Non-Invasive Fetal Electrocardiogram Monitoring Techniques: Potential and Future Research Opportunities in Smart Textiles
Abstract
1. Introduction
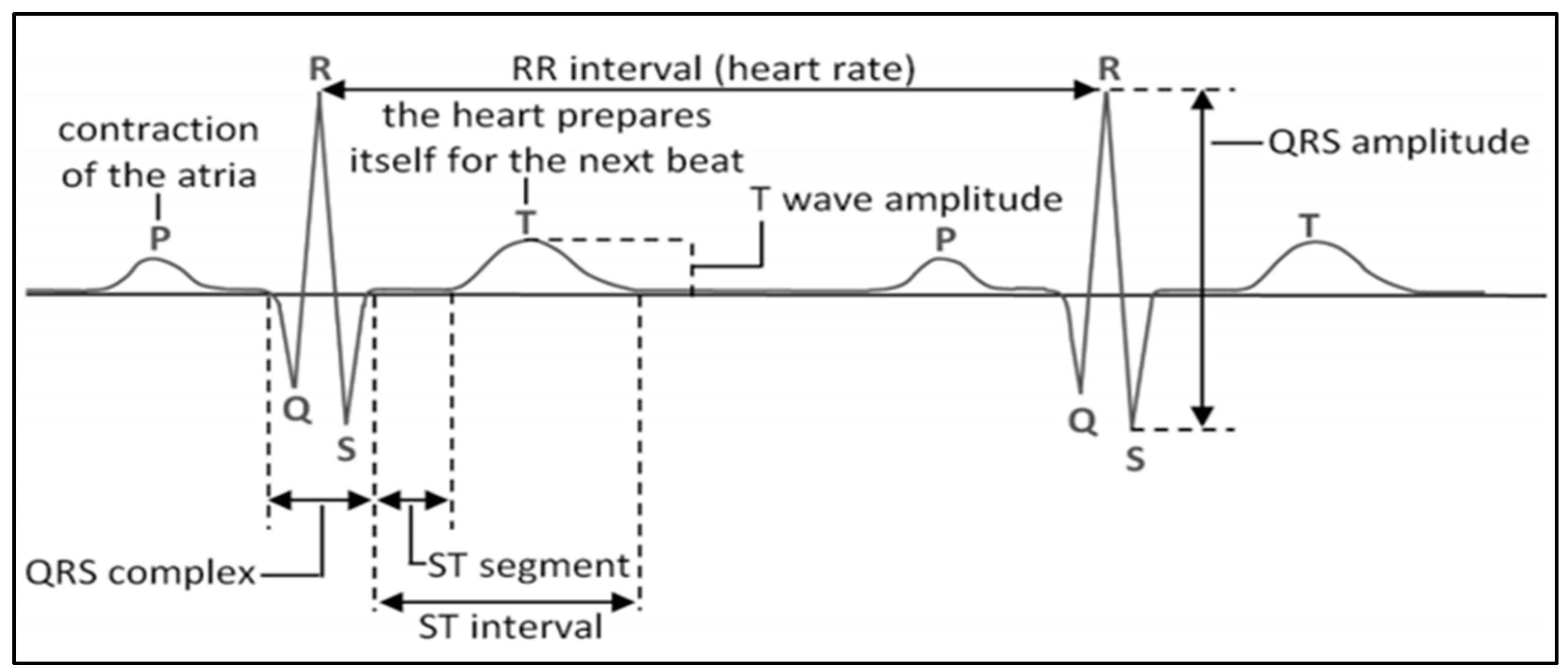
2. Background of FECG
3. Comparison of Fetal Heart Rate Monitoring Techniques
3.1. Photoplethysmography (PPG)
3.2. Cardiotocography (CTG)
3.3. Doppler Sound
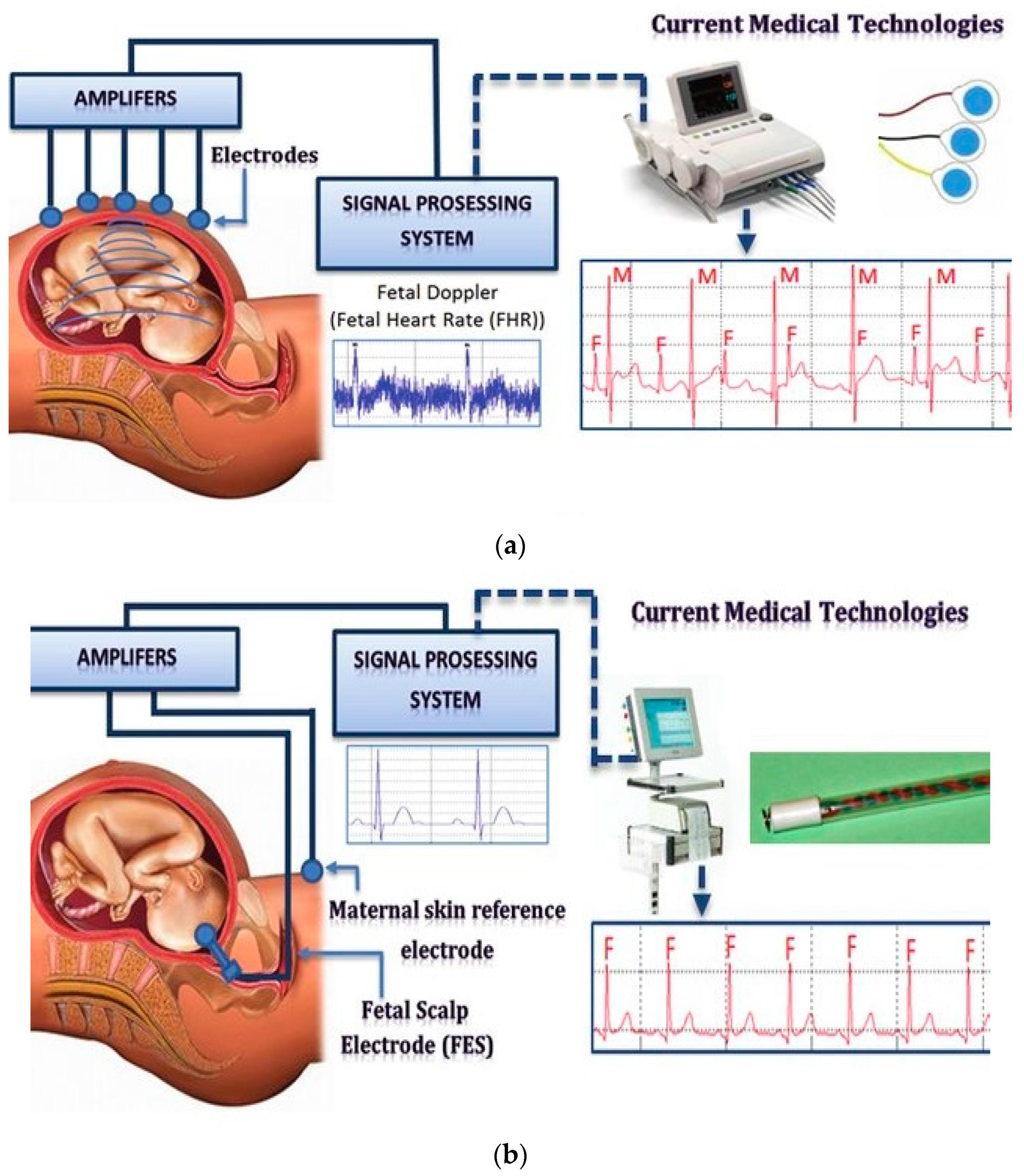
3.4. Fetalelectrocardiography (FECG)
4. Importance of Electrode Configurations for FECG Measurement
4.1. Electrode Configurations
4.2. Electrode Types
4.3. Current ECG Monitoring Development with Smart Textile Electrodes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ungureanu, G.; Gussi, I.; Wolf, W.; Țarălungă, D.; Pasca, S.; Strungaru, R. Prenatal Telemedicine Advances in Fetal Monitoring. 2011. Available online: https://scholar.google.com.hk/scholar?hl=en&as_sdt=0%2C5&q=Ungureanu%2C+Georgeta+%26+Gussi%2C+Ilinca+%26+Wolf%2C+Werner+%26+%C8%9Aar%C4%83lung%C4%83%2C+Drago%C8%99+%26+Pasca%2C+Sever+%26+Strungaru%2C+Rodica.+%282011%29.+Prenatal+Telemedicine+Advances+in+Fetal+Monitoring.+10.5772%2F13533.&btnG= (accessed on 23 June 2021). [CrossRef]
- Hasan, M.A.; Reaz, M.B.I.; Ibrahimy, M.I.; Hussain, M.S.; Uddin, J. Detection and processing techniques of FECG signal for fetal monitoring. Biol. Proced. Online 2009, 11, 263–295. [Google Scholar] [CrossRef]
- Chez, B.F.; Baird, S.M. Electronic Fetal Heart Rate Monitoring: Where Are We Now? J. Perinat. Neonatal Nurs. 2011, 25, 180–192. [Google Scholar] [CrossRef]
- Neilson, J.P. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database Syst. Rev. 2006, 3. [Google Scholar] [CrossRef]
- Freeman, R.K.; Garite, T.J.; Nageotte, M.P.; Miller, L.A. Fetal Heart Rate Monitoring; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Peters, C.H.; ten Broeke, E.D.; Andriessen, P.; Vermeulen, B.; Berendsen, R.C.; Wijn, P.F.; Oei, S.G. Beat-to-beat detection of fetal heart rate: Doppler ultrasound cardiotocography compared to direct ECG cardiotocography in time and frequency domain. Physiol. Meas. 2004, 25, 585. [Google Scholar] [CrossRef] [PubMed]
- Vullings, R.; Peters, C.; Mischi, M.; Oei, G.; Bergmans, J. Maternal ECG removal from non-invasive fetal ECG recordings. In Proceedings of the EMBS’06. 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 1394–1397. [Google Scholar]
- Hon, E.H. Apparatus for continuous monitoring of the fetal heart rate. Yale J. Biol. Med. 1960, 32, 397. [Google Scholar]
- Fetal Heart Monitoring. Journal of Obstetric, Gynecologic & Neonatal Nursing. 2018. Available online: https://www.jognn.org/article/S0884-2175(18)30322-8/fulltext (accessed on 23 June 2021). [CrossRef]
- Jezewski, J.; Roj, D.; Wrobel, J.; Horoba, K. A novel technique for fetal heart rate estimation from Doppler ultrasound signal. Biomed. Eng. Online 2011, 10. [Google Scholar] [CrossRef]
