Fischer–Tropsch Synthesis for Light Olefins from Syngas: A Review of Catalyst Development
Abstract
:1. Introduction
2. Catalysts for Fischer–Tropsch to Olefins (FTO)
2.1. Catalyst Active Metal E-ffects
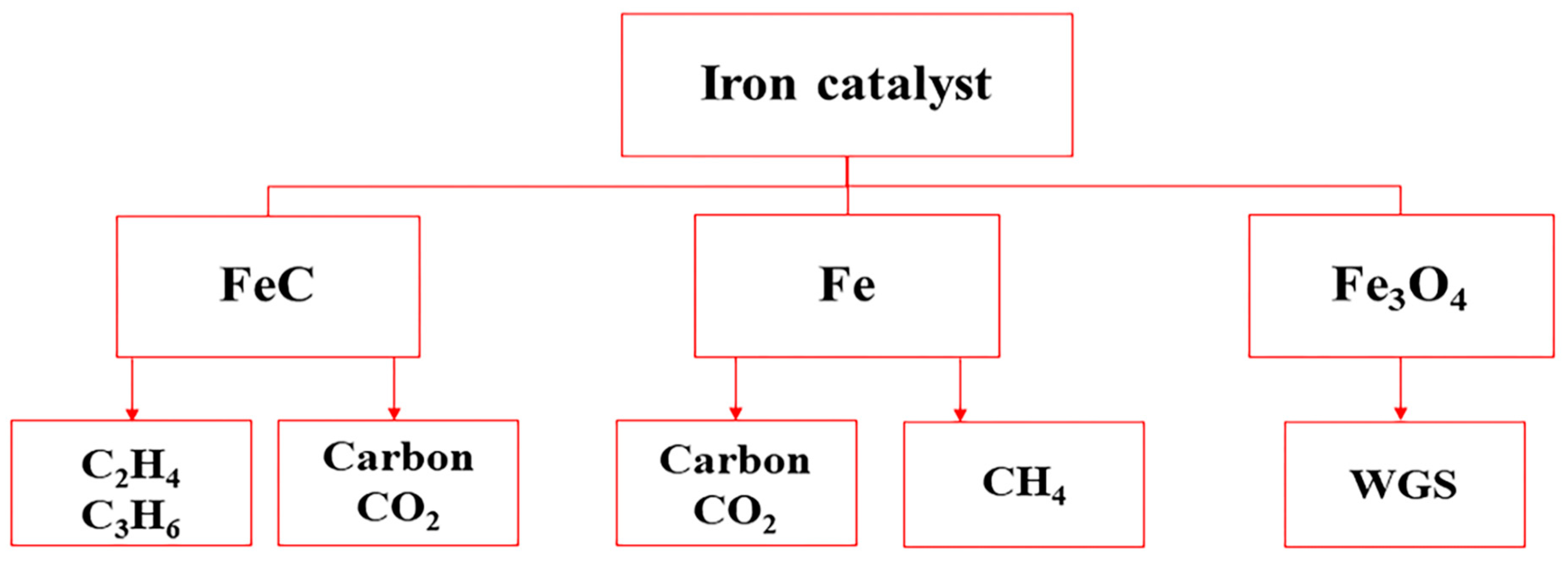
2.1.1. Iron-Based Catalysts
2.1.2. Cobalt-Based Catalysts
2.2. Catalysts’ Basicity Effects
2.3. Catalyst Dispersion Effects
2.4. Metal Support Interaction Effects
2.4.1. Carbon Nanotubes Supported Catalysts
2.4.2. Alumina-, Silica-, and Titania-Supported Catalysts
2.5. Promotion Effects
2.6. Deactivation of Iron and Cobalt Catalysts
2.6.1. Active Phase Oxidation
2.6.2. Carbidization
2.6.3. Surface Carbon Formation
2.6.4. Sintering
2.6.5. Poisoning of Sulfur, Nitrogen, and Alkali Metals
2.6.6. Surface Reconstruction and Attrition
2.7. Fischer–Tropsch Synthesis Plants
2.7.1. Techno-Economic Analysis
2.7.2. Fischer–Tropsch Synthesis Plants; Lifecycle Assessment
3. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadrameli, S. Thermal/catalytic cracking of hydrocarbons for the production of olefins: A state-of-the-art review I: Thermal cracking review. Fuel 2015, 140, 102–115. [Google Scholar] [CrossRef]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New trends in olefin production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Alotaibi, F.M.; Gonzalez-Cortes, S.; Alotibi, M.F.; Xiao, T.; Al-Megren, H.; Yang, G.; Edwards, P.P. Enhancing the production of light olefins from heavy crude oils: Turning challenges into opportunities. Catal. Today 2018, 317, 86–98. [Google Scholar] [CrossRef]
- van der Laan, G.P.; Beenackers, A.A. Hydrocarbon selectivity model for the gas–solid Fischer–Tropsch synthesis on precipitated iron catalysts. Ind. Eng. Chem. Res. 1999, 38, 1277–1290. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S. Energy efficient methanol-to-olefins process. Chem. Eng. Res. Des. 2018, 131, 41–54. [Google Scholar] [CrossRef]
- Ail, S.S.; Dasappa, S. Biomass to liquid transportation fuel via Fischer Tropsch synthesis–Technology review and current scenario. Renew. Sustain. Energy Rev. 2016, 58, 267–286. [Google Scholar] [CrossRef]
- Zafari, R.; Abdouss, M.; Zamani, Y. Effect of Mn and reduced graphene oxide for the Fischer–Tropsch reaction: An efficient catalyst for the production of light olefins from syngas. React. Kinet. Mech. Catal. 2020, 129, 707–724. [Google Scholar] [CrossRef]
- Di, Z.; Zhao, T.; Feng, X.; Luo, M. A Newly Designed Core-Shell-Like Zeolite Capsule Catalyst for Synthesis of Light Olefins from Syngas via Fischer–Tropsch Synthesis Reaction. Catal. Lett. 2019, 149, 441–448. [Google Scholar] [CrossRef]
- Lewis, P.E. Gas to Liquids: Beyond Fischer Tropsch. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 12–14 October 2013. [Google Scholar]
- Zhang, J.; Chen, J.; Ren, J.; Li, Y.; Sun, Y. Support effect of Co/Al2O3 catalysts for Fischer–Tropsch synthesis. Fuel 2003, 82, 581–586. [Google Scholar] [CrossRef]
- Sachtler, W.M.H. The Second Rideal Lecture. What makes a catalyst selective? Faraday Discuss. Chem. Soc. 1981, 72, 7–31. [Google Scholar] [CrossRef]
- Ponec, V. Surface composition and catalysis on alloys. Surf. Sci. 1979, 80, 352–366. [Google Scholar] [CrossRef]
- Biloen, P.; Helle, J.; Van den Berg, F.; Sachtler, W. On the activity of Fischer-Tropsch and methanation catalysts: A study utilizing isotopic transients. J. Catal. 1983, 81, 450–463. [Google Scholar] [CrossRef]
- Teimouri, Z.; Abatzoglou, N.; Dalai, A.K. Kinetics and Selectivity Study of Fischer–Tropsch Synthesis to C5+ Hydrocarbons: A Review. Catalysts 2021, 11, 330. [Google Scholar] [CrossRef]
- Filot, I.A.; Broos, R.J.; van Rijn, J.P.; van Heugten, G.J.; van Santen, R.A.; Hensen, E.J. First-principles-based microkinetics simulations of synthesis gas conversion on a stepped rhodium surface. ACS Catal. 2015, 5, 5453–5467. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for Fischer–Tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef] [Green Version]
- Lødeng, R.; Lunder, O.; Lein, J.-E.; Dahl, P.I.; Svenum, I.-H. Synthesis of light olefins and alkanes on supported iron oxide catalysts. Catal. Today 2018, 299, 47–59. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Kazak, V.O.; Pankina, G.V.; Perfiliev, Y.D.; Li, T.; Virginie, M.; Khodakov, A.Y. Influence of copper and potassium on the structure and carbidisation of supported iron catalysts for Fischer–Tropsch synthesis. Catal. Sci. Technol. 2017, 7, 2325–2334. [Google Scholar] [CrossRef]
- de Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer–Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Koeken, A.C.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Effects of sodium and sulfur on catalytic performance of supported iron catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Catal. 2013, 303, 22–30. [Google Scholar] [CrossRef]
- Cheng, K.; Virginie, M.; Ordomsky, V.V.; Cordier, C.; Chernavskii, P.A.; Ivantsov, M.I.; Paul, S.; Wang, Y.; Khodakov, A.Y. Pore size effects in high-temperature Fischer–Tropsch synthesis over supported iron catalysts. J. Catal. 2015, 328, 139–150. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, C.; Liu, C.; Wei, Y.; Cheruvathur, A.V.; Dugulan, A.I.; Niemantsverdriet, J.W.; Liu, X.; He, Y.; Qing, M.; et al. Relationship between Iron Carbide Phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and Catalytic Performances of Fe/SiO2 Fischer–Tropsch Catalysts. ACS Catal. 2018, 8, 3304–3316. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, X.; Chen, W.; Wang, D.; Song, N.; Qian, G.; Duan, X.; Yang, J.; Chen, D.; Yuan, W. Tailoring of Fe/MnK-CNTs Composite Catalysts for the Fischer–Tropsch Synthesis of Lower Olefins from Syngas. Ind. Eng. Chem. Res. 2018, 57, 11554–11560. [Google Scholar] [CrossRef]
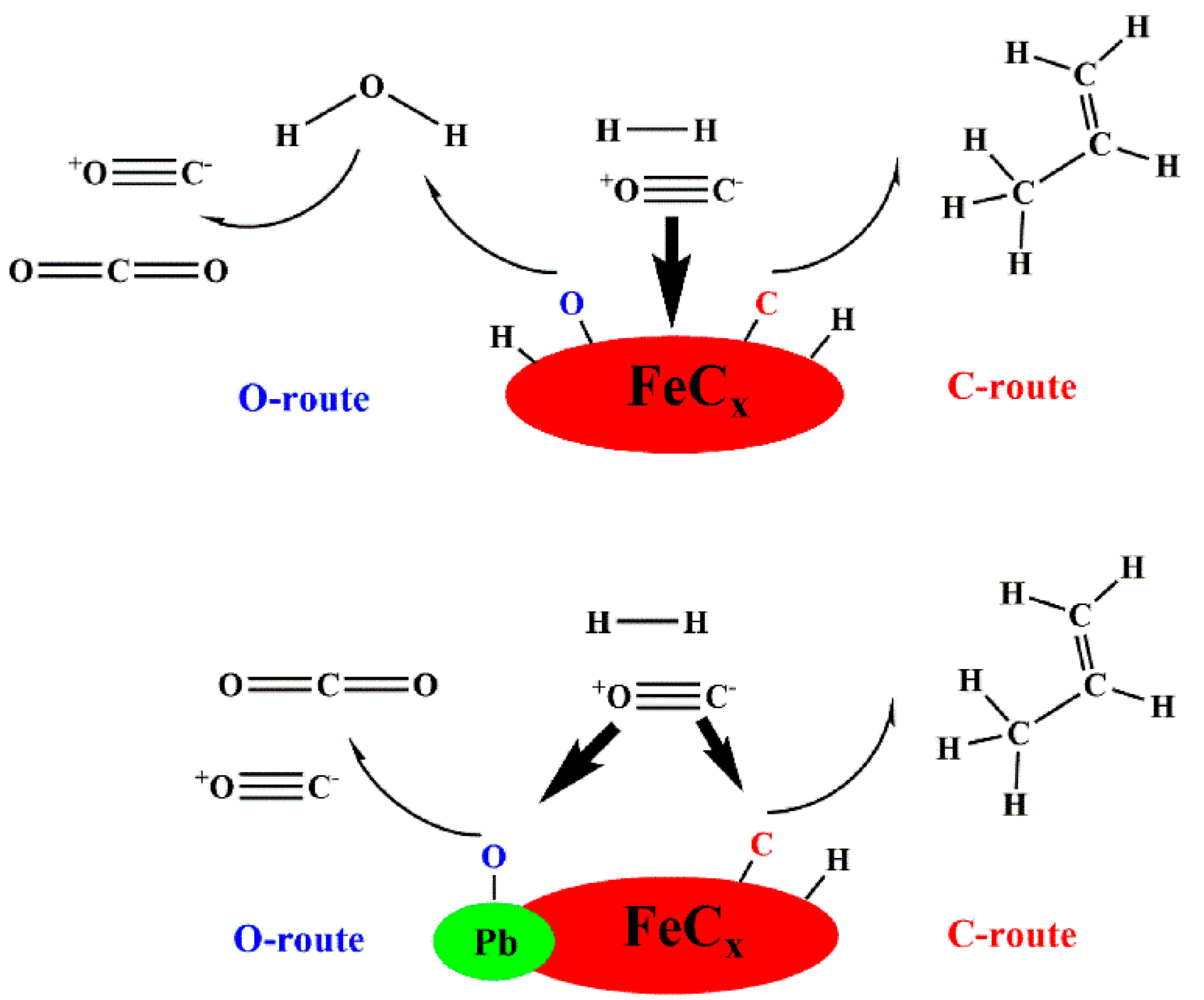
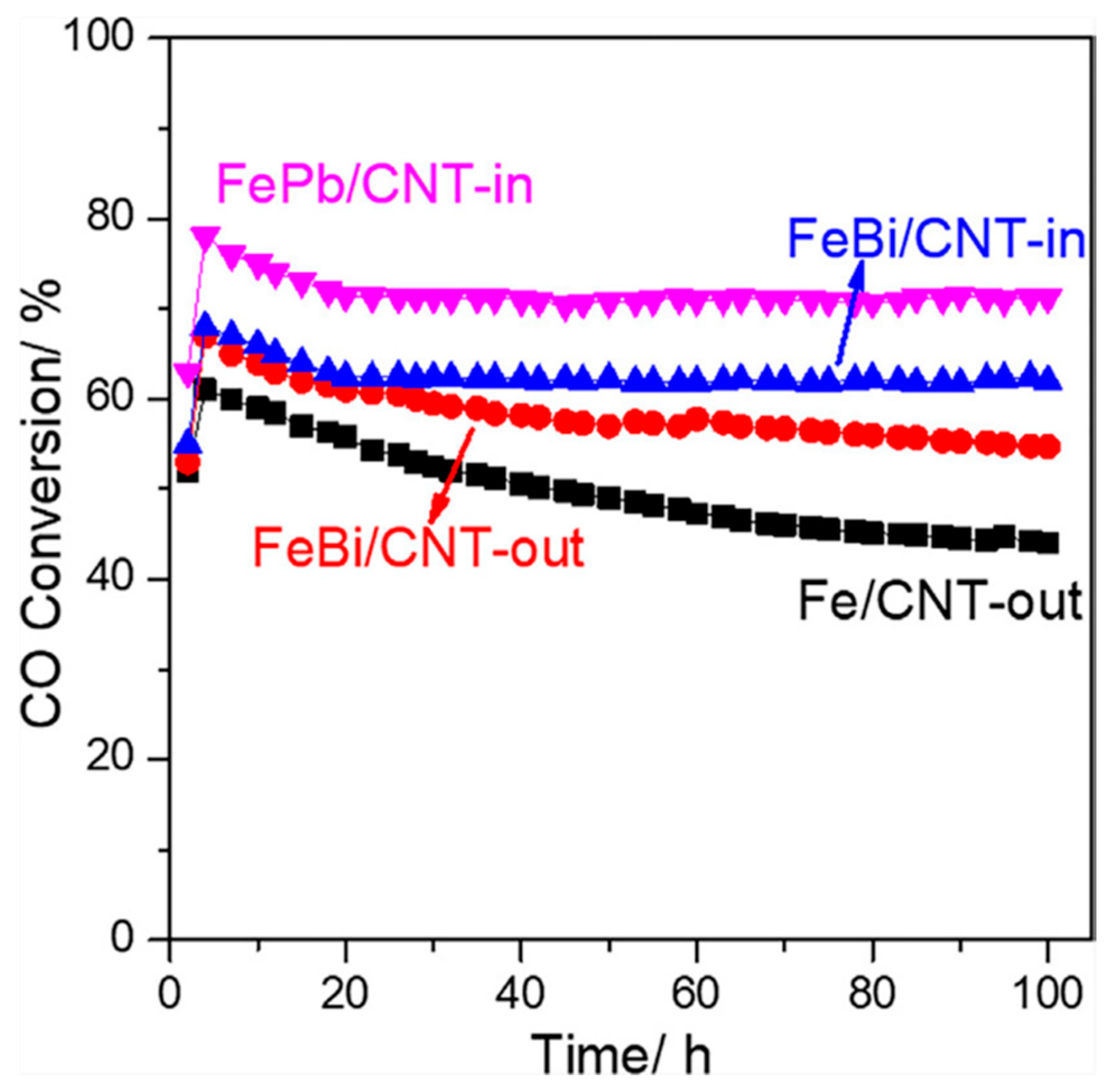
- Gu, B.; Ordomsky, V.V.; Bahri, M.; Ersen, O.; Chernavskii, P.A.; Filimonov, D.; Khodakov, A.Y. Effects of the promotion with bismuth and lead on direct synthesis of light olefins from syngas over carbon nanotube supported iron catalysts. Appl. Catal. B Environ. 2018, 234, 153–166. [Google Scholar] [CrossRef]
- Gu, B.; He, S.; Peron, D.V.; Pedrolo, D.R.S.; Moldovan, S.; Ribeiro, M.C.; Lobato, B.; Chernavskii, P.A.; Ordomsky, V.V.; Khodakov, A.Y. Synergy of nanoconfinement and promotion in the design of efficient supported iron catalysts for direct olefin synthesis from syngas. J. Catal. 2019, 376, 1–16. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, X.; Ji, J.; Duan, X.; Qian, G.; Zhou, X.; Chen, D.; Yuan, W. Modified carbon nanotubes by KMnO4 supported iron Fischer–Tropsch catalyst for the direct conversion of syngas to lower olefins. J. Mater. Chem. A 2015, 3, 4560–4567. [Google Scholar] [CrossRef]
