Biosynthesis of Copper Nanoparticles from Acacia cornigera and Annona purpurea and Their Insecticidal Effect against Tribolium castaneum
Abstract
1. Introduction
2. Materials and Methods
2.1. Biosynthesis of Copper Nanoparticles (CuNPs)
2.2. CuNPs Physicochemical Characterization
2.3. Determination of Total Phenols and Flavonoids
2.4. Insecticidal Activity of CuNPs
2.5. Determination of Cell Viability
2.6. Statistical Analysis
3. Results
3.1. Synthesis of Copper Nanoparticles from Plants’ Extracts
3.2. Insecticidal Activity of Copper Nanoparticles from Plants’ Extracts
3.3. Determination of Cell Viability
3.4. Characterization of Cu-Nanoparticles in Optimal Conditions
3.4.1. Scanning Electron Microscopy
3.4.2. Dynamic Light Scattering (DLS)
3.5. Determination of Total Phenols and Flavonoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel, D. Pesticides and Pest control. In Integrated Pest Management: Innovation-Development Process; Springer Science: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Dhaliwall, G.S.; Jindal, V.; Dhawan, A.K. Insect pest problems and crop losses: Changing trends. Indian J. Ecol. 2010, 37, 1–7. [Google Scholar]
- Stathas, I.G.; Sakellaridis, A.C.; Papadelli, M.; Kapolos, J.; Papadimitriou, K.; Stathas, G.J. The Effects of Insect Infestation on Stored Agricultural Products and the Quality of Food. Foods 2023, 12, 2046. [Google Scholar] [CrossRef] [PubMed]
- Hiruy, B.; Getu, E. Host type and textures on the survival of Tribolium castaneum (Coleoptera: Tenebrionidae) parental and filial generations. J. Entomol. Zool. 2018, 6, 622–626. [Google Scholar]