- Black, R.S.; Campbell, S. Cardiotocography versus Doppler. Ultrasound Obstet. Gynecol. 1997, 9, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Crowe, J.; Piéri, J.F.; Quartero, H.; Hayes-Gill, B.; James, D.; Shakespeare, S. Monitoring the fetal heart non-invasively: A review of methods. J. Perinat. Med. 2001, 29, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Strasburger, J.F.; Cheulkar, B.; Wakai, R.T. Magnetocardiography for fetal arrhythmias. Heart Rhythm Off. J. Heart Rhythm Soc. 2008, 5, 1073. [Google Scholar] [CrossRef]
- Adithya, P.C.; Sankar, R.; Moreno, W.A.; Hart, S. Trends in fetal monitoring through phonocardiography: Challenges and future directions. Biomed. Signal Process. Control 2017, 33, 289–305. [Google Scholar] [CrossRef]
- Andreotti, F.; Riedl, M.; Himmelsbach, T.; Wedekind, D.; Wessel, N.; Stepan, H.; Schmieder, C.; Jank, A.; Malberg, H.; Zaunseder, S. Robust fetal ECG extraction and detection from abdominal leads. Physiol. Meas. 2014, 35, 1551–1568. [Google Scholar] [CrossRef]
- Assaleh, K. Extraction of fetal electrocardiogram using adaptive neuro-fuzzy interference systems. IEEE Trans. Biomed. Eng. 2007, 54, 59–68. [Google Scholar] [CrossRef]
- Behar, J.; Johnson, A.; Clifford, G.D.; Oster, J. A comparison of single channel fetal ECG extraction methods. Ann. Biomed. Eng. 2014, 42, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Gao, X. On the improved correlative prediction scheme for aliased electrocardiogram (ECG) data compression. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; pp. 6180–6183. [Google Scholar]
- Ghodsi, M.; Hassani, H.; Sanei, S. Extracting fetal heart signal from noisy maternal ECG by singular spectrum analysis. J. Stat. Interface Spec. Issue Appl. SSA 2010, 3, 399–411. [Google Scholar] [CrossRef]
- Hyvarinen, A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 1999, 10, 626–634. [Google Scholar] [CrossRef]
- Immanuel, J.J.R.; Prabhu, V.; Christopheraj, V.J.; Sugumar, D.; Vanathi, P.T. Separation of maternal and fetal ECG signals from the mixed source signal using FASTICA. Procedia Eng. 2012, 30, 356–363. [Google Scholar] [CrossRef]
- Jafari, M.G.; Chambers, J.A. Fetal electrocardiogram extraction by sequential source separation in the wavelet domain. IEEE Trans. Biomed. Eng. 2005, 52, 390–400. [Google Scholar] [CrossRef][Green Version]
- Kropfl, M.; Modre-Osprian, R.; Schreier, G.; Hayn, D. A robust algorithm for fetal QRS detection using non-invasive maternal abdominal ECGs. Comput. Cardiol. 2013, 40, 313–316. [Google Scholar]
- Kumar, P.; Sharma, S.K.; Prasad, S. Detection of FECG from multivariate abdominal recordings using wavelets and neuro-fuzzy systems. Int. J. Eng. Adv. Technol. Stud. 2013, 2, 45–51. [Google Scholar]
- Melillo, P.; Santoro, D.; Vadursi, M. Detection and compensation of inter-channel time offsets in indirect fetal ECG sensing. IEEE Sens. J. 2014, 14, 2327–2334. [Google Scholar] [CrossRef]
- Panigrahy, D.; Sahu, P.K. Extraction of fetal electrocardiogram (ECG) by extended state Kalman filtering and adaptive neuro-fuzzy inference system (ANFIS) based on single channel abdominal recording. Sadhana 2015, 40, 1091–1104. [Google Scholar] [CrossRef]
