Effects of Vortioxetine on Sleep Architecture of Adolescents with Major Depressive Disorder
Abstract
:1. Introduction
1.1. Depression and Insomnia in Adolescents
1.2. Sleep Abnormalities in Adolescent Depression
1.3. The Effects of Antidepressants on Sleep Architecture
1.4. Vortioxetine—Pharmacokinetic/Molecular Mechanisms Related to Sleep
2. Results
2.1. Comparison of Questionnaire Indices before and after Treatment
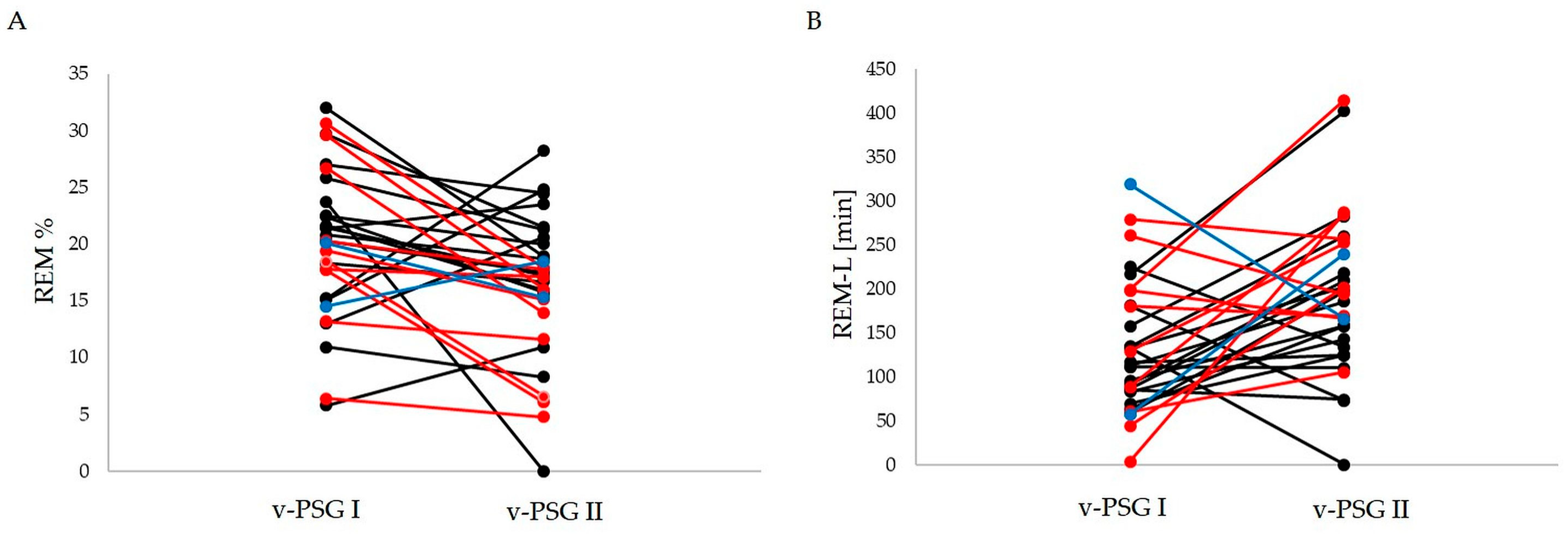
2.2. Comparison of Sleep-Related Indices before and after Treatment
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Ethical Statement
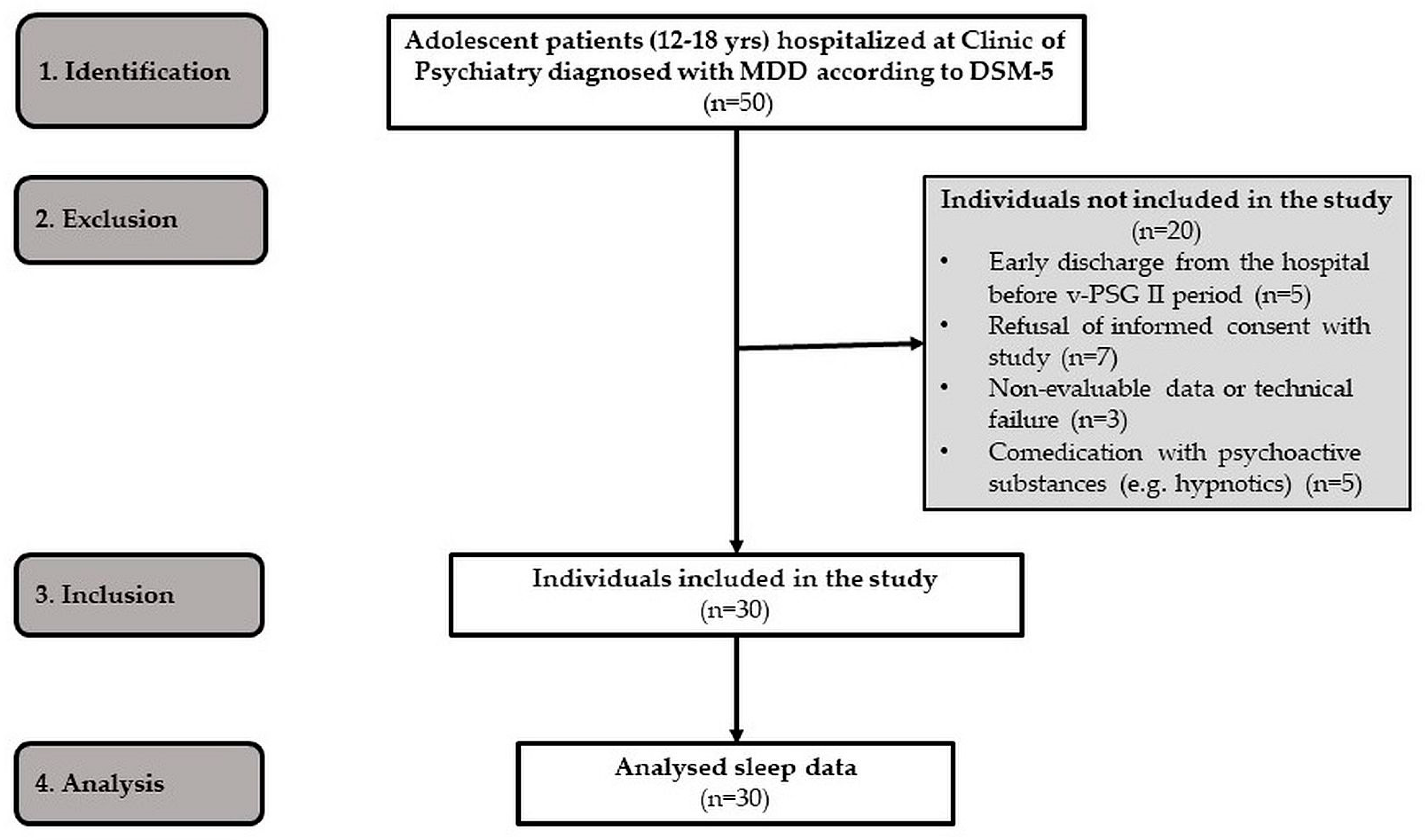
4.2. Participants
4.3. Clinical Assessment
4.3.1. The Children´s Depression Inventory
4.3.2. The Athens Insomnia Scale
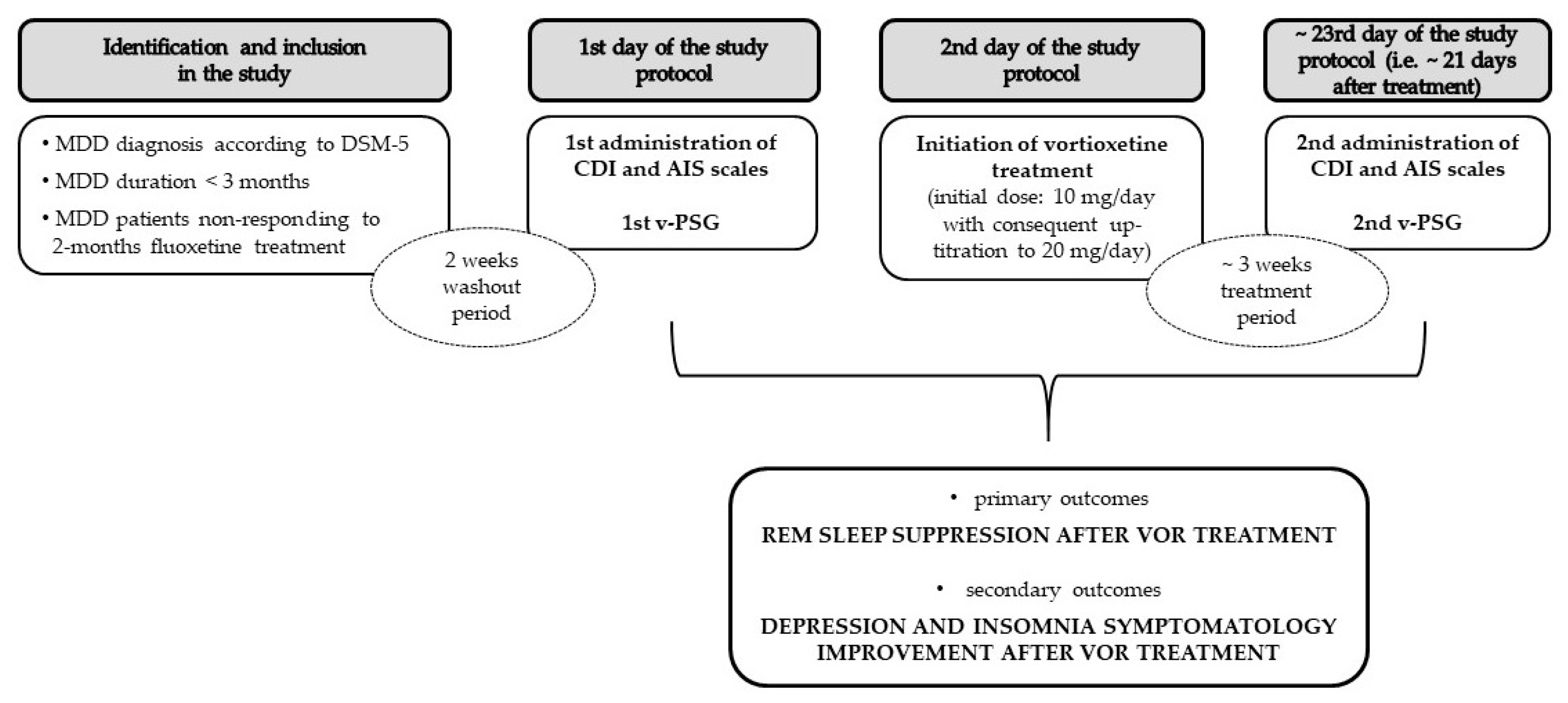
4.4. Protocol
4.5. Pharmacotherapy
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, M.M.; Beresford, B.; Bland, M.; Fraser, L.K. Prevalence and Incidence of Anxiety and Depression Among Children, Adolescents, and Young Adults With Life-Limiting Conditions: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Urrila, A.S.; Paunio, T.; Palomäki, E.; Marttunen, M. Sleep in adolescent depression: Physiological perspectives. Acta Physiol. 2015, 213, 758–777. [Google Scholar] [CrossRef] [PubMed]
- Palagini, L.; Baglioni, C.; Ciapparelli, A.; Gemignani, A.; Riemann, D. REM sleep dysregulation in depression: State of the art. Sleep Med. Rev. 2013, 17, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Palagini, L.; Hertenstein, E.; Riemann, D.; Nissen, C. Sleep, insomnia and mental health. J. Sleep Res. 2022, 31, e13628. [Google Scholar] [CrossRef]