- Duarte, S.; Magro, A.; Tomás, J.; Hilário, C.; Ferreira, R.B.; Carvalho, M.O. Antifungal Activity of Benzoquinones Produced by Tribolium castaneum in Maize-Associated Fungi. Insects 2022, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, M.; Wang, Y.; Song, W.; Tang, P. Identification and functional analysis of cytochrome P450 CYP346 family genes associated with phosphine resistance in Tribolium castaneum. Pestic. Biochem. Phys. 2020, 168, 104622. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Parthasarathy, R.; Bai, H.; Woithe, K.; Kaussmann, M.; Nauen, R.; Harrison, D.A.; Palli, S.R. A brain-specific cytochrome P450 responsible for the majority of deltamethrin resistance in the QTC279 strain of Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2010, 107, 8557–8562. [Google Scholar] [CrossRef] [PubMed]
- Alif, A.A.S.; Thangapandiyan, S. Comparative bioassay of silver nanoparticles and malathion on infestation of red flour beetle, Tribolium castaneum. J. Basic Appl. Zool. 2019, 80, 55. [Google Scholar]
- Mukhtar, M.M.; Mustapha, M.A.; Aliyu, M.; Ibrahim, S.S. Multiple Pesticide Resistance in Rust-Red Flour Beetle (Tribolium castaneum, Herbst 1797) from Northern Nigeria Is Probably Driven by Metabolic Mechanisms. Agrochemicals 2023, 2, 170–180. [Google Scholar] [CrossRef]
- Rauf, A.; Wilkins, R.M. Malathion-resistant Tribolium castaneum has enhanced response to oxidative stress, immunity, and fitness. Pestic. Biochem. Physiol. 2022, 184, 105128. [Google Scholar] [CrossRef]
- Julio, A.H.; Gigliolli, A.A.; Cardoso, K.A.; Drosdoski, S.D.; Kulza, R.A.; Seixas, F.A.; Ruvolo-Takasusuki, M.C.; de Souza, C.G.; Lapenta, A.S. Multiple resistance to pirimiphos-methyl and bifenthrin in Tribolium castaneum involves the activity of lipases, esterases, and laccase2. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 195, 27–43. [Google Scholar] [CrossRef]
- Gonzalez-Mendoza, D.; Valdez-Salas, B.; Bernardo-Mazariegos, E.; Tzintzun-Camacho, O.; Gutiérrez-Miceli, F.; Ruíz-Valdiviezo, V.; Rodríguez-Hernández, L.; Sanchez-Viveros, G. Influence of Monometallic and Bimetallic Phytonanoparticles on Physiological Status of Mezquite. Open Life Sci. 2019, 14, 62–68. [Google Scholar] [CrossRef]