- Poian, G.D.; Bernardini, R.; Rinaldo, R. Separation and analysis of fetal-ECG signals from compressed sensed abdominal ECG recordings. IEEE Trans. Biomed. Eng. 2016, 63, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Reza, S.; Shamsollahi, M.B.; Jutten, C.; Clifford, G.D. A nonlinear Bayesian filtering framework for ECG denoising. IEEE Trans. Biomed. Eng. 2007, 54, 2172–2185. [Google Scholar]
- Rooijakkers, M.J.; Rabotti, C.; de Lau, H.; Oei, S.G.; Bergmans, J.W.M.; Mischi, M. Feasibility study of a new method for low-complexity fetal movement detection from abdominal ECG recordings. IEEE J. Biomed. Health Inform. 2016, 20, 1361–1368. [Google Scholar] [CrossRef]
- Selvaraj, R.; Kanagaraj, B. A multi-stage adaptive singular value decomposition approach for fetal ECG signal extraction in multichannel input system for prenatal health monitoring. Asian J. Inf. Technol. 2016, 15, 1049–1055. [Google Scholar]
- Sameni, R.; Clifford, G.D. A review of fetal ECG signal processing; issues and promising directions. Open Pacing Electrophysiol. Ther. J. 2010, 3, 4. [Google Scholar] [CrossRef]
- von Steinburg, S.P.; Boulesteix, A.; Lederer, C.; Grunow, S.; Schiermeier, S.; Hatzmann, W.; Daumer, M. What is the “normal” fetal heart rate. PeerJ 2013. [Google Scholar] [CrossRef]
- Silva, I.; Behar, J.; Sameni, R.; Zhu, T.-T.; Oster, J.; Clifford, G.D.; Moody, G.B. Noninvasive fetal ECG: The PhysioNet/computing in cardiology challenge 2013. In Proceedings of the IEEE Conference of Computing in Cardiology (CinC), Zaragoza, Spain, 22–25 September 2013; pp. 149–152. [Google Scholar]
- Song, S.; Rooijakkers, M.J.; Harpe, P.; Rabotti, C.; Mischi, M.; van Roermund, A.H.M.; Cantatore, E. A noise reconfigurable current-reuse resistive feedback amplifier with signal-dependent power consumption for fetal ECG monitoring. IEEE Sens. J. 2016, 16, 8304–8313. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Hurnanen, T.; Koskinen, J.; Eriksson, J.; Pänkäälä, M.; Teräs, M.; Koivisto, T. A real-time approach for heart rate monitoring using a Hilbert transform in seismocardiograms. Physiol. Meas. 2016, 37, 1885–1909. [Google Scholar] [CrossRef]
- Yeh, H.-M.; Chang, Y.-C.; Lin, C.; Yeh, C.-H.; Lee, C.-N.; Shyu, M.-K.; Hung, M.-H.; Hsiao, P.-N.; Wang, Y.-H.; Tseng, Y.-H.; et al. A new method to derive fetal heart rate from maternal abdominal electrocardiogram monitoring fetal heart rate during cesarean section. PLoS ONE 2015, 10, e0117509. [Google Scholar]
- Zheng, W.; Li, X.-L.; Wei, X.-Y.; Liu, H.-X. Foetal ECG extraction by support vector regression. Electron. Lett. 2016, 52, 506–507. [Google Scholar]
- Adam, J. The Future of Fetal Monitoring. Rev. Obstet. Gynecol. 2012, 5, e132. [Google Scholar]
- Hasan, M.; Ibrahimy, M.; Reaz, M. Fetal ECG Extraction from Maternal Abdominal ECG Using Neural Network. J. Softw. Eng. Appl. 2009, 2, 330–334. [Google Scholar] [CrossRef]
- Graatsma, E.M.; Jacod, B.C.; Van Egmond, L.A.J.; Mulder, E.J.H.; Visser, G.H.A. Fetal electrocardiography: Feasibility of long-term fetal heart rate recordings. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 334338. [Google Scholar] [CrossRef]
- Graatsma, E.M. Monitoring of Fetal Heart Rate and Uterine Activity; Utrecht University: Utrecht, The Netherlands, 2010. [Google Scholar]