- Palagini, L.; Bastien, C.H.; Marazziti, D.; Ellis, J.G.; Riemann, D. The key role of insomnia and sleep loss in the dysregulation of multiple systems involved in mood disorders: A proposed model. J. Sleep Res. 2019, 28, e12841. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Li, R.; Zhang, M.-Q.; Zhang, Z.; Qu, W.-M.; Huang, Z.-L. The Neurobiological Mechanisms and Treatments of REM Sleep Disturbances in Depression. Curr. Neuropharmacol. 2015, 13, 543–553. [Google Scholar] [CrossRef]
- Steiger, A.; Pawlowski, M. Depression and Sleep. Int. J. Mol. Sci. 2019, 20, 607. [Google Scholar] [CrossRef]
- Augustinavicius, J.L.S.; Zanjani, A.; Zakzanis, K.K.; Shapiro, C.M. Polysomnographic features of early-onset depression: A meta-analysis. J. Affect. Disord. 2014, 158, 11–18. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- Gazea, M.; Del Rio-Bermudez, C.; Nissen, C.; Adamantidis, A.R. Chapter 17—Functions and Circuits of REM Sleep. In Handbook of Sleep Research; Dringenberg, H.C., Ed.; Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, pp. 249–267. [Google Scholar]
- Drakatos, P.; Olaithe, M.; Verma, D.; Ilic, K.; Cash, D.; Fatima, Y.; Higgins, S.; Young, A.H.; Chaudhuri, K.R.; Steier, J.; et al. Periodic limb movements during sleep: A narrative review. J. Thorac. Dis. 2021, 13, 6476–6494. [Google Scholar] [CrossRef]
- Figorilli, M.; Puligheddu, M.; Congiu, P.; Ferri, R. The Clinical Importance of Periodic Leg Movements in Sleep. Curr. Treat. Options Neurol. 2017, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Boz, S.; Lanquart, J.P.; Mungo, A.; Delhaye, M.; Loas, G.; Hein, M. Risk of Excessive Daytime Sleepiness Associated to Major Depression in Adolescents. Psychiatr. Q. 2021, 92, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Wichniak, A.; Wierzbicka, A.; Walęcka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr. Psychiatry Rep. 2017, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Hutka, P.; Krivosova, M.; Muchova, Z.; Tonhajzerova, I.; Hamrakova, A.; Mlyncekova, Z.; Mokry, J.; Ondrejka, I. Association of Sleep Architecture and Physiology with Depressive Disorder and Antidepressants Treatment. Int. J. Mol. Sci. 2021, 22, 1333. [Google Scholar] [CrossRef]