- Roe, D.P.; Xu, R.; Roberts, C.B. Influence of a carbon nanotube support and supercritical fluid reaction medium on Fe-catalyzed Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2017, 543, 141–149. [Google Scholar] [CrossRef]
- Lu, J.; Yang, L.; Xu, B.; Wu, Q.; Zhang, D.; Yuan, S.; Zhai, Y.; Wang, X.; Fan, Y.; Hu, Z. Promotion effects of nitrogen doping into carbon nanotubes on supported iron Fischer–Tropsch catalysts for lower olefins. ACS Catal. 2014, 4, 613–621. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Q.; Oyunkhand, E.; Ding, S.; Yamane, N.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Nitrogen-rich mesoporous carbon supported iron catalyst with superior activity for Fischer-Tropsch synthesis. Carbon 2018, 130, 304–314. [Google Scholar] [CrossRef]
- Li, C.; Sayaka, I.; Chisato, F.; Fujimoto, K. Development of high performance graphite-supported iron catalyst for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2016, 509, 123–129. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, M.; Liu, B.; Xu, Y.; Liu, X. Insights into the influence of support and potassium or sulfur promoter on iron-based Fischer–Tropsch synthesis: Understanding the control of catalytic activity, selectivity to lower olefins, and catalyst deactivation. Catal. Sci. Technol. 2017, 7, 1245–1265. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.-F.; Zhang, Y. The effect of pore size or iron particle size on the formation of light olefins in Fischer–Tropsch synthesis. RSC Adv. 2015, 5, 29002–29007. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Luo, Y.; Gu, B.; Carvalho, A.; Chernavskii, P.A.; Cheng, K.; Khodakov, A.Y. Soldering of iron catalysts for direct synthesis of light olefins from syngas under mild reaction conditions. ACS Catal. 2017, 7, 6445–6452. [Google Scholar] [CrossRef]
- Ni, Z.; Qin, H.; Kang, S.; Bai, J.; Wang, Z.; Li, Y.; Zheng, Z.; Li, X. Effect of graphitic carbon modification on the catalytic performance of Fe@SiO2-GC catalysts for forming lower olefins via Fischer-Tropsch synthesis. J. Colloid Interface Sci. 2018, 516, 16–22. [Google Scholar] [CrossRef]
- Gong, W.; Ye, R.-P.; Ding, J.; Wang, T.; Shi, X.; Russell, C.K.; Tang, J.; Eddings, E.G.; Zhang, Y.; Fan, M. Effect of copper on highly effective Fe-Mn based catalysts during production of light olefins via Fischer-Tropsch process with low CO2 emission. Appl. Catal. B Environ. 2020, 278, 119302. [Google Scholar] [CrossRef]
- Feyzi, M.; Khodaei, M.M.; Shahmoradi, J. Effect of sulfur on the catalytic performance of Fe–Ni/Al2O3 catalysts for light olefins production. J. Taiwan Inst. Chem. Eng. 2014, 45, 452–460. [Google Scholar] [CrossRef]
- LI, S.-y.; Shuai, L.; Zhang, Y.-H.; Li, J.-L.; Liu, Z.-N.; Li, W. Syngas-derived olefins over iron-based catalysts: Effects of basic properties of MgO nanocrystals. J. Fuel Chem. Technol. 2018, 46, 1342–1351. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, X.; Liu, X. Supported Fe/MnOx catalyst with Ag doping for remarkably enhanced catalytic activity in Fischer–Tropsch synthesis. Catal. Sci. Technol. 2018, 8, 1953–1970. [Google Scholar] [CrossRef]
- Botes, G.F.; Bromfield, T.C.; Coetzer, R.L.; Crous, R.; Gibson, P.; Ferreira, A.C. Development of a chemical selective iron Fischer Tropsch catalyst. Catal. Today 2016, 275, 40–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Tu, J.; Wang, T.; Li, X. One-pot synthesis of promoted porous iron-based microspheres and its Fischer–Tropsch performance. Appl. Catal. A Gen. 2015, 499, 139–145. [Google Scholar] [CrossRef]
- Zhao, M.; Yan, C.; Jinchang, S.; Qianwen, Z. Modified iron catalyst for direct synthesis of light olefin from syngas. Catal. Today 2018, 316, 142–148. [Google Scholar] [CrossRef]
- Zhang, S.; Li, D.; Liu, Y.; Zhang, Y.; Wu, Q. Zirconium Doped Precipitated Fe-Based Catalyst for Fischer–Tropsch Synthesis to Light Olefins at Industrially Relevant Conditions. Catal. Lett. 2019, 149, 1486–1495. [Google Scholar] [CrossRef]
- Wang, D.; Chen, B.; Duan, X.; Chen, D.; Zhou, X. Iron-based Fischer–Tropsch synthesis of lower olefins: The nature of χ-Fe5C2 catalyst and why and how to introduce promoters. J. Energy Chem. 2016, 25, 911–916. [Google Scholar] [CrossRef]
- Liu, J.-X.; Wang, P.; Xu, W.; Hensen, E.J. Particle size and crystal phase effects in Fischer-Tropsch catalysts. Engineering 2017, 3, 467–476. [Google Scholar] [CrossRef]
- Sahir, A.H.; Zhang, Y.; Tan, E.C.; Tao, L. Understanding the role of Fischer–Tropsch reaction kinetics in techno-economic analysis for co-conversion of natural gas and biomass to liquid transportation fuels. Biofuels Bioprod. Biorefining 2019, 13, 1306–1320. [Google Scholar] [CrossRef]
- Fu, T.; Li, Z. Review of recent development in Co-based catalysts supported on carbon materials for Fischer–Tropsch synthesis. Chem. Eng. Sci. 2015, 135, 3–20. [Google Scholar] [CrossRef]
- Xing, C.; Sun, J.; Yang, G.; Shen, W.; Tan, L.; Zhu, P.; Wei, Q.; Li, J.; Kyodo, M.; Yang, R. Tunable isoparaffin and olefin synthesis in Fischer–Tropsch synthesis achieved by composite catalyst. Fuel Process. Technol. 2015, 136, 68–72. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Kang, S.-H.; Kim, J.-H.; Lee, Y.-J.; Jun, K.-W. Fischer-Tropsch synthesis on Co-Al2O3-(promoter)/ZSM5 hybrid catalysts for the production of gasoline range hydrocarbons. Korean J. Chem. Eng. 2015, 32, 1993–1998. [Google Scholar] [CrossRef]
- Sage, V.; Sun, Y.; Hazewinkel, P.; Bhatelia, T.; Braconnier, L.; Tang, L.; Chiang, K.; Batten, M.; Burke, N. Modified product selectivity in Fischer-Tropsch synthesis by catalyst pre-treatment. Fuel Process. Technol. 2017, 167, 183–192. [Google Scholar] [CrossRef]
- Pedersen, E.Ø.; Svenum, I.-H.; Blekkan, E.A. Mn promoted Co catalysts for Fischer-Tropsch production of light olefins—An experimental and theoretical study. J. Catal. 2018, 361, 23–32. [Google Scholar] [CrossRef]
- Nabaho, D.; Niemantsverdriet, J.H.; Claeys, M.; van Steen, E. Hydrogen spillover in the Fischer–Tropsch synthesis: An analysis of platinum as a promoter for cobalt–alumina catalysts. Catal. Today 2016, 261, 17–27. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, L.; Sun, Z. Fischer-Tropsch synthesis in a microchannel reactor using mesoporous silica supported bimetallic Co-Ni catalyst: Process optimization and kinetic modeling. Chem. Eng. Process. Process Intensif. 2017, 119, 44–61. [Google Scholar] [CrossRef]