- Karvounis, E.C.; Papaloukas, C.; Fotiadis, D.I.; Michalis, L.K. Fetal heart rate extraction from composite maternal ECG using complex continuous wavelet transform. In Computers in Cardiology; IEEE: Piscataway, NJ, USA, 2004; pp. 737–740. [Google Scholar]
- Zhongliang, L.U.O. Fetal Electrocardiogram Extraction using Blind Source Separation and Empirical Mode Decomposition. J. Comput. Inf. Syst. 2012, 8, 4825–4833. [Google Scholar]
- Zheng, W.; Liu, H.; He, A.; Ning, X.; Cheng, J. Singlelead fetal electrocardiogram estimation by means of combining Rpeak detection, resampling and comb filter. Med. Eng. Phys. 2010, 32, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Martínek, R.; Zidek, J. A System for Improving the Diagnostic Quality of Fetal Electrocardiogram. Prz. Elektrotech. 2012, 164–173. Available online: https://scholar.google.com.tw/scholar?hl=en&as_sdt=0%2C5&q=46.%09Mart%C3%ADnek%2C+R.%2C+%26+Zidek%2C+J.+%282012%29.+A+System+for+Improving+the+Diagnostic+Quality+of+Fetal+Electrocardiogram.+Przegl%C4%85d+El-ektrotechniczny%2C+164-173.&btnG= (accessed on 23 June 2021).
- Sree, T.H.; Garimella, M.; Bandari, A.; Patel, I. Microcontroller Based Fetal Heart Rate Monitoring using Intelligent Biosystem. In Proceedings of the 3rd International Conference on Electronics, Biomedical Engineering and Its Applications (ICEBEA’2013), Singapore, 29–30 April 2013. [Google Scholar]
- Sun, Y.; Hu, S.; Azorin-Peris, V.; Greenwald, S.; Chambers, J.; Zhu, Y. Motion-compensated noncontact imaging photoplethysmography to monitor cardiorespiratory status during exercise. J. Biomed. Opt. 2011, 16, 077010. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Khanokh, B.; Slovik, Y. The difference in pulse transit time to the toe and finger measured by photoplethysmography. Physiol. Meas. 2002, 23, 85. [Google Scholar] [CrossRef] [PubMed]
- Gan, K.B.; Ali, M.M.; Zahedi, E. Two Channel Abdominal PPG Instrumentation. In Proceedings of the 4th Kuala Lumpur International Conference on Biomedical Engineering 2008, Kuala Lumpur, Malaysia, 25–28 June 2008; Springer: Berlin/Heidelberg, Germany, 2008; pp. 691–693. [Google Scholar]
- Gan, K.B.; Zahedi, E.; Ali, M.A.M. Transabdominal fetal heart rate detection using NIR photopleythysmography: Instrumentation and clinical results. IEEE Trans. Biomed. Eng. 2009, 56, 2075–2082. [Google Scholar] [PubMed]
- Gan, K.B.; Zahedi, E.; Ali, M.M. Feasibility of Fetal Photoplethysmography Signal Extraction using Adaptive Noise Cancelling. In Proceedings of the 3rd Kuala Lumpur International Conference on Biomedical Engineering 2006, Kuala Lumpur, Malaysia, 11–14 December 2006; Springer: Berlin/Heidelberg, Germany, 2006; pp. 387–390. [Google Scholar]
- Ahsan, K.; Emad, I.; Sayaka, O.; Yoshitaka, K. Validation of beat by beat fetal heart signals acquired from four-channel fetal phonocardiogram with fetal electrocardiogram in healthy late pregnancy. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Heart Rate. Available online: http://fabacademy.org/archives/2013/students/contonente.javier/week16/week16.html (accessed on 16 February 2021).