- Kolla, B.P.; Mansukhani, M.P.; Bostwick, J.M. The influence of antidepressants on restless legs syndrome and periodic limb movements: A systematic review. Sleep Med. Rev. 2018, 38, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Højer, A.-M.; Buchberg, J.; Areberg, J.; Nutt, D.J. Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: Part 1, a pharmacokinetic/pharmacodynamic comparison with paroxetine in healthy men. J. Psychopharmacol. 2015, 29, 1085–1091. [Google Scholar] [CrossRef]
- Sanchez, C.; Asin, K.E.; Artigas, F. Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol. Ther. 2015, 145, 43–57. [Google Scholar] [CrossRef]
- Areberg, J.; Petersen, K.B.; Chen, G.; Naik, H. Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin. Pharmacol. Toxicol. 2014, 115, 552–559. [Google Scholar] [CrossRef]
- Chen, G.; Højer, A.-M.; Areberg, J.; Nomikos, G. Vortioxetine: Clinical Pharmacokinetics and Drug Interactions. Clin. Pharmacokinet. 2018, 57, 673–686. [Google Scholar] [CrossRef]
- Findling, R.L.; Robb, A.S.; DelBello, M.P.; Huss, M.; McNamara, N.K.; Sarkis, E.H.; Scheffer, R.E.; Poulsen, L.H.; Chen, G.; Lemming, O.M.; et al. A 6-Month Open-Label Extension Study of Vortioxetine in Pediatric Patients with Depressive or Anxiety Disorders. J. Child Adolesc. Psychopharmacol. 2018, 28, 47–54. [Google Scholar] [CrossRef]
- Findling, R.L.; DelBello, M.P.; Zuddas, A.; Emslie, G.J.; Ettrup, A.; Petersen, M.L.; Schmidt, S.N.; Rosen, M. Vortioxetine for Major Depressive Disorder in Adolescents: 12-Week Randomized, Placebo-Controlled, Fluoxetine-Referenced, Fixed-Dose Study. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 1106–1118.e2. [Google Scholar] [CrossRef] [PubMed]
