.; Mead, J.; Roos, C.; Lowis, C.; Griffiths, B.; Mucur, P.; Herf, M.; Brown, T.; Hanifin, J.P.; Warfield, B.; et al. Luox: Novel Open-Access and Open-Source Web Platform for Calculating and Sharing Physiologically Relevant Quantities for Light and Lighting [Version 1; Peer Review: 2 Approved]. Open Res. 2021, 6, 69. [Google Scholar] [CrossRef]
- Mongrain, v.; Carrier, J.; Dumont, M. Circadian and Homeostatic Sleep Regulation in Morningness-Eveningness. J. Sleep Res. 2006, 15, 162–166. [Google Scholar] [CrossRef]
- Rufiange, M.; Dumont, M.; Lachapelle, P. Correlating Retinal Function with Melatonin Secretion in Subjects with an Early or Late Circadian Phase. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2491–2499. [Google Scholar]
- Wright, K.P.; McHill, A.W.; Birks, B.R.; Griffin, B.R.; Rusterholz, T.; Chinoy, E.D. Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Curr. Biol. 2013, 23, 1554–1558. [Google Scholar] [CrossRef] [Green Version]
- Ricketts, E.J.; Joyce, D.S.; Rissman, A.J.; Burgess, H.J.; Colwell, C.S.; Lack, L.C.; Gradisar, M. Electric Lighting, Adolescent Sleep and Circadian Outcomes, and Recommendations for Improving Light Health. Sleep Med. Rev. 2022, 64, 101667. [Google Scholar] [CrossRef]
- Van Der Lely, S.; Frey, S.; Garbazza, C.; Wirz-Justice, A.; Jenni, O.G.; Steiner, R.; Wolf, S.; Cajochen, C.; Bromundt, V.; Schmidt, C. Blue Blocker Glasses as a Countermeasure for Alerting Effects of Evening Light-Emitting Diode Screen Exposure in Male Teenagers. J. Adolesc. Health 2015, 56, 113–119. [Google Scholar] [CrossRef]
- Gasperetti, C.E.; Dolsen, M.R.; Harvey, A.G. The Influence of Intensity and Timing of Daily Light Exposure on Subjective and Objective Sleep in Adolescents with an Evening Circadian Preference. Sleep Med. 2020, 79, 166–174. [Google Scholar] [CrossRef]
- Heath, M.; Sutherland, C.; Bartel, K.; Gradisar, M.; Williamson, P.; Lovato, N.; Micic, G. Does One Hour of Bright or Short-Wavelength Filtered Tablet Screenlight Have a Meaningful Effect on Adolescents’ Pre-Bedtime Alertness, Sleep, and Daytime Functioning? Chronobiol. Int. 2014, 31, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Perrault, A.A.; Bayer, L.; Peuvrier, M.; Afyouni, A.; Ghisletta, P.; Brockmann, C.; Spiridon, M.; Hulo Vesely, S.; Haller, D.M.; Pichon, S.; et al. Reducing the Use of Screen Electronic Devices in the Evening Is Associated with Improved Sleep and Daytime Vigilance in Adolescents. Sleep 2019, 42, zsz125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Norren, D.; Vos, J.J. Light Damage to the Retina: An Historical Approach. Eye 2015, 30, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of Blue Light-Induced Eye Hazard and Protective Measures: A Review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Tejedor, J.; Marchena, M.; Ramírez, L.; García-Ayuso, D.; Gómez-Vicente, V.; Sánchez-Ramos, C.; de la Villa, P.; Germain, F. Removal of the Blue Component of Light Significantly Decreases Retinal Damage after High Intensity Exposure. PLoS ONE 2018, 13, e0194218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Q.; Lin, H.; Wu, J.; Wu, Z.; Qu, S.; Bi, Y. The Protective Effects of Blue Light-Blocking Films with Different Shielding Rates: A Rat Model Study. Transl. Vis. Sci. Technol. 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Ziegelberger, G.; Miller, S.A.; O’Hagan, J.; Okuno, T.; Schulmeister, K.; Sliney, D.; Stuck, B.; Croft, R.; Feychting, M.; Green, A.C.; et al. Light-Emitting Diodes (LEDS): Implications for Safety. Health Phys. 2020, 118, 549–561. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, H.; Yu, A.; Xie, J. Association between Sunlight Exposure and Risk of Age-Related Macular Degeneration: A Meta-Analysis. BMC Ophthalmol. 2018, 18, 331. [Google Scholar] [CrossRef] [Green Version]