- Subekti, N.; Saputri, R. The application of Cinnamomum aromaticum nanoparticle and chlorpyrifos for controlling Tribolium castaneum. AIP Conf. Proc. 2019, 2155, 020018. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abd El-Hack, M.E.; Taha, A.E.; Fouda, M.M.G.; Ajarem, J.S.; N. Maodaa, S.; Allam, A.A.; Elshaer, N. Ecofriendly Synthesis and Insecticidal Application of Copper Nanoparticles against the Storage Pest Tribolium castaneum. Nanomaterials 2020, 10, 587. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Murthy, H.A.; Ahmed, H.M.; Islam, M.N.; Prasad, R. Phytogenic synthesis of metal/metal oxide nanoparticles for degradation of dyes. J. Renew. Mater. 2021, 10, 1911–1930. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Taghdiri, M.; Makari, V.; Rahimi-Nasrabadi, M. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1249–1254. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Valdez-Salas, B.; Ceceña-Duran, C.; Tzintzun-Camacho, O.; Gutiérrez-Miceli, F.; Grimaldo-Juarez, O.; González-Mendoza, D. Silver nanoparticles from Prosopis glandulosa and their potential application as biocontrol of Acinetobacter calcoaceticus and Bacillus cereus. Chem. Speciat. Bioavailab. 2016, 29, 1–5. [Google Scholar] [CrossRef]
- Rajendran, M.; Muthukumarasamy, E.; Paulraj, B. Antioxidant Assay of Gold and silver nanoparticles from edible basidiomycetes mushroom fungi. Free Radic. Antioxid. 2017, 7, 137–142. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- León-Jiménez, E.; Valdés-Salas, B.; González-Mendoza, D.; Tzintzun-Camacho, O. Synthesis and insecticide activity of Cunanoparticles from Prosopis juliflora (Sw) DC and Pluchea sericea (Nutt) on Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Rev. Soc. Entomol. Arg. 2019, 78, 1851–7471. [Google Scholar] [CrossRef]