- De Diego-Adeliño, J.; Crespo, J.M.; Mora, F.; Neyra, A.; Iborra, P.; Gutiérrez-Rojas, L.; Salonia, S.F. Vortioxetine in major depressive disorder: From mechanisms of action to clinical studies. An updated review. Expert Opin. Drug Saf. 2022, 21, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Bennabi, D.; Haffen, E.; Van Waes, V. Vortioxetine for Cognitive Enhancement in Major Depression: From Animal Models to Clinical Research. Front. Psychiatry 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Leiser, S.C.; Pehrson, A.L.; Robichaud, P.J.; Sanchez, C. Multimodal antidepressant vortioxetine increases frontal cortical oscillations unlike escitalopram and duloxetine—A quantitative EEG study in rats. Br. J. Pharmacol. 2014, 171, 4255–4272. [Google Scholar] [CrossRef]
- Nissen, T.D.; Laursen, B.; Viardot, G.; l’Hostis, P.; Danjou, P.; Sluth, L.B.; Gram, M.; Bastlund, J.F.; Christensen, S.R.; Drewes, A.M. Effects of Vortioxetine and Escitalopram on Electroencephalographic Recordings—A Randomized, Crossover Trial in Healthy Males. Neuroscience 2020, 424, 172–181. [Google Scholar] [CrossRef]
- Liguori, C.; Ferini-Strambi, L.; Izzi, F.; Mari, L.; Manfredi, N.; D’Elia, A.; Mercuri, N.B.; Placidi, F. Preliminary evidence that vortioxetine may improve sleep quality in depressed patients with insomnia: A retrospective questionnaire analysis. Br. J. Clin. Pharmacol. 2019, 85, 240–244. [Google Scholar] [CrossRef]
- Cao, B.; Park, C.; Rosenblat, J.D.; Chen, Y.; Iacobucci, M.; Subramaniapillai, M.; Mansur, R.B.; Zuckerman, H.; Lee, Y.; McIntyre, R.S. Changes in sleep predict changes in depressive symptoms in depressed subjects receiving vortioxetine: An open-label clinical trial. J. Psychopharmacol. 2019, 33, 1388–1394. [Google Scholar] [CrossRef]