- O’Hagan, J.B.; Khazova, M.; Price, L.L.A. Low-Energy Light Bulbs, Computers, Tablets and the Blue Light Hazard. Eye 2016, 30, 230–233. [Google Scholar] [CrossRef] [Green Version]
- Bullough, J.D.; Bierman, A.; Rea, M.S. Evaluating the Blue-Light Hazard from Solid State Lighting. Int. J. Occup. Saf. Ergon. 2017, 25, 311–320. [Google Scholar] [CrossRef]
- Nield, K. CIE Position Statement on the Blue Light Hazard. Color Res. Appl. 2019, 44, 672–673. [Google Scholar] [CrossRef]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-Emitting Diodes (LED) for Domestic Lighting: Any Risks for the Eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef]
- McAdams, H.; Kaiser, E.A.; Igdalova, A.; Haggerty, E.B.; Cucchiara, B.; Brainard, D.H.; Aguirre, G.K. Selective Amplification of IpRGC Signals Accounts for Interictal Photophobia in Migraine. Proc. Natl. Acad. Sci. USA 2020, 117, 17320–17329. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.R. Exposure to Artificial Light at Night and Risk of Cancer: Where Do We Go from Here? Br. J. Cancer 2021, 124, 1467–1468. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saenz, A.; de Miguel, A.S.; Espinosa, A.; Costas, L.; Aragonés, N.; Tonne, C.; Moreno, V.; Pérez-Gómez, B.; Valentin, A.; Pollán, M.; et al. Association between Outdoor Light-at-Night Exposure and Colorectal Cancer in Spain. Epidemiology 2020, 31, 718–727. [Google Scholar] [CrossRef]
- Garcia-Saenz, A.; de Miguel, A.S.; Espinosa, A.; Valentin, A.; Aragonés, N.; Llorca, J.; Amiano, P.; Sánchez, V.M.; Guevara, M.; Capelo, R.; et al. Evaluating the Association between Artificial Light-at-Night Exposure and Breast and Prostate Cancer Risk in Spain (MCC-Spain Study). Environ. Health Perspect. 2018, 126, 044011. [Google Scholar] [CrossRef]
- Lawrenson, J.G.; Hull, C.C.; Downie, L.E. The Effect of Blue-Light Blocking Spectacle Lenses on Visual Performance, Macular Health and the Sleep-Wake Cycle: A Systematic Review of the Literature. Ophthalmic Physiol. Opt. 2017, 37, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, A.L.; Wolffsohn, J.S. Digital Eye Strain: Prevalence, Measurement and Amelioration. BMJ Open. Ophthalmol. 2018, 3, 146. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Downie, L.E.; Anderson, A.J. Do Blue-Blocking Lenses Reduce Eye Strain From Extended Screen Time? A Double-Masked Randomized Controlled Trial. Am. J. Ophthalmol. 2021, 226, 243–251. [Google Scholar] [CrossRef]
- Xiang, Z.Y.; Zou, H.D. Recent Epidemiology Study Data of Myopia. J. Ophthalmol. 2020, 2020, 4395278. [Google Scholar] [CrossRef]
- Xiong, S.; Sankaridurg, P.; Naduvilath, T.; Zang, J.; Zou, H.; Zhu, J.; Lv, M.; He, X.; Xu, X. Time Spent in Outdoor Activities in Relation to Myopia Prevention and Control: A Meta-Analysis and Systematic Review. Acta Ophthalmol. 2017, 95, 551–566. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.L.; Liu, Y.F.; Wang, G.; Shao, Y.Q.; Yu, C.X.; Yang, Z.; Zhou, Z.R.; Han, X.; Gong, X.; Qian, K.W.; et al. The Role of IpRGCs in Ocular Growth and Myopia Development. Sci. Adv. 2022, 8, 9027. [Google Scholar] [CrossRef]
- Wong, N.A.; Bahmani, H. A Review of the Current State of Research on Artificial Blue Light Safety as It Applies to Digital Devices. Heliyon 2022, 8, e10282. [Google Scholar] [CrossRef]
- Viola, A.U.; James, L.M.; Schlangen, L.J.M.; Dijk, D.J. Blue-Enriched White Light in the Workplace Improves Self-Reported Alertness, Performance and Sleep Quality. Scand. J. Work Environ. Health 2008, 34, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Keis, O.; Helbig, H.; Streb, J.; Hille, K. Influence of Blue-Enriched Classroom Lighting on Students’ Cognitive Performance. Trends Neurosci. Educ. 2014, 3, 86–92. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, S.H.; Suh, I.B.; Jang, J.W.; Jhoo, J.H.; Lee, J.H. Positive Effect of Timed Blue-Enriched White Light on Sleep and Cognition in Patients with Mild and Moderate Alzheimer’s Disease. Sci. Rep. 2021, 11, 10174. [Google Scholar] [CrossRef] [PubMed]