- Mendez-Trujillo, V.; Valdez-Salas, B.; Carrillo-Beltran, M.; Curiel-Alvarez, M.A.; Tzintzun-Camacho, O.; Ceceña-Duran, C.; Gonzalez-Mendoza, D. Green Synthesis of Bimetallic nanoparticles from Prosopis juliflora (sw) dc., and its effect against cotton mealybug, Phenacoccus solenopsis (hemiptera: Pseudococcidae). Phyton Int. J. Exp. Bot. 2019, 88, 269–275. [Google Scholar] [CrossRef]
- Halimi, M.; Nasrabadi, M.; Soleamani, N.; Rouhani, N. Green synthesis of nanosilver particles from extract of Dracocephalum lindbergii. Asian J. Nanosci. Mater. 2018, 1, 19–24. [Google Scholar] [CrossRef]
- Ullah, S.; Shujaat, N.; Khan, R.U.; Khan, R.A.; Bilal, H.; Khan, M.; Bilal, H.; Khan, I.U.; Ahmad, M.; Khan, M.; et al. Ruellia nudiflora-mediated biological synthesis of silver nanoparticles and their potential antioxidant, antifungal and antibacterial applications against selected multidrug resistant bacteria. Pak-Euro J. Med. Life Sci. 2021, 4, 229–239. [Google Scholar]
- Amer, N.R.; Lawler, S.P.; Zohdy, N.M.; Younes, A.; ElSayed, W.M.; Wos, G.; Abdelrazek, S.; Omer, H.; Connon, R.E. Copper Exposure Affects Anti-Predatory Behaviour and Acetylcholinesterase Levels in Culex pipiens (Diptera, Culicidae). Insects 2022, 13, 1151. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Pittarate, S.; Perumal, V.; Rajula, J.; Thungrabeab, M.; Mekchay, S.; Krutmuang, P. Larvicidal and Antifeedant Effects of Copper Nano-Pesticides against Spodoptera frugiperda (J.E. Smith) and Its Immunological Response. Insects 2022, 13, 1030. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal Efficacy of Microbial-Mediated Synthesized Copper Nano-Pesticide against Insect Pests and Non-Target Organisms. Int. J. Environ. Res. Public Health 2021, 18, 10536. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-T.; Wang, J.-C. Properties Related to the HLB Value of Hybrid Thermoelectric Nanofluids at Different Temperatures. Polymers 2024, 16, 509. [Google Scholar] [CrossRef]