- Zohdi-Fasaei, H.; Atashi, H.; Tabrizi, F.F.; Mirzaei, A.A. Modeling and optimization of Fischer-Tropsch synthesis over Co-Mn-Ce/SiO2 catalyst using hybrid RSM/LHHW approaches. Energy 2017, 128, 496–508. [Google Scholar] [CrossRef]
- Zafari, R.; Abdouss, M.; Zamani, Y.; Tavasoli, A. An Efficient Catalyst for Light Olefins Production from CO Hydrogenation: Synergistic Effect of Zn and Ce Promoters on Performance of Co–Mn/SiO2 Catalyst. Catal. Lett. 2017, 147, 2475–2486. [Google Scholar] [CrossRef]
- Zhou, W.-G.; Liu, J.-Y.; Wu, X.; Chen, J.-F.; Zhang, Y. An effective Co/MnOx catalyst for forming light olefins via Fischer–Tropsch synthesis. Catal. Commun. 2015, 60, 76–81. [Google Scholar] [CrossRef]
- Li, Z.; Lin, T.; Yu, F.; An, Y.; Dai, Y.; Li, S.; Zhong, L.; Wang, H.; Gao, P.; Sun, Y. Mechanism of the Mn Promoter via CoMn Spinel for Morphology Control: Formation of Co2C Nanoprisms for Fischer–Tropsch to Olefins Reaction. ACS Catal. 2017, 7, 8023–8032. [Google Scholar] [CrossRef]
- Lin, T.; Gong, K.; Wang, C.; An, Y.; Wang, X.; Qi, X.; Li, S.; Lu, Y.; Zhong, L.; Sun, Y. Fischer–Tropsch synthesis to olefins: Catalytic performance and structure evolution of Co2C-based catalysts under a CO2 environment. ACS Catal. 2019, 9, 9554–9567. [Google Scholar] [CrossRef]
- Phaahlamohlaka, T.N.; Dlamini, M.W.; Mogodi, M.W.; Kumi, D.O.; Jewell, L.L.; Billing, D.G.; Coville, N.J. A sinter resistant Co Fischer-Tropsch catalyst promoted with Ru and supported on titania encapsulated by mesoporous silica. Appl. Catal. A Gen. 2018, 552, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Du, J.; Wang, B.; Zhang, Y.; Liu, C.; Xiong, H.; Sun, F.; Chen, S.; Li, J. Plasma-assisted preparation of highly dispersed cobalt catalysts for enhanced Fischer–Tropsch synthesis performance. ACS Catal. 2018, 8, 6177–6185. [Google Scholar] [CrossRef]
- Bertella, F.; Lopes, C.W.; Foucher, A.C.; Agostini, G.; Concepción, P.; Stach, E.A.; Martínez, A.n. Insights into the Promotion with Ru of Co/TiO2 Fischer–Tropsch Catalysts: An In Situ Spectroscopic Study. ACS Catal. 2020, 10, 6042–6057. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Nisa, M.U.; Lv, J.; Li, Z. ZIF-67 as precursor to prepare high loading and dispersion catalysts for Fischer-Tropsch synthesis: Particle size effect. Fuel 2019, 241, 802–812. [Google Scholar] [CrossRef]
- Zafari, R.; Abdouss, M.; Zamani, Y. Application of response surface methodology for the optimization of light olefins production from CO hydrogenation using an efficient catalyst. Fuel 2019, 237, 1262–1273. [Google Scholar] [CrossRef]
- Pour, A.N.; Karimi, J.; Taghipoor, S.; Gholizadeh, M.; Hashemian, M. Fischer–Tropsch synthesis over CNT-supported cobalt catalyst: Effect of magnetic field. J. Iran. Chem. Soc. 2017, 14, 1477–1488. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Maksimov, S.V.; Maslakov, K.I.; Isaikina, O.Y.; Chernavskii, P.A.; Kazantsev, R.V.; Eliseev, O.L.; Savilov, S.S. Fischer-Tropsch synthesis over carbon-encapsulated cobalt and iron nanoparticles embedded in 3D-framework of carbon nanotubes. J. Catal. 2020, 389, 270–284. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Xu, Y.; Lin, Q.; Jiang, F.; Liu, X. Insight into the Intrinsic Active Site for Selective Production of Light Olefins in Cobalt-Catalyzed Fischer–Tropsch Synthesis. ACS Catal. 2019, 9, 7073–7089. [Google Scholar] [CrossRef]
- Kitakami, O.; Sato, H.; Shimada, Y.; Sato, F.; Tanaka, M. Size effect on the crystal phase of cobalt fine particles. Phys. Rev. B 1997, 56, 13849. [Google Scholar] [CrossRef]
- Tan, K.F.; Xu, J.; Chang, J.; Borgna, A.; Saeys, M. Carbon deposition on Co catalysts during Fischer–Tropsch synthesis: A computational and experimental study. J. Catal. 2010, 274, 121–129. [Google Scholar] [CrossRef]
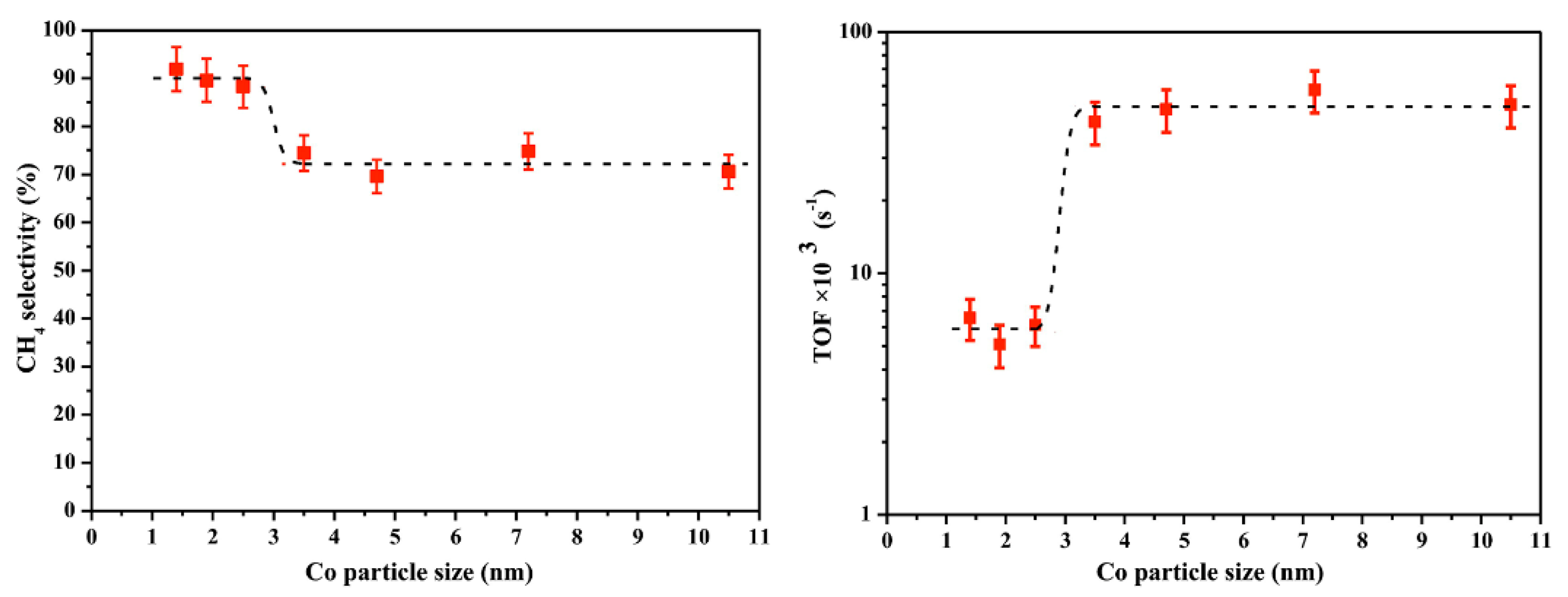
- Dai, Y.; Zhao, Y.; Lin, T.; Li, S.; Yu, F.; An, Y.; Wang, X.; Xiao, K.; Sun, F.; Jiang, Z. Particle size effects of cobalt carbide for Fischer–Tropsch to olefins. ACS Catal. 2018, 9, 798–809. [Google Scholar] [CrossRef]
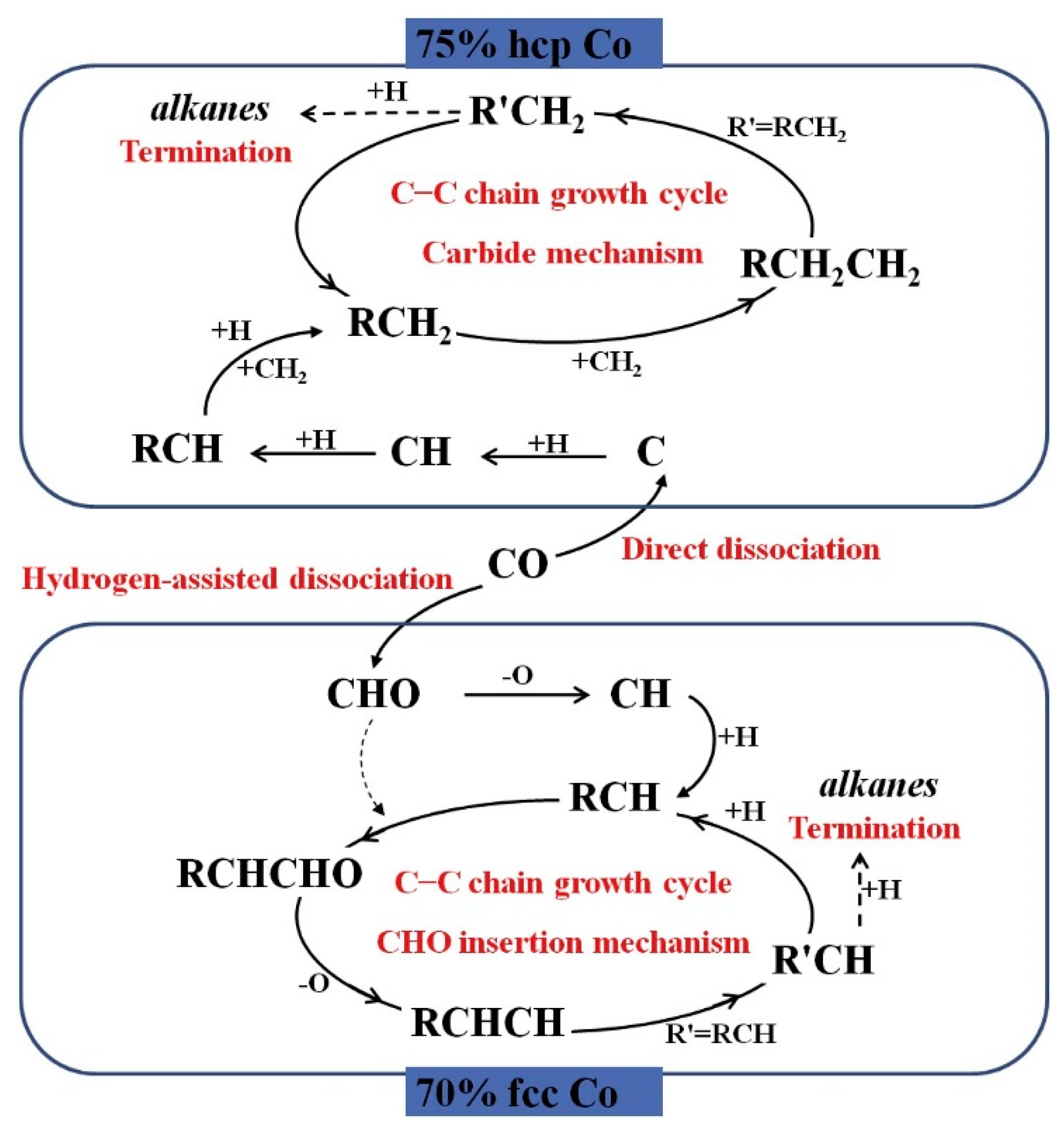
- Zhang, R.; Kang, L.; Liu, H.; Wang, B.; Li, D.; Fan, M. Crystal facet dependence of carbon chain growth mechanism over the Hcp and Fcc Co catalysts in the Fischer-Tropsch synthesis. Appl. Catal. B Environ. 2020, 269, 118847. [Google Scholar] [CrossRef]
- Su, H.-Y.; Zhao, Y.; Liu, J.-X.; Sun, K.; Li, W.-X. First-principles study of structure sensitivity of chain growth and selectivity in Fischer–Tropsch synthesis using HCP cobalt catalysts. Catal. Sci. Technol. 2017, 7, 2967–2977. [Google Scholar] [CrossRef]
- Bae, J.-S.; Hong, S.Y.; Park, J.C.; Rhim, G.B.; Youn, M.H.; Jeong, H.; Kang, S.W.; Yang, J.-I.; Jung, H.; Chun, D.H. Eco-friendly prepared iron-ore-based catalysts for Fischer-Tropsch synthesis. Appl. Catal. B Environ. 2019, 244, 576–582. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Zhang, C.; Chang, Q.; Wang, J.; Wang, X.; Lv, Z.; Dong, W.; Yang, Y.; Li, Y. Effect of alkalis on iron-based Fischer-Tropsch synthesis catalysts: Alkali-FeOx interaction, reduction, and catalytic performance. Appl. Catal. A Gen. 2016, 528, 131–141. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Wen, X.; Yang, Y.; Xu, J.; Li, Y. Effect of potassium promoter on phase transformation during H2 pretreatment of a Fe2O3 Fischer Tropsch synthesis catalyst precursor. Catal. Today 2020, 343, 101–111. [Google Scholar] [CrossRef]
- Xiong, H.; Motchelaho, M.A.; Moyo, M.; Jewell, L.L.; Coville, N.J. Effect of Group I alkali metal promoters on Fe/CNT catalysts in Fischer–Tropsch synthesis. Fuel 2015, 150, 687–696. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Zhang, C.; Wang, J.; Dong, W.; Yang, Y.; Li, Y. Alkalis in iron-based Fischer–Tropsch synthesis catalysts: Distribution, migration and promotion. J. Chem. Technol. Biotechnol. 2017, 92, 1472–1480. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Skiles, S.; Yang, F.; Yan, Z.; Goodman, D.W. Particle size effects in Fischer–Tropsch synthesis by cobalt. Catal. Today 2012, 181, 75–81. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Zhang, Y. Selectively forming light olefins via macroporous iron-based Fischer–Tropsch catalysts. React. Kinet. Mech. Catal. 2016, 119, 457–468. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Chen, N.; Ma, Q.; Fan, S.; Zhao, T.; Tsubaki, N. Effects of zinc on Fe-based catalysts during the synthesis of light olefins from the Fischer-Tropsch process. Chin. J. Catal. 2016, 37, 510–516. [Google Scholar] [CrossRef]