- Doghramji, K.; Jangro, W.C. Adverse Effects of Psychotropic Medications on Sleep. Sleep Med. Clin. 2016, 11, 503–514. [Google Scholar] [CrossRef]
- Leiser, S.C.; Iglesias-Bregna, D.; Westrich, L.; Pehrson, A.L.; Sanchez, C. Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: Part 2, pharmacological interactions in rodents suggest a role of serotonin-3 receptor antagonism. J. Psychopharmacol. 2015, 29, 1092–1105. [Google Scholar] [CrossRef]
- Riga, M.S.; Sanchez, C.; Celada, P.; Artigas, F. Sub-chronic vortioxetine (but not escitalopram) normalizes brain rhythm alterations and memory deficits induced by serotonin depletion in rats. Neuropharmacology 2020, 178, 108238. [Google Scholar] [CrossRef]
- Bonaventure, P.; Kelly, L.; Aluisio, L.; Shelton, J.; Lord, B.; Galici, R.; Miller, K.; Atack, J.; Lovenberg, T.W.; Dugovic, C. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J. Pharmacol. Exp. Ther. 2007, 321, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Lahmeyer, H.W.; Poznanski, E.O.; Bellur, S.N. EEG sleep in depressed adolescents. Am. J. Psychiatry 1983, 140, 1150–1153. [Google Scholar] [CrossRef]
- Kutcher, S.; Williamson, P.; Marton, P.; Szalai, J. REM Latency in Endogenously Depressed Adolescents. Br. J. Psychiatry 1992, 161, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Picchietti, M.A.; Picchietti, D.L. Restless Legs Syndrome and Periodic Limb Movement Disorder in Children and Adolescents. Semin. Pediatr. Neurol. 2008, 15, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Krone, L.B.; Wulff, K.; Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 74–89. [Google Scholar] [CrossRef]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM–5), 5th ed.; American Psychiatric Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- WHO Adolescent-Health. 2016. Available online: https://www.who.int/ (accessed on 9 October 2023).