- Korukonda, J.R.; Korumilli, T.O. Au nanoparticles by instant green chemicals and optimising key synthesis parameters using Taguchi method. Micro Nano Lett. 2019, 14, 1198–1203. [Google Scholar] [CrossRef]
- Alshammari, S.O.; Mahmoud, S.Y.; Farrag, E.S. Synthesis of Green Copper Nanoparticles Using Medicinal Plant Krameria sp. Root Extract and Its Applications. Molecules 2023, 28, 4629. [Google Scholar] [CrossRef]
- Prathap, N.; Dravid, N.; Kaarmukhilnilavan, S.R.; Shivakumar, M.S.; Venkatesan, S.; Shaik, M.R.; Shaik, B. Copper Oxide Nanoparticles Synthesized from Indigofera linnaei Ali and This Plant’s Biological Applications. Inorganics 2023, 11, 462. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Ye, J.; Chen, Y.; Wang, R.; Jin, D.; Liu, Y. Mechanism Study on Nanoparticle Negative Surface Charge Modification by Ascorbyl Palmitate and Its Improvement of Tumor Targeting Ability. Molecules 2022, 27, 4408. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Beato, M.; Usseglio, V.L.; Camina, J.L.; Zygadlo, J.A.; Dambolena, J.; Zunino, M. Phenolic compounds as controllers of Sitophilus zeamais: A look at the structure-activity relationship. J. Stored Prod. Res. 2022, 99, 102038. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Photocatalytic and antibacterial activityof CuO nanoparticlesbiosynthesized using Verbascum thapsus leaves extract. Optik 2020, 204, 164230. [Google Scholar] [CrossRef]
- Avis Tresa, B.; Antony, R. Green synthesis of silver doped nano metal oxides of zinc & copper for antibacterial properties, adsorption, catalytic hydrogenation & photodegradation of aromatics. J. Environ. Chem. Eng. 2019, 7, 102840. [Google Scholar]