- Cho, J.M.; Ahn, C.I.; Pang, C.; Bae, J.W. Fischer–Tropsch synthesis on Co/AlSBA-15: Effects of hydrophilicity of supports on cobalt dispersion and product distributions. Catal. Sci. Technol. 2015, 5, 3525–3535. [Google Scholar] [CrossRef]
- Anderson, R.B.; Hall, W.K.; Krieg, A.; Seligman, B. Studies of the Fischer--Tropsch Synthesis. V. Activities and Surface Areas of Reduced and Carburized Cobalt Catalysts. J. Am. Chem. Soc. 1949, 71, 183–188. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Khoshandam, B. Carbon nanotube synthesis via the catalytic chemical vapor deposition of methane in the presence of iron, molybdenum, and iron–molybdenum alloy thin layer catalysts. Results Phys. 2017, 7, 3826–3837. [Google Scholar] [CrossRef]
- Li, Z.; Liu, R.; Xu, Y.; Ma, X. Enhanced Fischer–Tropsch synthesis performance of iron-based catalysts supported on nitric acid treated N-doped CNTs. Appl. Surf. Sci. 2015, 347, 643–650. [Google Scholar] [CrossRef]
- Okoye-Chine, C.; Mbuya, C.; Ntelane, T.; Moyo, M.; Hildebrandt, D. The effect of silanol groups on the metal-support interactions in silica-supported cobalt Fischer-Tropsch catalysts. A temperature programmed surface reaction. J. Catal. 2020, 381, 121–129. [Google Scholar] [CrossRef]
- Atashi, H.; Siami, F.; Mirzaei, A.; Sarkari, M. Kinetic study of Fischer–Tropsch process on titania-supported cobalt–manganese catalyst. J. Ind. Eng. Chem. 2010, 16, 952–961. [Google Scholar] [CrossRef]
- Duan, X.; Wang, D.; Qian, G.; Walmsley, J.C.; Holmen, A.; Chen, D.; Zhou, X. Fabrication of K-promoted iron/carbon nanotubes composite catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Energy Chem. 2016, 25, 311–317. [Google Scholar] [CrossRef]
- Zhou, X.; Ji, J.; Wang, D.; Duan, X.; Qian, G.; Chen, D.; Zhou, X. Hierarchical structured α-Al 2 O 3 supported S-promoted Fe catalysts for direct conversion of syngas to lower olefins. Chem. Commun. 2015, 51, 8853–8856. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, J.; Dugulan, A.I.; Holmen, A.; Chen, D.; de Jong, K.P.; Louwerse, M.J. Size and promoter effects in supported iron Fischer–Tropsch catalysts: Insights from experiment and theory. ACS Catal. 2016, 6, 3147–3157. [Google Scholar] [CrossRef]
- Li, T.; Virginie, M.; Khodakov, A.Y. Effect of potassium promotion on the structure and performance of alumina supported carburized molybdenum catalysts for Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2017, 542, 154–162. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, D.; Liu, X. Conversion of syngas toward aromatics over hybrid Fe-based Fischer-Tropsch catalysts and HZSM-5 zeolites. Appl. Catal. A Gen. 2018, 552, 168–183. [Google Scholar] [CrossRef]
- Muleja, A.A.; Yao, Y.; Glasser, D.; Hildebrandt, D. Variation of the short-chain paraffin and olefin formation rates with time for a cobalt Fischer–Tropsch catalyst. Ind. Eng. Chem. Res. 2017, 56, 469–478. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Qing, M.; Liu, C.-L.; Dong, W.-S. Direct synthesis of lower olefins from syngas via Fischer–Tropsch synthesis catalyzed by a dual-bed catalyst. Mol. Catal. 2020, 485, 110824. [Google Scholar] [CrossRef]
- Yang, J.; Fang, X.; Xu, Y.; Liu, X. Investigation of the deactivation behavior of Co catalysts in Fischer–Tropsch synthesis using encapsulated Co nanoparticles with controlled SiO2 shell layer thickness. Catal. Sci. Technol. 2020, 10, 1182–1192. [Google Scholar] [CrossRef]
- Savost’yanov, A.; Yakovenko, R.; Narochnyi, G.; Zubkov, I.; Sulima, S.; Soromotin, V.; Mitchenko, S. Deactivation of a Commercial Co–Al2O3/SiO2 Catalyst in Fischer–Tropsch Synthesis under High-Pressure and Gas Recycling Conditions. Pet. Chem. 2020, 60, 81–91. [Google Scholar] [CrossRef]
- Gorimbo, J.; Muleja, A.; Liu, X.; Hildebrandt, D. Fischer–Tropsch synthesis: Product distribution, operating conditions, iron catalyst deactivation and catalyst speciation. Int. J. Ind. Chem. 2018, 9, 317–333. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Pendyala, V.R.R.; Sparks, D.E.; Shafer, W.D.; Thomas, G.A.; MacLennan, A.; Hu, Y.; Davis, B.H. Fischer-Tropsch synthesis. Effect of KCl contaminant on the performance of iron and cobalt catalysts. Catal. Today 2018, 299, 28–36. [Google Scholar] [CrossRef]
- Pendyala, V.R.R.; Shafer, W.D.; Jacobs, G.; Martinelli, M.; Sparks, D.E.; Davis, B.H. Fischer–Tropsch synthesis: Effect of ammonia on product selectivities for a Pt promoted Co/alumina catalyst. RSC Adv. 2017, 7, 7793–7800. [Google Scholar] [CrossRef] [Green Version]
- Tsakoumis, N.E.; Rønning, M.; Borg, Ø.; Rytter, E.; Holmen, A. Deactivation of cobalt based Fischer–Tropsch catalysts: A review. Catal. Today 2010, 154, 162–182. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Sparks, D.E.; Todic, B.; Bukur, D.B.; Davis, B.H. Quantitative comparison of iron and cobalt based catalysts for the Fischer-Tropsch synthesis under clean and poisoning conditions. Catal. Today 2020, 343, 125–136. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Carvalho, A.; Legras, B.; Paul, S.; Virginie, M.; Sushkevich, V.L.; Khodakov, A.Y. Effects of co-feeding with nitrogen-containing compounds on the performance of supported cobalt and iron catalysts in Fischer–Tropsch synthesis. Catal. Today 2016, 275, 84–93. [Google Scholar] [CrossRef]
- Cheng, K.; Ordomsky, V.V.; Legras, B.; Virginie, M.; Paul, S.; Wang, Y.; Khodakov, A.Y. Sodium-promoted iron catalysts prepared on different supports for high temperature Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2015, 502, 204–214. [Google Scholar] [CrossRef]
- Gavrilović, L.; Brandin, J.; Holmen, A.; Venvik, H.J.; Myrstad, R.; Blekkan, E.A. Fischer-Tropsch synthesis—Investigation of the deactivation of a Co catalyst by exposure to aerosol particles of potassium salt. Appl. Catal. B Environ. 2018, 230, 203–209. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; van Dillen, A.J.; de Jong, K.P. Cobalt particle size effects in the Fischer− Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Kamata, H.; Ohara, H.; Izumi, Y.; Ong, D.S.W.; Chang, J.; Poh, C.K.; Chen, L.; Borgna, A. Low-Olefin Production Process Based on Fischer–Tropsch Synthesis: Process Synthesis, Optimization, and Techno-Economic Analysis. Ind. Eng. Chem. Res. 2020, 59, 8728–8739. [Google Scholar] [CrossRef]