- Cesarelli, M.; Romano, M.; Bifulco, P.; Fedele, F.; Bracale, M. An algorithm for the recovery of fetal heart rate series from CTG data. Comput. Biol. Med. 2007, 37, 663669. [Google Scholar] [CrossRef]
- Williams, B.; Arulkumaran, S. Cardiotocography and medicolegal issues. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 457–466. [Google Scholar] [CrossRef]
- Ugwumadu, A. Understanding cardiotocographic patterns associated with intrapartum fetal hypoxia and neurologic injury. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 509–536. [Google Scholar] [CrossRef]
- Alfirevic, Z.; Devane, D.; Gyte, G.M. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst. Rev. 2006, 3. [Google Scholar] [CrossRef]
- Paul, H.; Rik, V.; Alexander, K.; Jan, B.; van Judith, L.; Piero, T.; Massimo, M. Doppler Ultrasound Technology for Fetal Heart Rate Monitoring: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019. [Google Scholar] [CrossRef]
- Pregnancy and Antental Care. Available online: https://steemit.com/health/@doctorhealth/pregnancy-antenatal-care-and-counselling (accessed on 10 February 2021).
- Voicu, I.; Ménigot, S.; Kouamé, D.; Girault, J. New Estimators and Guidelines for Better Use of Fetal Heart Rate Estimators with Doppler Ultrasound Devices. Comput. Math. Methods Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.; Foulquière, K.; Kaluzynski, K.; Tranquart, F.; Fignon, A.; Pourcelot, D.; Berson, M.; Pourcelot, L. The DopFet system: A new ultrasonic Doppler system for monitoring and characterization of fetal movement. Ultrasound Med. Biol. 2000, 26, 1117–1124. [Google Scholar] [CrossRef]
- Jeżewski, J.; Wróbel, J.; Horoba, K.; Cholewa, D.; Gacek, A.; Kupka, T.; Matonia, A. Monitoring of mechanical and electrical activity of fetal heart: The nature of signals. Arch. Perinat. Med. 2002, 8, 40–46. [Google Scholar]
- Van Geijn, H.P.; Copray, F.J.A. A Critical Appraisal of Fetal Surveillance; Academic Hospital of the Free University: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Crowe, J.A.; Herbert, J.M.; Huang, X.B.; Reed, N.; Woolfson, M.S.; Rassi, D.; Zhuravlev, Y.E.; Emery, S.J. Sequential recording of the abdominal fetal electrocardiogram and magnetocardiogram. Physiol. Meas. 1995, 16, 43–47. [Google Scholar] [CrossRef]
- Goodlin, R.C. History of fetal monitoring. Am. J. Obstet. Gynecol. 1979, 133, 323–352. [Google Scholar] [CrossRef]
- Solum, T.; Ingemarsson, I.; Nygren, A. The accuracy of abdominal ECG for fetal electronic monitoring. J. Perinat. Med. 1980, 8, 142–149. [Google Scholar] [CrossRef]
- Khamene, A.; Negahdaripour, S. A new method for the extraction of fetal ECG from the composite abdominal signal. Biomed. Eng. IEEE Trans. 2000, 47, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.; Sameni, R.; Ward, J.; Robinson, J.; Wolfberg, A.J. Clinically accurate fetal ECG parameters acquired from maternal abdominal sensors. Am. J. Obstet. Gynecol. 2011, 205, 47.e1–47.e5. [Google Scholar] [CrossRef]
- Kahankova, R.; Martinek, R.; Jaros, R.; Behbehani, K.; Matonia, A.; Jezewski, M.; Behar, J.A. A Review of Signal Processing Techniques for Non-Invasive Fetal Electrocardiography. IEEE Rev. Biomed. Eng. 2020, 13, 51–73. [Google Scholar] [CrossRef]
- Monica Healthcare. Available online: http://www.monicahealthcare.com/ (accessed on 6 August 2019).
- Mindchild. Available online: http://www.mindchild.com/ (accessed on 6 August 2019).
- Nemo Healthcare, Community Research and Development Information Service. Available online: https://nemohealthcare.com/en/ (accessed on 6 August 2019).