- Chan-Chan, L.H.; Martínez-Barbosa, M.E.; Vásquez-Alfaro, M.M.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M.; Lagarda-Diaz, I.; Maldonado, A. Segmented Poly(urea) urethane Nanoparticles: Size Optimization Using Taguchi Experimental Design and Nanoprecipitation Method. Curr. Nanosci. 2021, 17, 70–80. [Google Scholar] [CrossRef]
- Shahzad, K.; Manzoor, F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 2019, 44, 1–11. [Google Scholar] [CrossRef]
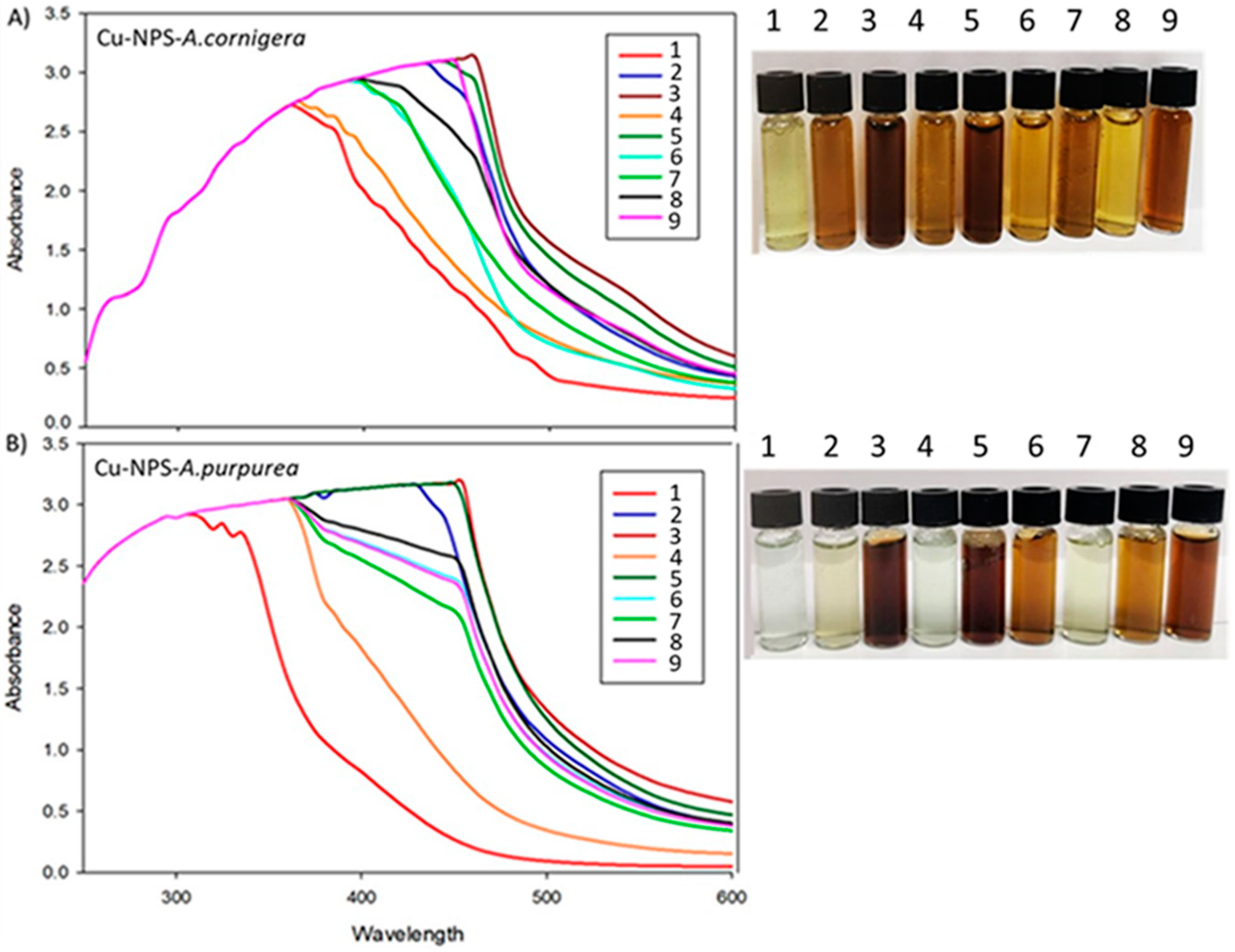

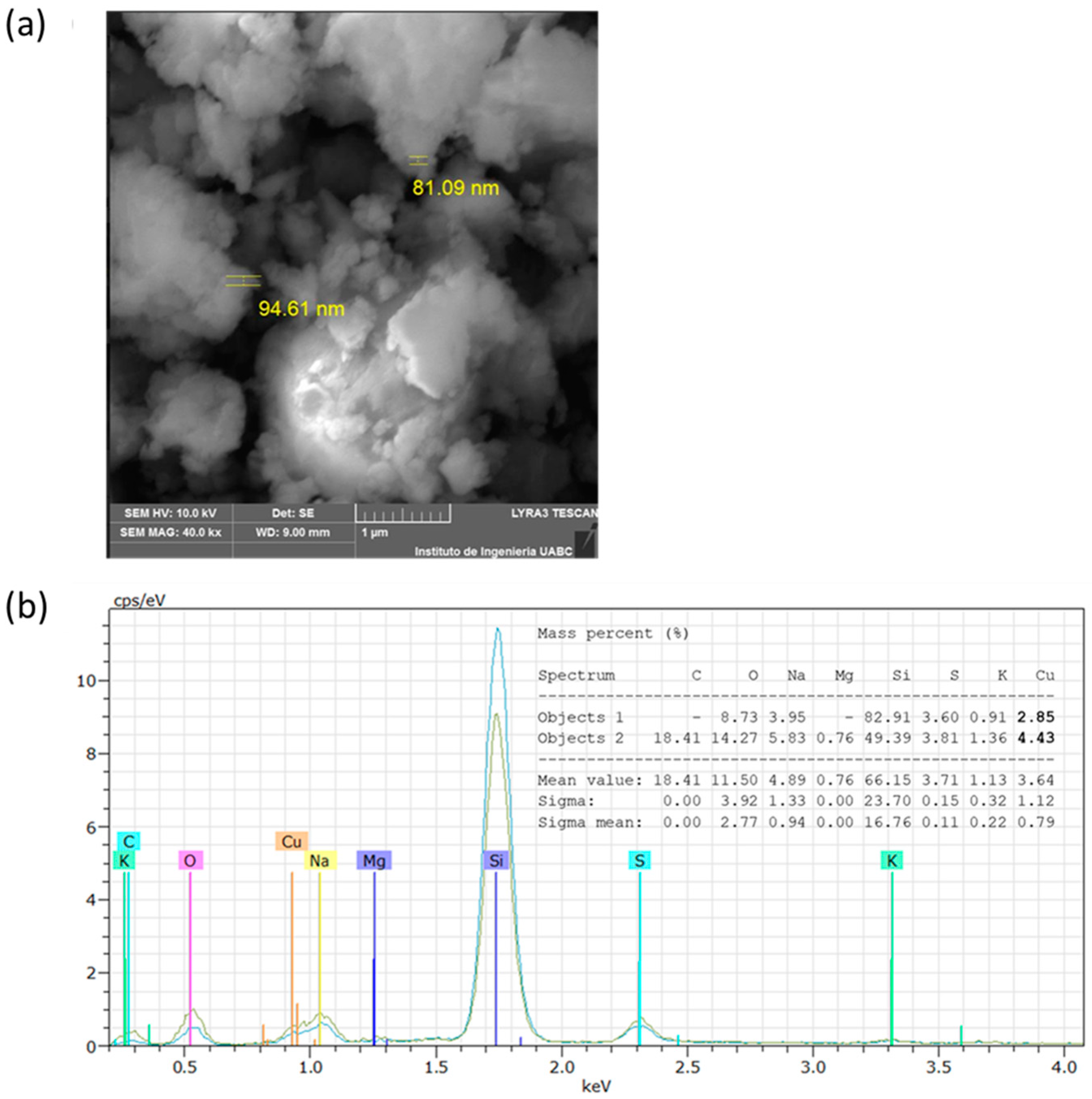
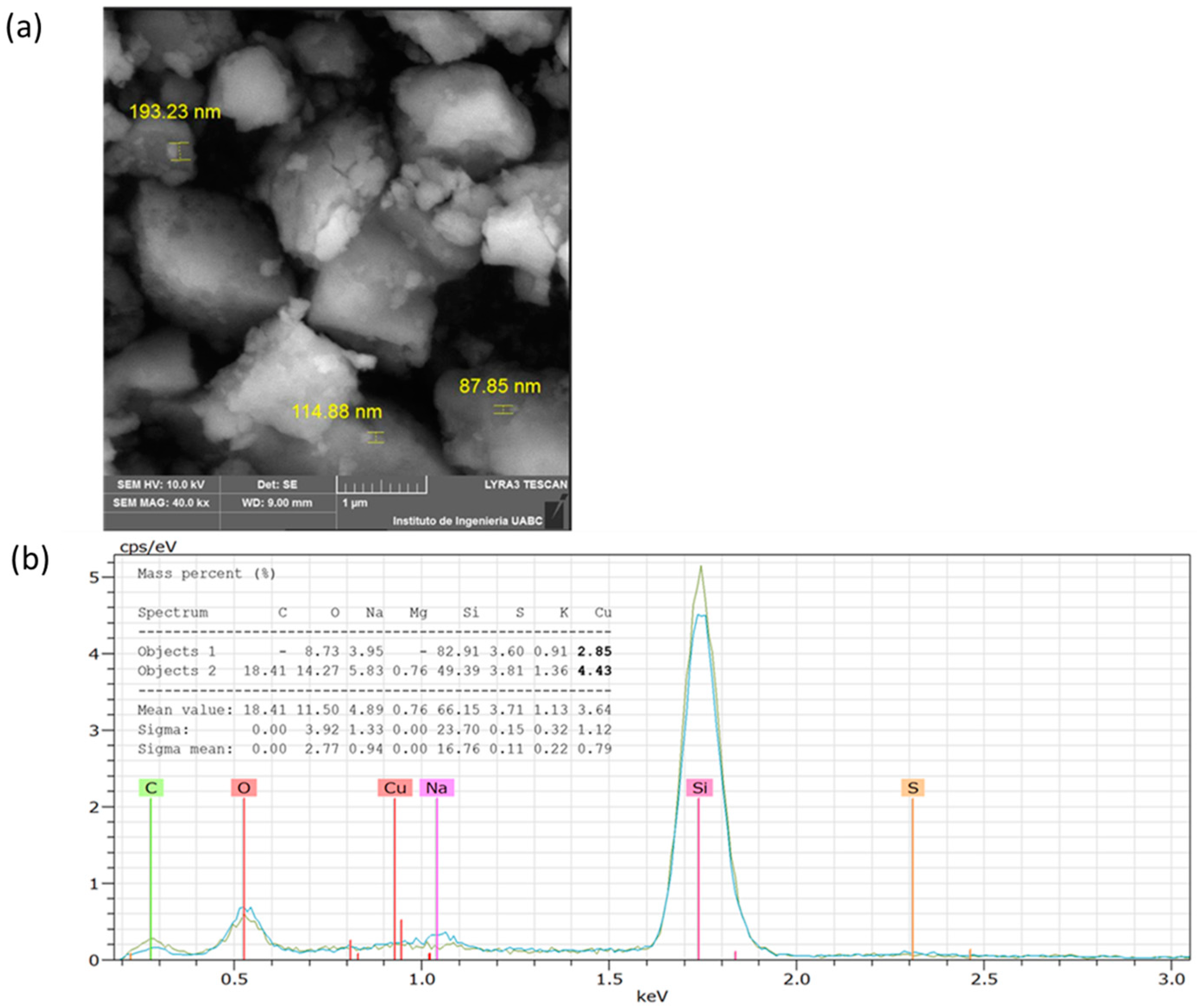
| Experiment | Temperature (°C) | pH | Concentration (% v/v) | Time (min) | CuNPs A. cornigera | CuNPs A. purpurea |
|---|---|---|---|---|---|---|
| % Mortality | ||||||
| 1 | 40 | 5 | 50 | 20 | 33 ± 11.55 a | 27 ± 5.77 a |
| 2 | 40 | 7 | 75 | 30 | 66 ± 5.77 b | 55 ± 5.77 b |
| 3 | 40 | 9 | 100 | 40 | 43 ± 20.82 b | 37 ± 11.55 c |
| 4 | 50 | 5 | 75 | 40 | 26 ± 5.77 a | 23 ± 8.45 a |
| 5 | 50 | 7 | 100 | 20 | 56 ± 5.77 b | 47 ± 5.77 b |
| 6 | 50 | 9 | 50 | 30 | 80 ± 10.00 c | 70 ± 10.00 c |
| 7 | 60 | 5 | 100 | 30 | 76 ± 11.55 c | 63 ± 10.00 c |
| 8 | 60 | 7 | 50 | 40 | 53 ± 6.87 b | 43 ± 5.77 ab |
| 9 | 60 | 9 | 75 | 20 | 26 ± 5.77 a | 23 ± 5.77 a |
| Factor | Level | Description of Levels | % Mortality | |
|---|---|---|---|---|
| CuNPs A. cornigera | CuNPs A. purpurea | |||
| Temperature (°C) | 2 | 50 | 54 | 46.6 |
| pH | 2 | 7 | 58.3 | 49.3 |
| Extract concentration (% v/v) | 3 | 100 | 58.3 | 49 |
| Reaction time (min) | 2 | 30 | 74 | 62.6 |
| Expected result under optimal conditions | 91.6 | 77.3 | ||
| Experimental value | 90 ± 8.16 | 76.6 ± 4.71 | ||
| Particle | DLS | LDV | |
|---|---|---|---|
| Average Diameter (nm) | Zeta Potential ζ(mV) | Electrophretic Mobility U (μmcm/(Vs)) | |
| CuNPs A. cornigera | 26.28 | +9.6 | 0.75 |
| CuNPs A. purpurea | 29.91 | −32.7 | 2.55 |
| Treatments | Total Phenols (mg AG/g) | Total Flavonoids (mg Q/g) |