- Martinelli, M.; Gnanamani, M.K.; LeViness, S.; Jacobs, G.; Shafer, W.D. An overview of Fischer-Tropsch Synthesis: XtL processes, catalysts and reactors. Appl. Catal. A Gen. 2020, 608, 117740. [Google Scholar] [CrossRef]
- Do, T.N.; Kim, J. Green C2-C4 hydrocarbon production through direct CO2 hydrogenation with renewable hydrogen: Process development and techno-economic analysis. Energy Convers. Manag. 2020, 214, 112866. [Google Scholar] [CrossRef]
- Snehesh, A.S.; Mukunda, H.; Mahapatra, S.; Dasappa, S. Fischer-Tropsch route for the conversion of biomass to liquid fuels-Technical and economic analysis. Energy 2017, 130, 182–191. [Google Scholar] [CrossRef]
- Okeke, I.J.; Mani, S. Techno-economic assessment of biogas to liquid fuels conversion technology via Fischer-Tropsch synthesis. Biofuels Bioprod. Biorefining 2017, 11, 472–487. [Google Scholar] [CrossRef]
- Rafati, M.; Wang, L.; Dayton, D.C.; Schimmel, K.; Kabadi, V.; Shahbazi, A. Techno-economic analysis of production of Fischer-Tropsch liquids via biomass gasification: The effects of Fischer-Tropsch catalysts and natural gas co-feeding. Energy Convers. Manag. 2017, 133, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Borugadda, V.B.; Kamath, G.; Dalai, A.K. Techno-economic and life-cycle assessment of integrated Fischer-Tropsch process in ethanol industry for bio-diesel and bio-gasoline production. Energy 2020, 195, 116985. [Google Scholar] [CrossRef]
- Campanario, F.; Ortiz, F.G. Techno-economic assessment of bio-oil aqueous phase-to-liquids via Fischer-Tropsch synthesis and based on supercritical water reforming. Energy Convers. Manag. 2017, 154, 591–602. [Google Scholar] [CrossRef]
- Herz, G.; Reichelt, E.; Jahn, M. Techno-economic analysis of a co-electrolysis-based synthesis process for the production of hydrocarbons. Appl. Energy 2018, 215, 309–320. [Google Scholar] [CrossRef]
- Kreutz, T.G.; Larson, E.D.; Elsido, C.; Martelli, E.; Greig, C.; Williams, R.H. Techno-economic prospects for producing Fischer-Tropsch jet fuel and electricity from lignite and woody biomass with CO2 capture for EOR. Appl. Energy 2020, 279, 115841. [Google Scholar] [CrossRef]
- Santos, G.R.S.; Basha, O.M.; Wang, R.; Ashkanani, H.; Morsi, B. Techno-economic assessment of Fischer-Tropsch synthesis and direct methane-to-methanol processes in modular GTL reactors. Catal. Today 2020, 371, 93–112. [Google Scholar] [CrossRef]
- Dimitriou, I.; Goldingay, H.; Bridgwater, A.V. Techno-economic and uncertainty analysis of Biomass to Liquid (BTL) systems for transport fuel production. Renew. Sustain. Energy Rev. 2018, 88, 160–175. [Google Scholar] [CrossRef]
- Zang, G.; Sun, P.; Elgowainy, A.A.; Bafana, A.; Wang, M. Performance and cost analysis of liquid fuel production from H2 and CO2 based on the Fischer-Tropsch process. J. CO2 Util. 2021, 46, 101459. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Huang, Y.; Yi, Q.; Wei, G.-Q.; Kang, J.-X.; Li, W.-Y.; Feng, J.; Xie, K.-C. Energy use, greenhouse gases emission and cost effectiveness of an integrated high–and low–temperature Fisher–Tropsch synthesis plant from a lifecycle viewpoint. Appl. Energy 2018, 228, 1009–1019. [Google Scholar] [CrossRef]
- Van Vliet, O.P.; Faaij, A.P.; Turkenburg, W.C. Fischer–Tropsch diesel production in a well-to-wheel perspective: A carbon, energy flow and cost analysis. Energy Convers. Manag. 2009, 50, 855–876. [Google Scholar] [CrossRef]
- Navas-Anguita, Z.; Cruz, P.L.; Martin-Gamboa, M.; Iribarren, D.; Dufour, J. Simulation and life cycle assessment of synthetic fuels produced via biogas dry reforming and Fischer-Tropsch synthesis. Fuel 2019, 235, 1492–1500. [Google Scholar] [CrossRef]
- Li, M.; Zhao, W.; Xu, Y.; Zhao, Y.; Yang, K.; Tao, W.; Xiao, J. Comprehensive Life Cycle Evaluation of Jet Fuel from Biomass Gasification and Fischer–Tropsch Synthesis Based on Environmental and Economic Performances. Ind. Eng. Chem. Res. 2019, 58, 19179–19188. [Google Scholar] [CrossRef]
- Ortiz, F.G.; Alonso-Fariñas, B.; Campanario, F.; Kruse, A. Life cycle assessment of the Fischer-Tropsch biofuels production by supercritical water reforming of the bio-oil aqueous phase. Energy 2020, 210, 118648. [Google Scholar] [CrossRef]
- Zang, G.; Sun, P.; Elgowainy, A.; Bafana, A.; Wang, M. Life Cycle Analysis of Electrofuels: Fischer–Tropsch Fuel Production from Hydrogen and Corn Ethanol Byproduct CO2. Environ. Sci. Technol. 2021, 55, 3888–3897. [Google Scholar] [CrossRef] [PubMed]
- Okeke, I.J.; Sahoo, K.; Kaliyan, N.; Mani, S. Life cycle assessment of renewable diesel production via anaerobic digestion and Fischer-Tropsch synthesis from miscanthus grown in strip-mined soils. J. Clean. Prod. 2020, 249, 119358. [Google Scholar] [CrossRef]
- Liu, C.M.; Sandhu, N.K.; McCoy, S.T.; Bergerson, J.A. A life cycle assessment of greenhouse gas emissions from direct air capture and Fischer–Tropsch fuel production. Sustain. Energy Fuels 2020, 4, 3129–3142. [Google Scholar] [CrossRef] [Green Version]
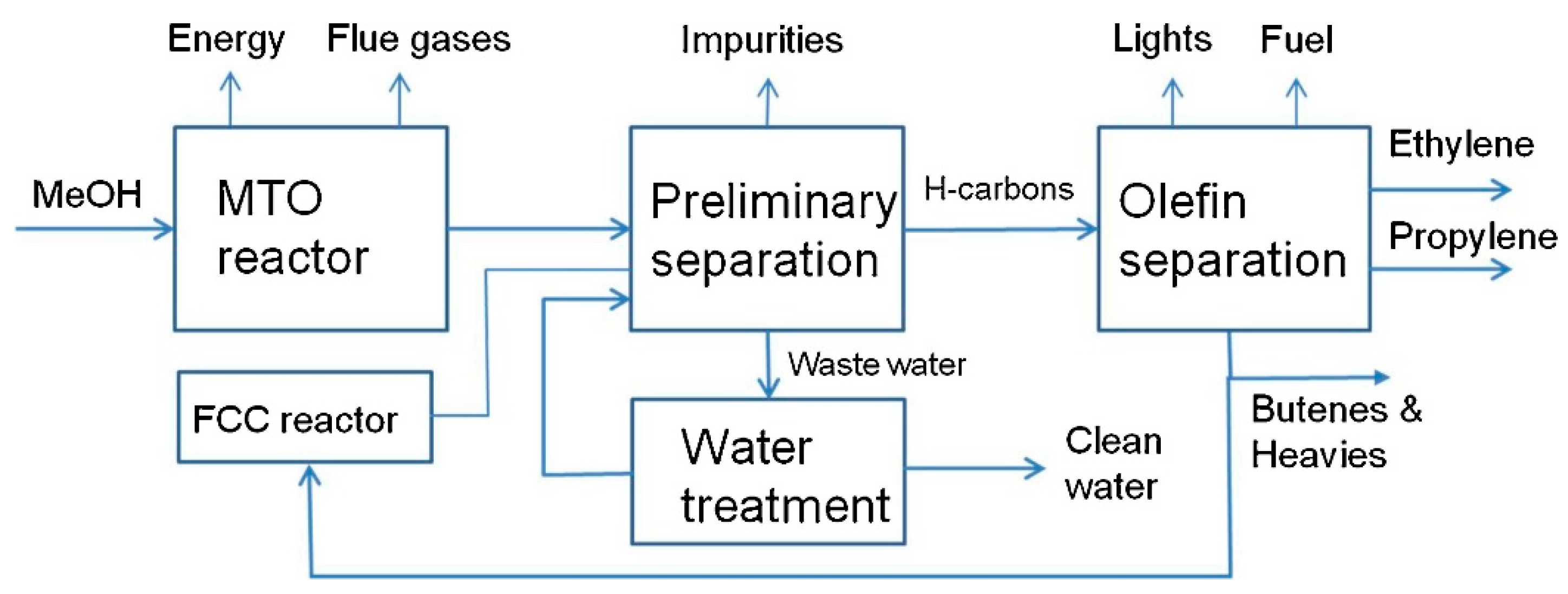
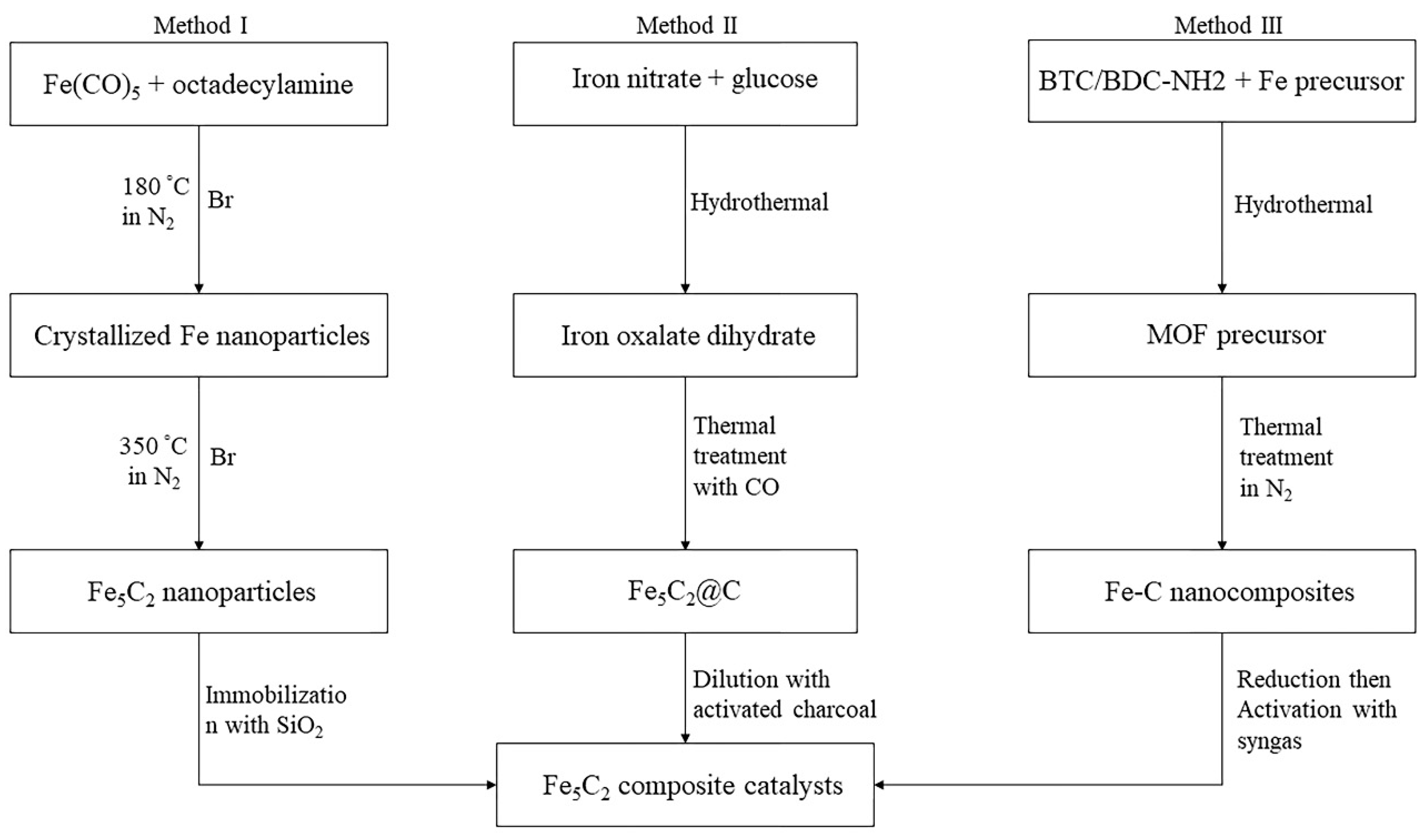

| Company | Carbon Source | Capacity (bpd) | Commissioning Date |
|---|---|---|---|
| Sasol | Coal | 2500 | 1955 |
| Sasol | Coal | 85,000 | 1980 |
| Sasol | Coal | 85,000 | 1982 |
| MossGas | Natural gas | 30,000 | 1992 |
| Shell | Natural gas | 12,500 | 1993 |
| Sasol/Qatar Petroleum | Natural gas | 34,000 | 2006 |
| Sasol Chevron | Natural gas | 34,000 | 2007 |
| Shell | Natural gas | 140,000 | 2009 |
| Sasol/USA | Natural gas | 96,000 | 2018 |
| Sasol/Canada | Natural gas | 96,000 | 2020 |
| Active Metal | Support | Promoter | Synthesis Method | Active Phase | C2-C4 Selectivity (%) | CO Conversion (%) | Reference |
|---|---|---|---|---|---|---|---|
| Fe | CNTs | Mn/K | Impregnation | FeMn2O4 before reduction | 51.7 | 30.1 | [23] |
| Fe Fe | CNTs CNTs | Bi Pb | Impregnation | Hägg χ-Fe5C2 or ε-Fe2C | 60.9 57.7 | 10.0 18.6 | [24] |
| Fe Fe | CNTs CNTs | Bi Pb/K | Impregnation | χ-Fe5C2 | 45–62.4 52.6–62 | 25.5–25.6 40.7–76.2 | [25] |
| Fe | CNTs | Mn/K | Impregnation | Hägg χ-Fe5C2 | 50.3 | 22.7 | [26] |
| Fe-Zn-Cu Fe | - CNTs | - K | Co-precipitation Deposition-precipitation | - | 35 42 | 45 16 | [27] |
| Fe | N-CNTs a | K | Impregnation | χ-Fe5C2 | 54.6 | 14.4 | [28] |
| Fe | NMCs b | - | Ultrasonic-impregnation | Fe5C2 and Fe2C | 33.9 | 92.6 | [29] |