- Cohen, W.R.; Ommani, S.; Hassan, S.; Mirza, F.G.; Solomon, M.; Brown, R.; Schifrin, B.S.; Himsworth, J.M.; Hayes-Gill, B.R. Accuracy and reliability of fetal heart rate monitoring using maternal abdominal surface electrodes. Acta Obstet. Gynecol. Scand. 2012, 91, 1306–1313. [Google Scholar] [CrossRef]
- Hon, E.; Hess, O. The clinical value of fetal electrocardiography. Am. J. Obstet. Gynecol. 1960, 79, 1012–1023. [Google Scholar] [CrossRef]
- Algunaidi, M.S.M.; Ali, M.M.; Gan, K.B.; Zahedi, E. Fetal heart rate monitoring based on adaptive noise cancellation and maternal QRS removal window. Eur. J. Sci. Res. 2009, 27, 565–575. [Google Scholar]
- Goell, P.; Rai, S.; Chandra, M.; Gupta, V.K. Analysis of LMS Algorithm in Wavelet Domain. In Proceedings of the Conference on Advances in Communication and Control Systems (CAC2S 2013), Dehradun, India, 6–8 April 2013. [Google Scholar]
- Unser, M.; Aldroubi, A. A review of wavelets in biomedical applications. Proc. IEEE 1996, 84, 626–638. [Google Scholar] [CrossRef]
- Abdulhay, E.W.; Oweis, R.J.; Alhaddad, A.M.; Sublaban, F.N.; Radwan, M.A.; Almasaeed, H.M. Non-Invasive Fetal Heart Rate Monitoring Techniques: Review article. Biomed. Sci. Eng. 2014, 2, 53–67. [Google Scholar]
- Chia, E.L.; Ho, T.F.; Rauff, M.; Yip, W.C.L. Cardiac time intervals of normal fetuses using noninvasive fetal electrocardiography. Prenat. Diagn. 2005, 25, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Algunaidi, M.S.; Ali, M.M.; Islam, M.F. Evaluation of an improved algorithm for fetal QRS detection. Int. J. Phys. Sci. 2011, 6, 213–220. [Google Scholar]
- Karvounis, E.C.; Tsipouras, M.G.; Fotiadis, D.I.; Naka, K.K. An Automated Methodology for Fetal Heart Rate Extraction from the Abdominal Electrocardiogram. IEEE Trans. Inf. Technol. Biomed. 2007, 11, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Rooijakkers, M.J.; Rabotti, C.; Oei, S.G.; Mischi, M. Low-complexity R-peak detection for ambulatory fetal monitoring. Physiol. Meas. 2012, 33, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Rooijakkers, M.J.; Song, S.; Rabotti, C.; Oei, S.G.; Bergmans, J.W.M.; Cantatore, E.; Mischi, M. Influence of Electrode Placement on Signal Quality for Ambulatory Pregnancy Monitoring. Comput. Math. Methods Med. 2014, 2014, 960980. [Google Scholar] [CrossRef]
- Vullings, R.; Peters, C.H.L.; Sluijter, R.J.; Mischi, M.; Oei, S.G.; Bergmans, J.W.M. Dynamic segmentation and linear prediction for maternal ECG removal in antenatal abdominal recordings. Physiol. Meas. 2009, 30, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Jie-Min, Z.; Xiao-Lin, H.; Qun, G.; Tie-Bing, L.; Ping, L.; Ying, Z.; Hong-Xing, L. Some regularity on how to locate electrodes for higher fECG SNRs. Chin. Phys. B 2015, 24, 038702. [Google Scholar]
- Martens, S.M.M.; Rabotti, C.; Mischi, M.; Sluijter, R.J. A robust fetal ECG detection method for abdominal recordings. Physiol. Meas. 2007, 28, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Smith, M.J.; Thomas, M.; Green, A.R.; Cheng, F.; Oseku-Afful, S.; Wee, L.Y.; Fisk, N.M.; Gardiner, H.M. Non-invasive fetal electrocardiography in singleton and multiple pregnancies. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 668–678. [Google Scholar] [CrossRef]
- Oostendorp, T.F.; Van Oosterom, A.; Jongsma, H.W. The fetal ECG throughout the second half of gestation. Clin. Phys. Physiol. Meas. 1989, 10, 147–160. [Google Scholar] [CrossRef]
- Marchon, N.; Naik, G. Electrode positioning for monitoring Fetal ECG: A review. In Proceedings of the 2015 International Conference on Information Processing (ICIP), Pune, India, 16–19 December 2015; pp. 5–10. [Google Scholar] [CrossRef]
- Strazdienė, E.; Blaževič, P.; Vegys, A.; Dapkūnienė, K. New Tendencies of Wearable Electronics Application in Smart Clothing. Electron. Electr. Eng. 2007, 73, 21–24. [Google Scholar]
- Rodrigues, R. Fetal beat detection in abdominal ECG recordings: Global and time adaptive approaches. Physiol. Meas. 2014, 35, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Rakshit, M.; Sahu, P.K. An efficient method for fetal ECG extraction from single channel abdominal ECG. In Proceedings of the 2015 International Conference on Industrial Instrumentation and Control (ICIC), Pune, India, 28–30 May 2015; pp. 1083–1088. [Google Scholar] [CrossRef]
- Kennedy, R.G. Electronic fetal heart rate monitoring: Retrospective reflections on a twentieth-century technology. J. R. Soc. Med. 1998, 91, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ivanoska-Dacikj, A.; Stachewicz, U. Smart textiles and wearable technologies—Opportunities offered in the fight against pandemics in relation to current COVID-19 state. Rev. Adv. Mater. Sci. 2020, 59, 487–505. [Google Scholar] [CrossRef]
- Hughes-Riley, T.; Dias, T.; Cork, C. A Historical Review of the Development of Electronic Textiles. Fibers 2018, 6, 34. [Google Scholar] [CrossRef]
- Tsukada, Y.T.; Tokita, M.; Murata, H.; Hirasawa, Y.; Yodogawa, K.; Iwasaki, Y.-K.; Kasai, N.; Shimizu, W.; Nakashima, H.; Tsukada, S. Validation of wearable textile electrodes for ECG monitoring. Heart Vessels 2019, 34, 1203–1211. [Google Scholar] [CrossRef]