|---|---|---|
| A. cornigera extract | 2.578 ± 0.128 b | 0.460 ± 0.014 a |
| A. purpurea extract | 3.017 ± 0.168 a | 0.357 ± 0.004 b |
| A. cornigera CuNPs | 0.104 ± 0.004 c | 0.031 ± 0.003 c |
| A. purpurea CuNPs | 0.142 ± 0.007 c | 0.027 ± 0.001 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solorzano Toala, R.; Gutierrez-Miceli, F.; Valdez-Salas, B.; Beltran-Partida, E.; Gonzalez-Mendoza, D.; Tzintzun-Camacho, O.; Grimaldo-Juarez, O.; Basilio-Cortes, A. Biosynthesis of Copper Nanoparticles from Acacia cornigera and Annona purpurea and Their Insecticidal Effect against Tribolium castaneum. Reactions 2024, 5, 274-284. https://doi.org/10.3390/reactions5020013
Solorzano Toala R, Gutierrez-Miceli F, Valdez-Salas B, Beltran-Partida E, Gonzalez-Mendoza D, Tzintzun-Camacho O, Grimaldo-Juarez O, Basilio-Cortes A. Biosynthesis of Copper Nanoparticles from Acacia cornigera and Annona purpurea and Their Insecticidal Effect against Tribolium castaneum. Reactions. 2024; 5(2):274-284. https://doi.org/10.3390/reactions5020013
Chicago/Turabian StyleSolorzano Toala, Rogelio, Federico Gutierrez-Miceli, Benjamin Valdez-Salas, Ernesto Beltran-Partida, Daniel Gonzalez-Mendoza, Olivia Tzintzun-Camacho, Onecimo Grimaldo-Juarez, and Antobelli Basilio-Cortes. 2024. "Biosynthesis of Copper Nanoparticles from Acacia cornigera and Annona purpurea and Their Insecticidal Effect against Tribolium castaneum" Reactions 5, no. 2: 274-284. https://doi.org/10.3390/reactions5020013
APA StyleSolorzano Toala, R., Gutierrez-Miceli, F., Valdez-Salas, B., Beltran-Partida, E., Gonzalez-Mendoza, D., Tzintzun-Camacho, O., Grimaldo-Juarez, O., & Basilio-Cortes, A. (2024). Biosynthesis of Copper Nanoparticles from Acacia cornigera and Annona purpurea and Their Insecticidal Effect against Tribolium castaneum. Reactions, 5(2), 274-284. https://doi.org/10.3390/reactions5020013