- Kovacs, M. Manual for the Children’s Depression Inventory; Multi-Health Systems: North Tonawanda, NY, USA, 1992. [Google Scholar]
- Matthey, S.; Petrovski, P. The Children’s Depression Inventory: Error in cutoff scores for screening purposes. Psychol. Assess. 2002, 14, 146–149. [Google Scholar] [CrossRef]
- Chung, K.-F.; Kan, K.K.-K.; Yeung, W.-F. Assessing insomnia in adolescents: Comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Med. 2011, 12, 463–470. [Google Scholar] [CrossRef]
- Altamura, A.C.; Moro, A.R.; Percudani, M. Clinical pharmacokinetics of fluoxetine. Clin. Pharmacokinet. 1994, 26, 201–214. [Google Scholar] [CrossRef]
- Montano, C.B.; Jackson, W.C.; Vanacore, D.; Weisler, R.H. Practical Advice for Primary Care Clinicians on the Safe and Effective Use of Vortioxetine for Patients with Major Depressive Disorder (MDD). Neuropsychiatr. Dis. Treat. 2022, 18, 867–879. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Lipinska, G.; Thomas, K.G.F. The Interaction of REM Fragmentation and Night-Time Arousal Modulates Sleep-Dependent Emotional Memory Consolidation. Front. Psychol. 2019, 10, 1766. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, V.; Abeyratne, U.R.; Hukins, C.; Duce, B. A state transition-based method for quantifying EEG sleep fragmentation. Med. Biol. Eng. Comput. 2009, 47, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Doman, J.; Detka, C.; Hoffman, T.; Kesicki, D.; Monahan, J.P.; Buysse, D.J.; Reynolds, C.F., 3rd; Coble, P.A.; Matzzie, J.; Kupfer, D.J. Automating the sleep laboratory: Implementation and validation of digital recording and analysis. Int. J. Biomed. Comput. 1995, 38, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Hall, M.; Begley, A.; Cherry, C.R.; Houck, P.R.; Land, S.; Ombao, H.; Kupfer, D.J.; Frank, E. Sleep and treatment response in depression: New findings using power spectral analysis. Psychiatry Res. 2001, 103, 51–67. [Google Scholar] [CrossRef]
| Study | n (Number of Subjects) | Study Design | Treatment Duration Dosing Regimen | Results |
|---|---|---|---|---|
| Liguori et al., 2019 [27] | 15 adults with MDD and insomnia | A retrospective analysis of questionnaires (PSQI, ESS, BDI) | 6 months VOR at 10 mg | Improvements in subjective sleep complaints and reduction of depressive symptoms |
| Cao et al., 2019 [28] | 92 adults with MDD and 54 healthy controls | A post-hoc analysis of the clinical trial of sleep questionnaires (PSQI, ESS, ISI) | 8 weeks VOR at 10–20 mg | Improvements in sleep (predictive of AD response) and linearly correlated to depressive symptoms |
| Leiser et al., 2015 [30] | Animals (rats) | Bipolar sleep EEG | 1, 3, 7, 10 days VOR at 0.6 mg/kg (s.c. injection, drug-infused chow, or water) | ↓ REM sleep % (only acute effect) |
| Wilson et al., 2015 [17] | 19 healthy men | A randomized, double-blind, placebo-controlled (compared to paroxetine and placebo) PSG study | 2 days VOR at 20–40 mg paroxetine at 20 mg | ~↑ REM-L, ~↓ REM sleep % ↑ N1 stage % ~↑ WASO (at 40 mg dose) |
| Before Treatment | After Treatment | p-Value | Cohen’s d | |
|---|---|---|---|---|
| AIS | 7.53 ± 5.73 | 4.70 ± 3.91 | 0.002 | 0.6589 |
| CDI_A | 5.10 ± 2.80 | 3.00 ± 2.74 | <0.001 | 0.8866 |
| CDI_B | 1.93 ± 1.41 | 1.70 ± 1.11 | 0.007 | 0.6052 |
| CDI_C | 3.60 ± 2.27 | 2.70 ± 1.91 | 0.025 | 0.4412 |