- Paul, G.; Torah, R.; Beeby, S.; Tudor, J. A printed, dry electrode frank configuration vest for ambulatory vectorcardiographic monitoring. Smart Mater. Struct. 2016, 26, 025029. [Google Scholar] [CrossRef]
- Komolafe, A.; Torah, R.; Wei, Y.; Nunes-Matos, H.; Li, M.; Hardy, D.; Dias, T.; Tudor, M.; Beeby, S. Integrating Flexible Filament Circuits for E-Textile Applications. Adv. Mater. Technol. 2019, 4, 1900176. [Google Scholar] [CrossRef]
- Schwarz, A.; Hakuzimana, J.; Kaczynska, A.; Banaszczyk, J.; Westbroek, P.; McAdams, E.; Moody, G.; Chronis, Y.; Priniotakis, G.; De Mey, G.; et al. Gold coated para-aramid yarns through electroless deposition. Surf. Coat. Technol. 2010, 204, 1412–1418. [Google Scholar] [CrossRef]
- Odhiambo, S.A.; De Mey, G.; Hertleer, C.; Schwarz, A.; van Langenhove, L. Discharge characteristics of poly(3,4-ethylene dioxythiophene): Poly(styrenesulfonate) (PEDOT:PSS) textile batteries; comparison of silver coated yarn electrode devices and pure stainless steel filament yarn electrode devices. Text. Res. J. 2014, 84, 347–354. [Google Scholar] [CrossRef]
- An, X.; Stylios, G.K. A Hybrid Textile Electrode for Electrocardiogram (ECG) Measurement and Motion Tracking. Materials 2018, 11, 1887. [Google Scholar] [CrossRef]
- Achilli, A.; Pani, D.; Bonfiglio, A. Characterization of Screen-Printed Textile Electrodes Based on Conductive Polymer for ECG Acquisition; IEEE: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Lee, Y.-D.; Chung, W.-Y. Wireless sensor network based wearable smart shirt for ubiquitous health and activity monitoring. Sens. Actuator B Chem. 2009, 140, 390–395. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef]
- Kannaian, T.; Neelaveni, R.; Thilagavathi, G. Design and development of embroidered textile electrodes for continuous measurement of electrocardiogram signals. J. Ind. Text. 2013, 42, 303–318. [Google Scholar] [CrossRef]
- Alzaidi, A.; Zhang, L.; Bajwa, H. Smart textiles based wireless ECG system. In Proceedings of the 2012 IEEE Long Island Systems, Applications and Technology Conference (LISAT), Farmingdale, NY, USA, 4 May 2012; pp. 1–5. [Google Scholar] [CrossRef]
- Smart Fabrics, a Technology That Revolutionizes Experiences—Ignasi Sayol. Available online: https://nemohealthcare.com/en/ (accessed on 13 June 2020).
- Tang, D.H.; Gilligan, A.M.; Romero, K. Economic burden and disparities in healthcare resource use among adult patients with cardiac arrhythmia. Appl. Health Econ. Health Policy. 2014, 12, 59–71. [Google Scholar] [CrossRef]
- Steinberg, C.; Bennett, M.T.; Krahn, A.D. Extended ECG Monitoring. In Cardiac Arrhythmias, Pacing and Sudden Death; Kowey, P., Piccini, J.P., Naccarelli, G., Eds.; Springer: Cham, Switzerland, 2017; pp. 48–59. [Google Scholar]
- Priori, S.G. Survivors of out-of-hospital cardiac arrest with apparently normal heart. Need for definition and standardized clinical evaluation. Consensus Statement of the Joint Steering Committees of the Unexplained Cardiac Arrest Registry of Europe and of the Idiopathic Ventricular Fibrillation Registry of the United States. Circulation 1997, 95, 265–272. [Google Scholar]
- Tomson, T.T.; Passman, R. Current and Emerging Uses of Insertable Cardiac Monitors: Evaluation of Syncope and Monitoring for Atrial Fibrillation. Cardiol. Rev. 2017, 25, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Philippon, F.; Sanchez, M.; Fortier-Poisson, P.; O’Hara, G.; Molin, F.; Sarrazin, J.F.; Nault, I.; Blier, L.; Roy, K.; et al. A Novel Wearable Device for Continuous Ambulatory ECG Recording: Proof of Concept and Assessment of Signal Quality. Biosensors 2019, 9, 17. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Z.; Wu, T.; Zhang, Y. A wearable mobihealth care system supporting real-time diagnosis and alarm. Med. Biol. Eng. Comput. 2007, 45, 877–885. [Google Scholar] [CrossRef]
- Cho, G.; Jeong, K.; Paik, M.J.; Kwun, Y.; Sung, M. Performance evaluation of textile-based electrodes and motion sensors for smart clothing. IEEE Sens. J. 2011, 11, 3183–3193. [Google Scholar] [CrossRef]
- Pola, T.; Vanhala, J. Textile electrodes in ECG measurement. In Proceedings of the 2007 3rd International Conference on Intelligent Sensors, Sensor Networks and Information, Melbourne, QLD, Australia, 3–6 December 2007; pp. 635–639. [Google Scholar]
- Paul, G.; Torah, R.; Beeby, S.; Tudor, J. The development of screen-printed conductive networks on textiles for biopotential monitoring applications. Sens. Actuator A Phys. 2014, 206, 35–41. [Google Scholar] [CrossRef]
- Qin, H.; Li, J.; He, B.; Sun, J.; Li, L.; Qian, L. Novel Wearable Electrodes Based on Conductive Chitosan Fabrics and Their Application in Smart Garments. Materials 2018, 11, 370. [Google Scholar] [CrossRef] [PubMed]








| No. | Author | Number of Electrodes Used | Electrodes Placement | Parameters’ Specification | Number of Participants and Time of Data Recording (s) | Accuracy | Number of Gestation Weeks | Proposed Method for Extraction of FECG |
|---|---|---|---|---|---|---|---|---|
| 1. | Chia et al., 2005 [81] | 3 | Equilateral triangle formation of three electrodes on the abdomen of pregnant women. | N/A | Participants = 100 Recording = 10 min | Success rates for detecting the P, QRS, and T waves were 74.6%, 91.0%, and 79.3%, respectively | >18 weeks | Cancellation of maternal template in 1st and 2nd derivative of abdominal signals |