| Fe-Cu | Graphite | - | Co-precipitation | Fe7C3 | 37.8 | 44.9 | [30] |
| Fe Fe Fe Fe Fe Fe Fe Fe | AC CSiO2 c CSiO2 c SiC SiC γ-Al2O3 SiO2 TiO2 | K K K/S Na/S K - - K | Impregnation | χ-Fe5C2 and ε’-Fe2.2C | 21.7 26.5 51.7 51.4 19.7 16.1 19.5 17.3 | 48.9 32.2 11.8 10.3 57.1 10.0 16.7 67.7 | [31] |
| Fe Fe Fe Fe | mSiO2 d SiO2 SiO2-CO e SiO2-H2 e | - - - - | Impregnation | χ-Fe5C2 is dominant (above 36%) Fe3C and ε-Fe2.2C are low (less than 14%) | 12.8 15.2 18.3 14.1 | 15.4 28.5 76.9 51.6 | [21] |
| Fe | SiO2-E f | Mn | Impregnation | - | 54.6 | 50.5 | [32] |
| Fe Fe-Cu Fe Fe-Cu | SiO2 SiO2 SiO2 SiO2 | - - K K | Impregnation Co-impregnation Impregnation Co-impregnation | χ-Fe5C2 | 10.1 15.2 18.7 18.1 | 23 33.9 29.9 34.3 | [18] |
| Fe Fe | SiO2 SiO2 | Bi Pb | Impregnation | χ-Fe5C2 | 53 32 | 17 55 | [33] |
| Fe Fe2O3 | SiO2-GC g SiO2 | - - | Hydrothermal deposition | Hägg χ-Fe5C2 | 12.9 17.4 | 40.6 40.6 | [34] |
| Fe-Mn | SiO2 | Cu | Co-precipitation, impregnation | Hägg χ-Fe5C2 | 40.1 | 96.9 | [35] |
| Fe Fe Fe | Si-CO e Si-H2 e Si-Syngas e | - - - | Co-precipitation | Fe7C3, χ-Fe5C2 ε-Fe2C, χ-Fe5C2 χ-Fe5C2 | 30.8 15.0 17.1 | 50.8 33.1 22.3 | [22] |
| Fe | α-Al2O3 | S/Na | Impregnation | - | 50 | 66 | [20] |
| Fe-Ni | Al2O3 | K2S | Co-precipitation | - | 77.8 | 64.6 | [36] |
| Fe Fe Fe Fe | MgO-NS h MgO-NS MgO-NS MgOcubes | - - - - | Impregnation Deposition-precipitation Ultrasonic impregnation Ultrasonic impregnation | - | 14.6 15.5 29.6 21.5 | 55.6 38.0 35.5 35.7 | [37] |
| Fe | MnOx | Ag | Impregnation | χ-Fe5C2 | 35.4 | 50.3 | [38] |
| Fe | - | Na/S | Precipitation | - | 66 | 30 | [39] |
| Fe Fe Fe Fe Fe | - - - - - | - Na K Zn Mn | Solvothermal | - | 19.3 23.3 22.1 18.1 34.1 | 91.0 93.2 97.1 98.3 37.4 | [40] |
| Fe Fe | - - | Zn/Na Zn/K | Co-precipitation | - | 42.7 37.19 | 97.16 5.02 | [41] |
| Fe | - | Zr | Co-precipitation | - | 57 | 40.6 | [42] |
| Active Metal | Support | Promoter | Synthesis Method | C2-C4 Selectivity (%) | CO Conversion (%) | Reference |
|---|---|---|---|---|---|---|
| Co Co | MHZSM 5 a HZSM 5 | - - | - | 29.1 30.9 | 79.0 75.9 | [47] |
| Co | Al2O3/ZSM 5 | La | Co-precipitation | 24.1 | 20.7 | [48] |
| Co Co | γ-Al2O3 γ-Al2O3-PT b | Ru/La Ru/La | Impregnation | 11.2 15.9 | 45.8 43.7 | [49] |
| Co-Mn | γ-Al2O3 | - | Co-impregnation | 8–11 | 20–45 | [50] |
| Co Co | Al2O3 Al2O3 + Pt/Al2O3 | - Pt | Impregnation | 44.8–50.4 46.2–59.2 | 9.5 13 | [51] |
| Co-Ni | mSiO2 c | - | Impregnation | 26.8 | 19.7 | [52] |
| Co-Mn-Ce | SiO2 | - | Impregnation | 17.4 | 10.1 | [53] |
| Co | Mn/SiO2 | Zn/Ce | Impregnation | 10–36 | 17–31.8 | [54] |
| Co | MnOx | - | Co-precipitation | 26.5–42.2 | 42.3–45.3 | [55] |
| Co-Mn | - | - | Co-precipitation | 50 | 2.5 | [56] |
| Co-Mn | - | - | Co-precipitation | 37.7 | 30 | [57] |
| Co Co Co | TiO2 TiO2@mSiO2 c TiO2@mSiO2 | - - Ru | Deposition precipitation | 10.6–20.9 5.2–21.7 12.1–23.3 | 27.5–33.1 17–46.1 31.6–58.9 | [58] |
| Co Co | TiO2-C d TiO2-P e | Pt Pt | Co-impregnation | 5.4 6.2–7.0 | 28.6 66.3 | [59] |
| Co | TiO2 TiO2 | - Ru | Impregnation | 32.3 27.2–29.9 | 24.3 82.3–98.3 | [60] |
| Co | C f | - | - | 10.87–11.87 | 34.15–35.62 | [61] |
| Co-Mn | Al2O3 GNS g rGO h | - - - | Impregnation | 14–28 22–42 25–53 | 21–37 5.4–39.2 20.5–33.2 | [7] |
| Co-Mn | GNS g | - | Impregnation | 29.2 | 49 | [62] |
| Co | CNT | - | Impregnation | 29.5–18.9 | 84–75 | [63] |
| Co Co | CNT-800 i CNT-1000 | - - | Impregnation, Spark plasma sintering | 8.1 20.3 | 14.9 34.5 | [64] |
| Catalyst | Technique | Note | Reference |
|---|---|---|---|
| Fe/MgO a | CO2-TPD b | Surface basicity of catalyst based on desorption peaks: Moderate alkaline sites (Mg2+/O2+) around 160–400 °C Strong basic sites (unsaturated O2−) above 400 °C MgO nanosheet: Mg2+/O2+ around 350 °C Unsaturated O2− nearly 600 °C For Fe/MgO-c-UI, the ratio of medium/strong basicity is higher than that of Fe/MgO-ns-UI | [37] |
| Unmodified Fe ore K/Cu/iron ore Fe/Cu/K/SiO2 c | CO2-TPD | CO2 adsorbed on the alkali surface: 22 μmol/g 100 μmol/g 129 μmol/g Iron ore-based catalysts contain Al2O3 which is more acidic than SiO2 | [71] |
| Alkali promoted Fe/SiO2 | H2-TPR d | Reducibility of catalysts based on alkali type: The First step reduction: The lower temperature peak: Fe2O3 → Fe3O4 The first step reduction temperature increase in the order of Li > Na > K > Rb > Cs Subsequent reduction: The higher temperature peaks: Fe3O4 → FeO and FeO → α-Fe | [72] |
| K/α-Fe2O3 | H2-TPR | Reducibility of catalysts based on amount of alkali: The first reduction temperatures shift to higher temperature by increasing potassium levels. The second reduction temperatures decrease with increasing potassium. | [73] |
| Catalyst | Technique | Dispersion (%) | C2-C4 Selectivity (%) | Note | Reference |
|---|---|---|---|---|---|
| Co/TiO2 Co/TiO2@mSiO2 a CoRu/TiO2@mSiO2 | Pulse Chemisorption | 4.5–1.9 3.6–3.7 5.0–6.7 | 10.6–20.9 5.2–21.7 12.1–23.3 | TChemisorption = 350–450 °C FTS (T = 220–250 °C, P = 10 bar, H2/CO = 2, GHSV = 800 mLg−1h−1) | [58] |
| Co/CNT | H2−TPD | 8.2–10.8 | 29.5–18.9 | FTS (T = 220 °C, P = 20 bar, H2/CO = 2, GHSV = 40 mLg−1h−1) | [63] |
| CoPt/TiO2-C b CoPt/TiO2-P1 c CoPt/TiO2-P3 CoPt/TiO2-P4 | H2−TPD O2−titration | 20.4 26.9 27.8 73.7 | 5.4 6.2 6.5 7.0 | FTS (T = 210 °C, P = 10 bar, H2/CO = 2, GHSV = 4 SLg−1h−1) | [59] |
| 0CTAB-Co@C d 2CTAB-Co@C 4CTAB-Co@C 8CTAB-Co@C | H2−TPD | 32.05 20.07 37.07 38.51 | 10.87 11.87 11.21 11.27 | FTS (T = 230 °C, P = 20 bar, H2/CO = 2, GHSV = 6.75 SLg−1h−1) | [61] |
| Catalyst | Promoter | T (°C) | P (bar) | GHSV (Lh−1g−1) | H2/CO | C2-C4 Selectivity (%) | CO Conversion (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Fe/α-Al2O3 | S/Na | 340 | 20 | 3 | 1 | 50 | 60–66 | [20] |
| Fe/α-Al2O3-H a | S | 350 | 1 | 9 | 1 | 68 | 0.9 | [86] |
| Fe-Ni/Al2O3 | K2S | 340 | 1 | 3 | 2 | 77.8 | 64.6 | [36] |
| Fe/CNTs | Mn/K | 270 | 20 | 30 | 1 | 51.7 | 30.1 | [23] |
| Fe/CNTs | Bi Pb | 350 350 | 1 1 | 3.4 3.4 | 1 1 | 60.9 57.7 | 10 18.6 | [24] |
| Fe/CNTs-Confined b | Bi Bi Pb/K Pb/K | 350 350 350 350 | 10 1 10 1 | 17 3.4 17 3.4 | 1 1 1 1 | 45 62.4 52.6 62 | 60.2 25.6 76.2 40.7 | [25] |
| Fe/CNTs c | Mn/K | 270 | 20 | 30 | 1 | 50.3 | 22.7 | [26] |
| Fe/N-doped CNTs Fe/N-doped CNTs | - K | 300 300 | 1 1 | 4.2 4.2 | 1 1 | 46.7 54.6 | 14.4 16.5 | [28] |
| Fe/NMCs d | - | 340 | 10 | - | 1 | 33.9 | 92.6 | [29] |
| Fe/CNTs | K | 270 | 20 | 18 | 1 | 42.2 | 28.8 | [85] |
| Fe/CNF | Na/S | 350 | 1.85 | 12–24 | 10 | 50 | 10 | [87] |