| CDI_D | 6.10 ± 3.61 | 4.47 ± 3.12 | 0.013 | 0.5508 |
| CDI_E | 4.17 ± 2.61 | 2.83 ± 2.55 | 0.002 | 0.6536 |
| CDI_TS | 20.9 ± 11.3 | 14.1 ± 10.0 | <0.001 | 0.8061 |
| v-PSG I | v-PSG II | p-Value | Cohen’s d | |
|---|---|---|---|---|
| Conventional sleep-related indices | ||||
| TST (min) | 404.0 ± 49.9 | 391.0 ± 70.9 | 0.363 | 0.1689 |
| WASO | 51.4 ± 28.9 | 70.1 ± 57.4 | 0.152 | −0.3190 |
| SE% | 79.9 ± 9.34 | 76.1 ± 13.4 | 0.152 | 0.2884 |
| SL | 50.8 ± 39.1 | 52.2 ± 35.9 | 0.887 | −0.0322 |
| REM-L | 132.0 ± 75.3 | 193.0 ± 89.8 | 0.005 | −0.5569 |
| REM% | 20.10 ± 6.59 | 16.20 ± 6.33 | 0.009 | 0.5086 |
| RD | 3.12 ± 2.80 | 2.74 ± 2.29 | 0.931 | −0.0207 |
| N1% | 4.67 ± 2.22 | 6.00 ± 4.41 | 0.234 | −0.2951 |
| N2% | 47.10 ± 8.80 | 48.10 ± 8.76 | 0.461 | −0.1364 |
| N3% | 28.20 ± 7.99 | 29.60 ± 10.90 | 0.551 | −0.1478 |
| PLMI | 17.70 ± 21.30 | 32.00 ± 37.10 | 0.119 | −0.3320 |
| Sleep fragmentation indices | ||||
| REM fragmentation | 11.70 ± 7.07 | 12.50 ± 7.12 | 0.558 | −0.0997 |
| AI | 14.40 ± 7.05 | 17.50 ± 10.00 | 0.318 | −0.2129 |
| Spectral power indices | ||||
| Delta power (μV2) | 7.38 ± 0.19 | 7.40 ± 0.18 | 0.632 | −0.0933 |
| Delta power N1 (μV2) | 5.35 ± 0.45 | 5.38 ± 0.49 | 0.824 | −0.0431 |
| Delta power N2 (μV2) | 6.76 ± 0.25 | 6.77 ± 0.21 | 0.916 | 0.0205 |
| Delta power N3 (μV2) | 7.21 ± 0.20 | 7.24 ± 0.20 | 0.396 | −0.1660 |
| Delta power NREM (μV2) | 7.36 ± 0.18 | 7.38 ± 0.18 | 0.512 | −0.1280 |
| Delta power REM (μV2) | 5.88 ± 0.45 | 5.76 ± 0.43 | 0.382 | 0.1745 |
| Alpha power (μV2) | 6.70 ± 0.04 | 6.74 ± 0.22 | 0.257 | −0.2232 |
| Alpha power N1 (μV2) | 5.03 ± 0.49 | 5.06 ± 0.56 | 0.540 | −0.1217 |
| Alpha power N2 (μV2) | 6.52 ± 0.24 | 6.54 ± 0.28 | 0.768 | −0.0575 |
| Alpha power N3 (μV2) | 5.94 ± 0.48 | 6.03 ± 0.35 | 0.171 | −0.2710 |
| Alpha power NREM (μV2) | 6.67 ± 0.24 | 6.71 ± 0.22 | 0.253 | −0.2247 |
| Alpha power REM (μV2) | 5.17 ± 0.62 | 5.06 ± 0.76 | 0.374 | 0.1776 |
| Characteristics of MDD Adolescents (n = 30) | |
|---|---|
| Age (mean ± SD) | 15.0 ± 1.5 years |
| Female/male | 21/9 |
| Duration of MDD | <3 months |
| Drugs used prior to VOR | Fluoxetine |
| Severity of MDD | Moderate or severe |
| Comorbidities | None |
| Co-interventions | None |
| VOR—10 mg/day | 18 adolescents |
| VOR—15 mg/day | 10 adolescents |
| VOR—20 mg/day | 2 adolescents |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlyncekova, Z.; Hutka, P.; Visnovcova, Z.; Ferencova, N.; Kovacova, V.; Macejova, A.; Tonhajzerova, I.; Ondrejka, I. Effects of Vortioxetine on Sleep Architecture of Adolescents with Major Depressive Disorder. Clocks & Sleep 2023, 5, 627-638. https://doi.org/10.3390/clockssleep5040042
Mlyncekova Z, Hutka P, Visnovcova Z, Ferencova N, Kovacova V, Macejova A, Tonhajzerova I, Ondrejka I. Effects of Vortioxetine on Sleep Architecture of Adolescents with Major Depressive Disorder. Clocks & Sleep. 2023; 5(4):627-638. https://doi.org/10.3390/clockssleep5040042
Chicago/Turabian StyleMlyncekova, Zuzana, Peter Hutka, Zuzana Visnovcova, Nikola Ferencova, Veronika Kovacova, Andrea Macejova, Ingrid Tonhajzerova, and Igor Ondrejka. 2023. "Effects of Vortioxetine on Sleep Architecture of Adolescents with Major Depressive Disorder" Clocks & Sleep 5, no. 4: 627-638. https://doi.org/10.3390/clockssleep5040042