| 2. | Bergveld et al., 1986 [82] | 4 |  | Bandwidth = 0.2 to 120 Hz | Participants = 37 | - | Between 20 and 37 weeks | - |
| 3. | Algunaidi et al., 2011 [83] | 4 |  | Sampling Frequency = 256 Hz Resolution = 12 bits | Participants = 30 Recording = 60 s | - | Between 36 and 38 weeks | Peak detection Algorithm |
| 4. | Karvounis et al., 2007 [84] | 4 |  | Sampling Frequency = 300 Hz Resolution = 12 bits Gain = 7800 | Two scenarios were used: 8 Participants and recording of 60 s was performed. 5 Participants and recording of 15 min each was performed. | 97.47% | 20–41 weeks | 3-stage method: time frequency analysis, complex wavelet, and Heuristic algorithm |
| 5. | Emad. A. Ibrahim [15]. | 4 |  | N/A | N/A | 60% | 34 weeks | Fetal heart rates (FHR) extracted from fetal phonocardiography (FPCG) |
| 6. | Graatsma et al., 2008 [85] | 5 | - | Bandwidth = 1–70 Hz Sampling frequency = 300 Hz | Participants = 150 Time of recording = 15 h | - | Between 15 and 24 weeks | - |
| 7. | Rooijakkers et al., 2014 [86] | 6 | - | - | Participants = 5 Recording = 20 min each | - | 39 weeks | - |
| 8. | Vullings et al., 2009 [87] | 8 |  | Sampling frequency = 1 kHz Gain = 500 | Participants = 7 Recording = 10 min | 91% | 37–41 weeks | Weighted Averaging of MECG segments (WAMES) |
| 9. | Rooijakkers et al., 2012 [15] | 8 |  | Sampling Frequency = 1 kHz | - | 91% | 40 weeks during labor | Peak detection algorithm |
| 10. | Andreotti et al., 2014 [88] | 8 | - | - | Participants = 10 Recording = 20 min | 90% | 20–28 weeks | - |
| 11. | Zhang et al., 2014 [68] | 10 | - | - | Participants = 78 Recording = 24 s each | - | 3rd trimester | Adaptive R-wave detection algorithm |
| 12. | Martens et al., 2007 [89] | 13 |  | Gain = 20 | - | 85% | 9 weeks to labor | Sequential estimation method |
| 13. | Taylor et al., 2003 [90] | 12–16 electrodes |  | Sampling Frequency = 512 Hz | Participants = 241 Recording = 250 | 85% | Between 15 and 33 weeks | Linear regression to analyze QRS intervals |
| 14. | Oostendorp et al.,1989 [91] | 32 |  | Sampling Frequency = 500 Hz | Participants = 6 Recordings = 37 | - | Between 20 and 40 weeks | Homogeneous volume conduction model |
| 15. | Clifford et al., 2011 [92] | 32 | - | Sampling Frequency = 1 kHz | - | 91.2% | Between 35 and 41 weeks | - |
| No. of Electrodes | Discussion | Reference |
|---|---|---|
| Group 1: range of electrodes between 1 and 4 | Advantages: For NIFECG monitoring, the electrodes are placed on the abdomen of pregnant women. Hence, less are used for fetus monitoring, resulting in a simple procedure without any discomfort to pregnant women. Disadvantages: The data recorded does not provide detailed information about the growth of the fetus. | [15,81,82,83,84,85,86,87,88,89] |
| Group 2, comprises a range between 10 and 20 electrodes. | Advantages: It is evidenced that the more electrodes placed on the abdomen of pregnant women, the more comprehensive information can be gained about the fetus, thus helping in analyzing the well-being of the fetus efficiently and effectively. Disadvantages: Discomfort among the pregnant women due to several electrode placements on the abdomen of pregnant women. | [89,90] |
| Group 3, comprises a range of more than 20 electrodes | Advantages: Provides the detailed information about the growth of the fetus. Disadvantages: The deployment of 20 or more electrodes for FHR monitoring involves a complex setup procedure in addition to being expensive. Furthermore, results in skin irritation and severe discomfort to pregnant women due to gel used in setting up the electrodes to establish effective electrode configuration. | [91,92] |
| No. | Method | Material | System Integration | Reference |
|---|---|---|---|---|
| 1. | Knitting | Stainless steel | T-shirt | [113] |
| 2. | Knitting and Embroidery | Stainless steel filament, nylon fabric | T-shirt | [114] |
| 3. | Weaving and Knitting | Silver yarns | Chest band | [115] |
| 4. | Screen printing | Silver ink | Chest band | [116] |
| 5. | Electroless platting | Silver chloride | Smart garment | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggarwal, G.; Wei, Y. Non-Invasive Fetal Electrocardiogram Monitoring Techniques: Potential and Future Research Opportunities in Smart Textiles. Signals 2021, 2, 392-412. https://doi.org/10.3390/signals2030025
Aggarwal G, Wei Y. Non-Invasive Fetal Electrocardiogram Monitoring Techniques: Potential and Future Research Opportunities in Smart Textiles. Signals. 2021; 2(3):392-412. https://doi.org/10.3390/signals2030025
Chicago/Turabian StyleAggarwal, Geetika, and Yang Wei. 2021. "Non-Invasive Fetal Electrocardiogram Monitoring Techniques: Potential and Future Research Opportunities in Smart Textiles" Signals 2, no. 3: 392-412. https://doi.org/10.3390/signals2030025
APA StyleAggarwal, G., & Wei, Y. (2021). Non-Invasive Fetal Electrocardiogram Monitoring Techniques: Potential and Future Research Opportunities in Smart Textiles. Signals, 2(3), 392-412. https://doi.org/10.3390/signals2030025