| Fe/AC Fe/CSiO2 e Fe/SiC | K K Na/S | 300 300 300 | 10 10 2 | 2.2 2.2 2.2 | 1.1 1.1 1.1 | 21.7 26.5 51.4 | 48.9 32.2 10.3 | [31] |
| Fe/SiO2-E f | Mn | 300 | 10 | - | 1 | 54.6 | 50.5 | [32] |
| Fe/SiO2 | - Cu K Cu/K | 300 300 300 300 | 20 20 20 20 | 16 16 16 16 | 2 2 2 2 | 10.1 15.2 18.7 18.1 | 23 33.9 29.9 34.3 | [18] |
| Fe/SiO2 | Bi Pb | 350 350 | 1 1 | 3.4 3.4 | 1 1 | 53 32 | 17 55 | [33] |
| Fe/MnOx | Ag | 340 320 | 10 10 | 7.4 7.4 | 1.1 1.1 | 35.4 34.3 | 50.3 55 | [38] |
| Fe/MgO nanosheets Fe/MgO cubes | - - | 300 300 | 10 10 | 8 8 | 1 1 | 14.6–29.6 21.5 | 35.5–55.6 35.7 | [37] |
| Fe-Cu/Graphite | - | 260 | 20 | - | 1.1 | 37.8 | 44.9 | [30] |
| Fe | Na/S | 330 | 20 | 12.9 | 4 | 64.24 | 25 | [39] |
| Fe | - Na K Zn Mn | 280 280 280 280 280 | 20 20 20 20 20 | 3 3 3 3 3 | 1 1 1 1 1 | 19.3 23.3 22.1 18.1 34.1 | 91 93.2 97.1 98.3 37.4 | [40] |
| Fe | Zn/Na Zn/K | 350 350 | 20 20 | 3 3 | 2.7 2.7 | 42.7 37.19 | 95.09 95.02 | [41] |
| Fe | Zr | 280 | 10 | - | 1 | 57 | 40.6 | [42] |
| Mo/γ-Al2O3 | K | 300 | 10 | 6 | 2 | 21.8 | 4.2 | [88] |
| Catalyst | Promoter | T (°C) | P (bar) | GHSV (Lh−1g−1) | H2/CO | C2-C4 Selectivity (%) | CO Conversion (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Co-Meso-HZSM 5 a Co-HZSM 5 | - - | 240 240 | 1 1 | - - | 2 2 | 29.1 30.9 | 79 75.9 | [47] |
| Co/γ-Al2O3 Co/γ-Al2O3-PT b | Ru/La Ru/La | 220 220 | 20 20 | 4–6 4–6 | 2 2 | 11.2 15.9 | 45.8 43.7 | [49] |
| Co/γ-Al2O3 | Mn | 240 | 5 | - | 2.1 | 8–11 | 20–45 | [50] |
| Co-Al2O3/ZSM 5 | La | 240 | 20 | 4 | 2 | 24.1 | 20.7 | [48] |
| Co/MnOx Co/MnOx-BDO c | - - | 240 240 | 10 10 | 2.5 2.5 | 2 2 | 26.5 42.2 | 45.3 42.3 | [55] |
| Co-Mn/SiO2 | Zn/Ce | 260 | 1 | 4.5 | 1 | 10–36 | 80–90 | [54] |
| Co/Al2O3 Co/Al2O3 + Pt/Al2O3 | - Pt | 220 220 | 20 20 | 144 144 | 2 2 | 44.8–50.4 46.2–59.2 | 9.5 13 | [51] |
| Co/TiO2 Co/TiO2@mSiO2 d Co/TiO2@mSiO2 | - - Ru | 220–250 220–250 220–250 | 10 10 10 | 0.8 0.8 0.8 | 2 2 2 | 10.6–20.9 5.2–21.7 12.1–23.3 | 18.6–36.6 17–46.1 31.6–58.9 | [58] |
| Co/CNT | - | 220 | 20 | 0.04 | 2 | 18.9–29.5 | 5–84 | [63] |
| Co/CNT | - | 240 | 20 | 5 | 2 | 8.1–20.3 | 34.5–66.7 | [64] |
| CoPt/TiO2-C e CoPt/TiO2-P1 f CoPt/TiO2-P3 CoPt/TiO2-P4 | - - - - | 210 210 210 210 | 10 10 10 10 | 4 4 4 4 | 2 2 2 2 | 5.4 6.2 6.5 7.0 | 19.9 48.2 39.6 3.4 | [59] |
| 0CTAB-Co@C g 2CTAB-Co@C 4CTAB-Co@C 8CTAB-Co@C | - - - - | 230 230 230 230 | 20 20 20 20 | 6.75 6.75 6.75 6.75 | 2 2 2 2 | 10.87 11.87 11.21 11.27 | 35.62 34.15 36.20 40.08 | [61] |
| Catalyst | Time (h) | C2-C4 Selectivity (%) | Deactivation | Reference |
|---|---|---|---|---|
| FeZnNa/zeolite | 100 | 46.1 | Carbon deposition suggested by Raman spectroscopy | [91] |
| Co/SiO2 | 46–50 46–50 | 11 at 220 °C 14.5 at 240 °C | -At 240 °C, oxidation of metallic Co -At 220 °C, blocking of pore channel and active sites with heavier hydrocarbon -Note that with increasing thickness of SiO2 shell, the average pore size decreases accelerating deactivation | [92] |
| Co-Al2O3/SiO2 | 500 | 7 | -Carbonization -Pore clogging by heavy hydrocarbons resulted in the decreased specific surface area -Agglomeration of cobalt crystallite | [93] |
| FeCuK/SiO2 | 5000–10000 | 2.73–10.14 | Carbon deposition confirmed by XRD | [94] |
| FeMn-HZSM-5 FeK-HZSM-5 | - - | 28.5 6.4 | -Coke deposition -Heavy hydrocarbon over FeK catalyst | [89] |
| Fe-Zr | 10 | 57 | -Surface enriching of Zr covering iron carbide active sites based on XPS results | [42] |
| FeKS/CSiO2 a | 10 | 47.7–51.7 | -K-induced carbon deposition -Oxidation of χ-Fe5C2 to Fe3O4 | [31] |
| Fe-Si-Cu-Rb Pt-Co/Al2O3 | 573–662 1254–1327 | 25.7 at 100 ppm KCl 9.11 at 50 ppm KCl | -Investigating KCl poisoning -Site blocking by K and Cl ions -Electronic modification affecting CO/H2 adsorption | [95] |
| Pt-Co/Al2O3 | - | 7.3 at 1000 ppm NH3 | Investigating ammonia poisoning -Cobalt nitride formation -Decreasing selectivity from 10.5 to 7.3 | [96] |
| Process-Catalyst | Notes | Reference |
|---|---|---|
| FTS-Bioethanol plant -Fe/CNT pellet catalyst | -Conversion of biomass-derived syngas to syncrude (biogasoline and biodiesel) -Reactant flow: 3305 kg syngas/h, product capacity: 1000 kg syncrude/h -Net annual profit: 5.2 MUSD/year, internal rate of return: 107.9% -Environmental friendly process | [109] |
| FTS -Fe and Co catalyst | -Conversion of biomass to FT liquids -Overall thermal efficiency of biomass to FT liquids considering electricity output was in the range of 41.3–45.5% for Fe- and Co-based catalyst. -Co-feeding of natural gas and biomass reduces costs of biomass pretreatment and gasification. -Co-feeding of natural gas and biomass reduces costs of FT liquids about 30% (from $28.8 to $19–$20 per GJ of FT liquids). -Production of FT biofuels at oil price of $60/barrel is not economically feasible. | [108] |
| LTFT and SCWRa -Not mentioned | -Integrating LTFT with SCWR of bio-oil aqueous phase to produce biofuels and electricity -Plant capacity: 60 t/h, feeding concentration: 25 wt%, return rate: 10% -FT liquids: 0.93 Є/kg diesel, 0.26 Є/kg jet fuel, 1.20 Є/kg gasoline -Electricity selling price: 0.17 Є/kWh -Decrease in selling price by increasing plant size (20–200 t/h) | [110] |
| FTS and co-electrolysis b -Co catalyst | -Fuel production via Power-to-X process -Reduced numbers of reactors and heat exchanger compared to Power-to-X technologies b -Overall energetic efficiency: 68% (62% considering heat losses) -Focus on valuable products like waxes favors economic feasibility -Capital expenditure of the plant: 194,000 Є/bpd which is higher than that of commercial plants, e.g., Velocys (90,000 Є/bpd), Shell/Pearl (122,000 Є/bpd), and Sasol/Oryx (25,000–44,000 Є/bpd) -Availability and cost of renewable electricity affect the production cost | [111] |
| FTS -Not mentioned | -Conversion of lignite and woody biomass to jet fuel and electricity -Plant profitability is sensitive to biomass input fraction -High moisture content of biomass (43%) causes energy penalty -Co-firing of lignite and biomass is less profitable than solely biomass -Carbon-negative plants (only biomass input) are economically feasible at oil prices below $100/bbl with carbon emission price above $120/tonne CO2eq | [112] |