- Cremascoli, R.; Sparasci, D.; Giusti, G.; Cattaldo, S.; Prina, E.; Roveta, F.; Bruno, F.; Ghezzi, C.; Cerri, S.; Picascia, M.; et al. Effects of Circadian Phase Tailored Light Therapy on Sleep, Mood, and Cognition in Alzheimer’s Disease: Preliminary Findings in a Pivotal Study. Front. Physiol. 2022, 12, 2205. [Google Scholar] [CrossRef] [PubMed]
- Yelden, K.; James, L.M.; Duport, S.; Kempny, A.; Farmer, S.F.; Leff, A.P.; Playford, E.D. A Simple Intervention for Disorders of Consciousness- Is There a Light at the End of the Tunnel? Front. Neurol. 2022, 13, 1534. [Google Scholar] [CrossRef] [PubMed]
- Riemersma-van der Lek, R.F.; Swaab, D.F.; Twisk, J.; Hol, E.M.; Hoogendijk, W.J.; van Someren, E.J. Effect of Bright Light and Melatonin on Cognitive and Noncognitive Function in Elderly Residents of Group Care Facilities. JAMA 2008, 299, 2642. [Google Scholar] [CrossRef]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for Daytime, Evening, and Nighttime Indoor Light Exposure to Best Support Physiology, Sleep, and Wakefulness in Healthy Adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]
- Kompier, M.E.; Smolders, K.C.H.J.; de Kort, Y.A.W. A Systematic Literature Review on the Rationale for and Effects of Dynamic Light Scenarios. Build. Environ. 2020, 186, 107326. [Google Scholar] [CrossRef]
- Canazei, M.; Weninger, J.; Pohl, W.; Marksteiner, J.; Weiss, E.M. Effects of Dynamic Bedroom Lighting on Measures of Sleep and Circadian Rest-Activity Rhythm in Inpatients with Major Depressive Disorder. Sci. Rep. 2022, 12, 6137. [Google Scholar] [CrossRef] [PubMed]
- Ru, T.; Kompier, M.E.; Chen, Q.; Zhou, G.; Smolders, K.C.H.J. Temporal Tuning of Illuminance and Spectrum: Effect of a Full-Day Dynamic Lighting Pattern on Well-Being, Performance and Sleep in Simulated Office Environment. Build. Environ. 2023, 228, 109842. [Google Scholar] [CrossRef]
- Münch, M.; Wirz-Justice, A.; Brown, S.A.; Kantermann, T.; Martiny, K.; Stefani, O.; Vetter, C.; Wright, K.P.; Wulff, K.; Skene, D.J. The Role of Daylight for Humans: Gaps in Current Knowledge. Clocks Sleep 2020, 2, 61–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoop, M.; Stefani, O.; Bueno, B.; Matusiak, B.; Hobday, R.; Wirz-Justice, A.; Martiny, K.; Kantermann, T.; Aarts, M.P.J.; Zemmouri, N.; et al. Daylight: What Makes the Difference? Light. Res. Technol. 2020, 52, 423–442. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, I.; Sharifpour, R.; Vandewalle, G. Light as a Modulator of Non-Image-Forming Brain Functions—Positive and Negative Impacts of Increasing Light Availability. Clocks & Sleep 2023, 5, 116-140. https://doi.org/10.3390/clockssleep5010012
Campbell I, Sharifpour R, Vandewalle G. Light as a Modulator of Non-Image-Forming Brain Functions—Positive and Negative Impacts of Increasing Light Availability. Clocks & Sleep. 2023; 5(1):116-140. https://doi.org/10.3390/clockssleep5010012
Chicago/Turabian StyleCampbell, Islay, Roya Sharifpour, and Gilles Vandewalle. 2023. "Light as a Modulator of Non-Image-Forming Brain Functions—Positive and Negative Impacts of Increasing Light Availability" Clocks & Sleep 5, no. 1: 116-140. https://doi.org/10.3390/clockssleep5010012
APA StyleCampbell, I., Sharifpour, R., & Vandewalle, G. (2023). Light as a Modulator of Non-Image-Forming Brain Functions—Positive and Negative Impacts of Increasing Light Availability. Clocks & Sleep, 5(1), 116-140. https://doi.org/10.3390/clockssleep5010012