| FTS and DMTM c -Co catalyst | -Conversion of natural gas into liquid products -Unit cost of DMTM process is sensitive to the methane recycle ratio -Unit cost of FTS in MCR is less sensitive to the tailgas recycle ratios -Higher energy requirements compared to conventional GTL technologies d -For internal rate of return (IRR) above 10%, tailgas recycle ratio has to be above 8% at CO conversion of 80%, while the minimum methane recycle ratio of 60% is required for profitability -For profitability index (PI) >1, tailgas recycle ratio of 15% (at CO conversion of 80%) and minimum methane recycle ratio of 55% is required e | [113] |
| FTS, MTG, TIGAS f -Co catalyst | -Conversion of biomass to liquid hydrocarbon fuels via Biomass-to-liquid (BTL) process -Modelling of BTL systems for gasification of woody biomass -Overall energy efficiency of BTL: 37.9–47.9% lower heating value (LHV) -Production costs of BTL: 17.88–25.41 Є per GJ of produced fuels -BTL production costs is 8% higher than current market prices | [114] |
| FTS -Barium zirconate-based perovskite-type catalyst | -Conversion of H2 + CO2 to FT liquid fuels via electricity generated from renewable source -CO2 and H2 are provided by ethanol plant and electrolysis, respectively. -H2 price ($2/kg via electrolysis in 2020) has the largest impact on the minimum selling price of FT fuel ($5.4–5.9/gal) -Conversion of 223 metric ton H2/day and 2387 metric ton CO2/day into 351 metric ton/day of liquid FT fuel obtains overall energy efficiency of 57.5% LHV and 52.2% HHV g -CO2 and H2 prices are required to be $17.3/metric ton CO2 and $0.8/kg H2 to be cost-competitive with petroleum diesel price of $3.1/gal in 2050 | [115] |
| FTO h -FeMnCuK -Fe2O3 | -Conversion of natural gas into light olefins -Capital expenditure of the FTO plant: 170.8 MM$ for treatment of (360 MT/day and 18,849 MMBtu/day) of natural gas -Internal rate of return for FeMnCuK-based FTO plant: 20% -The levelized production cost: $679/MT in year 2012 | [103] |
| FTS -Not mentioned | -Conversion of biogas to drop-in diesel fuel in biogas-to-liquid (BgTL) plant -Minimum selling price of the FT drop-in fuels: $5.67/gal (feed capacity:2000 Nm3/h) -Increasing feed capacity to 20,000 Nm3/h reduces minimum selling price to $2.06/gal | [107] |
| FTS - | -Co-conversion of natural gas and biomass to transportation fuels -Hydrocracker increases the production of diesel and jet fuels -Minimum fuel selling price: $2.17–3.60 and $2.47–3.47 per GGE i with and without hydrocracker, respectively | [45] |
| Process | Notes | Reference |
|---|---|---|
| BDR a, FTS | -Conversion of biogas to liquid fuels -Functional unit of the LCA study is defined as 1 kg of synthetic biodiesel produced at plant -Lifecycle environmental profile of synthetic biodiesel is calculated and compared with conventional diesel -Evaluation of the plant in terms of global warming, cumulative non-renewable energy demand, ozone layer depletion, acidification, and eutrophication | [119] |
| LTFT HTFT b | -Conversion of coal to FT oil -Study focused on LCA of energy use, CO2 emission and cost input of FTS from coal and its competitor -Mining and washing of coal, and oil production cause the energy input and CO2 emission -The FTS plant from coal to oil is not beneficial compared to oil refinery pathway in terms of energy use and greenhouse gases emission | [117] |
| Gasification FTS | -Conversion of biomass to FT jet fuel -Lifecycle includes the stages of biomass growth, collection, transportation, plant construction and demolition, production, product distribution, and consumption -Application of steam for heat supply (case1) and power generation (case2) -Cases1 and 2 are better than the commercial plant due to reduced nonrenewable resource consumption and pollutant emissions, while production costs increase. -The pollution mitigation benefit of case1 and 2 are small, the consumption of CO2 is much fewer than in traditional processes -Case1 and 2 are sensitive to consumption of electricity and stalk, respectively | [120] |
| SCWR c LTFT HT c | -Production of biofuels via SCWR-LTFT and HT which process bio-oil aqueous phase and oil phase, respectively -Estimating the cradle-to-gate environmental impacts especially the global warming potential (GWP) -Hot water produced in the process is considered as a co-product to be used for district heating. The impact of catalyst is accounted for in the process to produce biofuels | [121] |
| FTS | -Conversion of H2 and CO2 into FT fuels -H2 is provided by water electrolysis with electricity from solar, wind, and nuclear sources -CO2 is provided by corm ethanol industry byproduct -investigation of greenhouse gas (GHG) emissions of FT fuel plant -Environmental impacts and GHG emissions of FT fuel plant are evaluated using GREET 2020 model d -Energy efficiency of FT fuel production: 58% | [122] |
| FTS | -Conversion of miscanthus biomass to biogas via anaerobic digestion -Production of drop-in FT biodiesel by FTS -Focus on emission of CO2, CH4, and NOx which contributes to global warming potential -Compared to commercial plants, the drop-in FT biodiesel reduces both GHG emissions (by 73%) and fossil fuel depletion (4.91 MJ/GGE), while potential of respiratory impacts, smog formation, acidification, and eutrophication is higher. | [123] |
| DAC e, FTS | -Conversion of CO2 (obtained by DAC) and H2 (obtained by electrolysis) into FT biodiesel -Evaluation of GHG emissions from the DAC-FTS to biodiesel plant -The electricity emissions factor used in the process is relatively low -The biodiesel plant is suggested to be conducted in regions with very low grid emission factors -The biodiesel is suggested to be co-located with a renewable energy facility | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahyazadeh, A.; Dalai, A.K.; Ma, W.; Zhang, L. Fischer–Tropsch Synthesis for Light Olefins from Syngas: A Review of Catalyst Development. Reactions 2021, 2, 227-257. https://doi.org/10.3390/reactions2030015
Yahyazadeh A, Dalai AK, Ma W, Zhang L. Fischer–Tropsch Synthesis for Light Olefins from Syngas: A Review of Catalyst Development. Reactions. 2021; 2(3):227-257. https://doi.org/10.3390/reactions2030015
Chicago/Turabian StyleYahyazadeh, Arash, Ajay K. Dalai, Wenping Ma, and Lifeng Zhang. 2021. "Fischer–Tropsch Synthesis for Light Olefins from Syngas: A Review of Catalyst Development" Reactions 2, no. 3: 227-257. https://doi.org/10.3390/reactions2030015
APA StyleYahyazadeh, A., Dalai, A. K., Ma, W., & Zhang, L. (2021). Fischer–Tropsch Synthesis for Light Olefins from Syngas: A Review of Catalyst Development. Reactions, 2(3), 227-257. https://doi.org/10.3390/reactions2030015









